Copyright
©2014 Baishideng Publishing Group Inc.
World J Med Genet. Nov 27, 2014; 4(4): 77-93
Published online Nov 27, 2014. doi: 10.5496/wjmg.v4.i4.77
Published online Nov 27, 2014. doi: 10.5496/wjmg.v4.i4.77
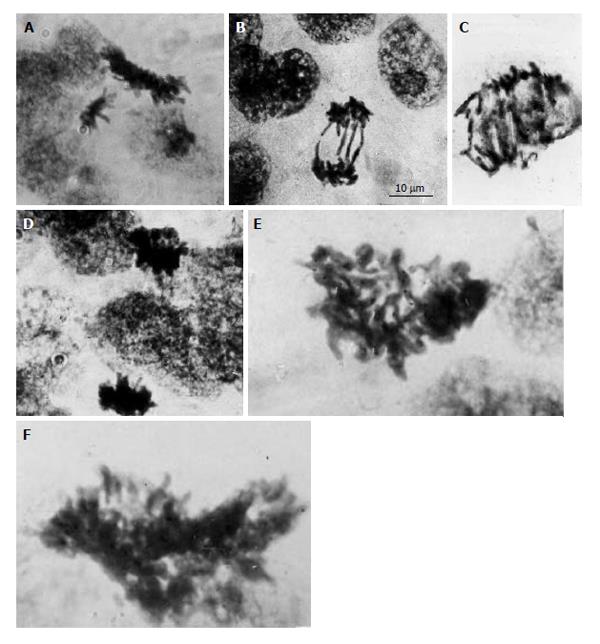
Figure 1 Diploid and polyploid mitoses in the junctional zone trophoblast cells in rat placenta at the 14th day of gestation.
A: Tetraploid metaphase and diploid anaphase; B: Diploid restitutional anaphase with multiple chromosome bridges; C: Polyploid restitutional anaphase with multiple chromosome bridges; D: Tetraploid normal anaphase; E: Octaploid metaphase; F: Hexadecaploid metaphase.
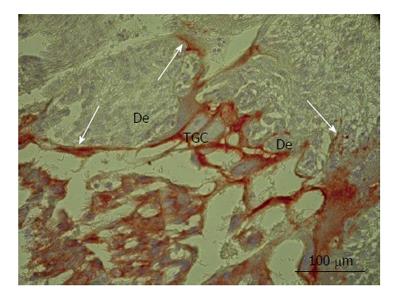
Figure 2 Secondary trophoblast giant cells of rat placenta at the 16th day of gestation.
Note massive long cytokeratin-positive sprouts that embrace wide zones of decidual tissue (De). TGC: Trophoblast giant cells.
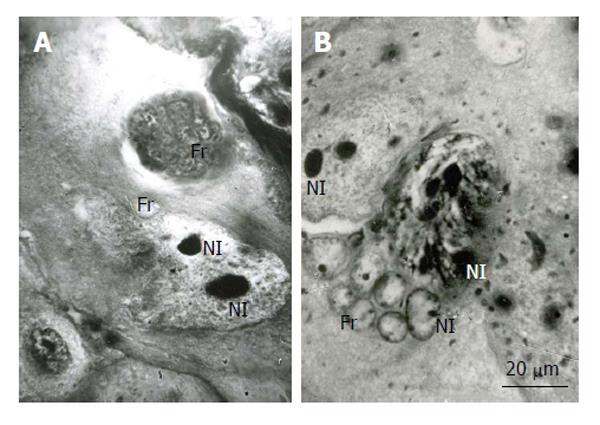
Figure 3 Fragmentation of the rat trophoblast giant cells.
A and B: First the giant nuclei fall into two large nuclei, the latter then breaks down into numerous small fragments (Fr); B: The giant nucleolus falls down into several nucleoli (Nl), then they form small nucleoli that seem to move into small nuclear fragments, some nucleoli in the nuclear fragments may be formed de novo. Heidenhain hematoxylin staining.
