Copyright
©2014 Baishideng Publishing Group Inc.
World J Med Genet. May 27, 2014; 4(2): 6-18
Published online May 27, 2014. doi: 10.5496/wjmg.v4.i2.6
Published online May 27, 2014. doi: 10.5496/wjmg.v4.i2.6