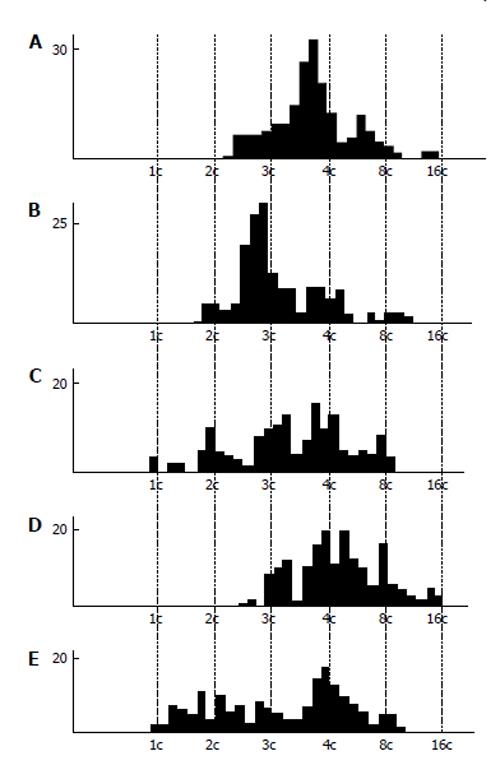
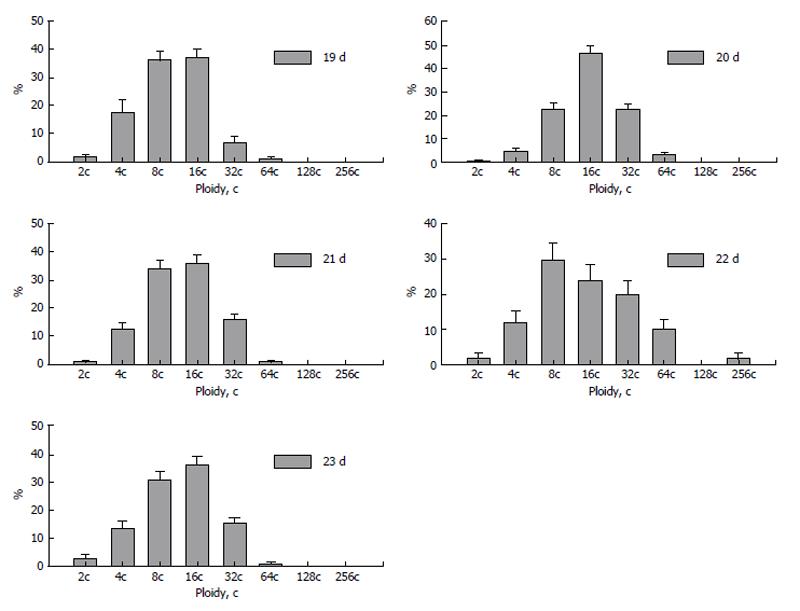
Figure 4 DNA content in the nuclear fragments of secondary trophoblast giant cells of mouse (A and B) and rat (C-E) at the 17th (A), 19th (B), 14th (C), 16th (D), and 18th (E) day of gestation.
Abscissa: The DNA content (arbitrary units, logarithmic scale), and ploidy, c; Ordinate: the number of nuclear fragments.
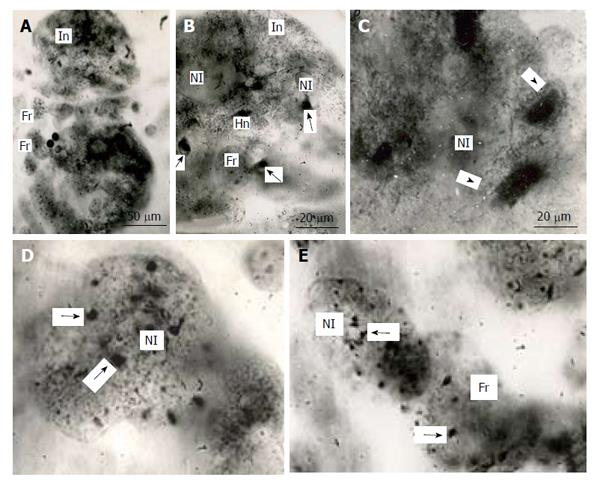
Figure 5 Distribution of heterochromatin blocks into nuclear fragments of mouse trophoblast giant cells.
A: The initial nucleus (In) of trophoblast giant cells (TGC) in the process of fragmentation; B: The initial nucleus contains large clear-cut heterochromatin blocks (Hn, arrows) near nucleoli (Nl); C: The nucleus contains non-classic polytene chromosomes with numerous distal loops (arrowheads); D and E: Nuclear fragments with small heterochromatin blocks (arrows) near nucleoli. Squash preparations, aceto-orcein staining. Fr: Nuclear fragments.
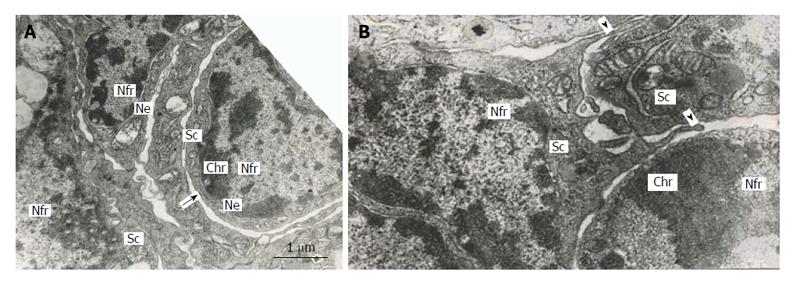
Figure 6 Subnuclear compartments in the rat trophoblast giant cells are separated from each other by forming rather wide channels of endoplasmic reticulum (A, arrow); some of them show intercellular junctions (A and B), some compartments (B) produce pseudopodia-like outgrowths (arrowheads) moving to other compartments.
Nfr: Nuclear fragments; Chr: Condensed chromatin; Sc: Subnuclear compartments; Ne: Nuclear envelope.
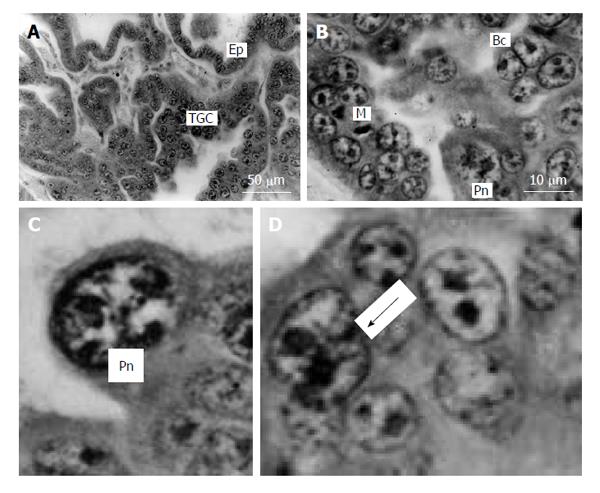
Figure 7 Silver fox placenta.
A and B: Trabeculae of trophoblast and folds of uterine glandular epithelium (Ep) mutually contact each other, trophoblast giant cells (TGC) are scattered in the fetal part of placenta between accumulations of proliferative cells; B: Mitotic (M), binucleate (Bc) and cells with polytene nuclei (Pn); C: Nucleus with non-classic polytene chromosomes; D: A polyploid nucleus in the beginning of fragmentation (arrow). Meyer hematoxylin staining.
Figure 8 Dynamics of polyploidization of the trophoblast cells in silver fox placenta.
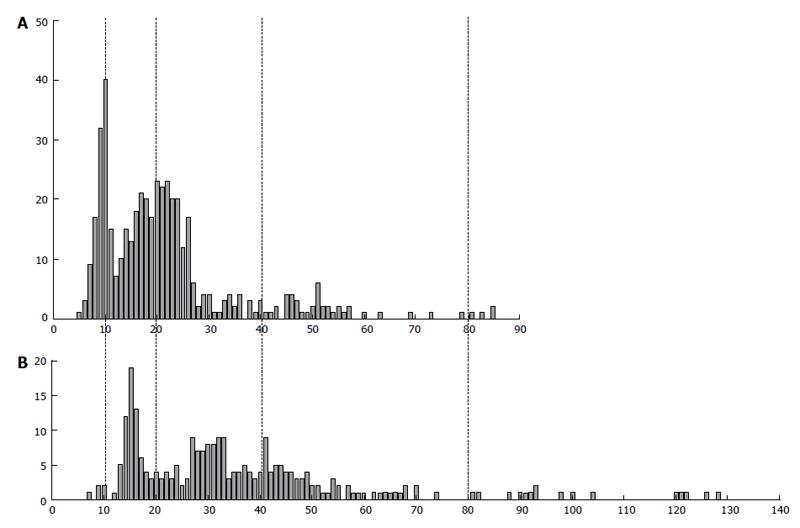
Figure 9 The difference in DNA content in human extravillous trophoblast cells invaded endometrium in placentae of the first trimester between two individuals (A and B).
Abscissa: The DNA content (arbitrary units); Ordinate: The number of cells.
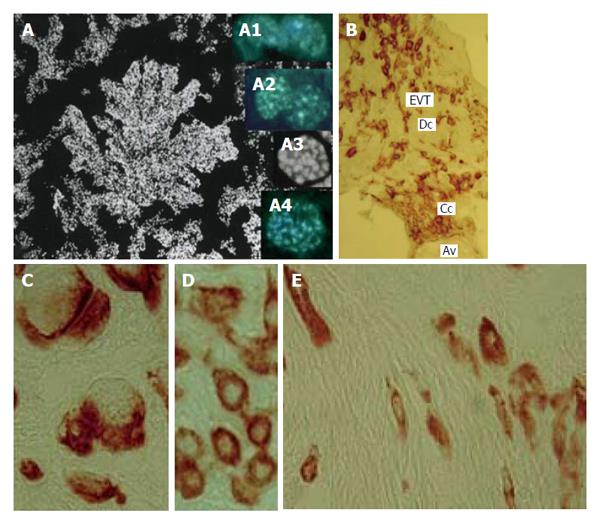
Figure 10 Human trophoblast cells undergoing polyploidization.
A: A squash spread of chorionic villus stained with 4’,6-diamidino-2-phenylindole; A1: Binucleate cell in interphase; A2: Binucleate cell in prophase; A3: Endometaphase; A4: Endoanaphase; B: A cell column (Cc) at the tip of anchoring villus (Av) generates a pool of extravillous trophoblast cells (EVT) capable for invasion of decidualized endometrium (Dc); C: Multinucleate EVT invaded decidua; D: Interstitial EVT of moderate ploidy; E: Small elongated low-ploidy EVT that reach myometrium.
- Citation: Zybina TG, Zybina EV. Genome variation in the trophoblast cell lifespan: Diploidy, polyteny, depolytenization, genome segregation. World J Med Genet 2014; 4(4): 77-93
- URL: https://www.wjgnet.com/2220-3184/full/v4/i4/77.htm
- DOI: https://dx.doi.org/10.5496/wjmg.v4.i4.77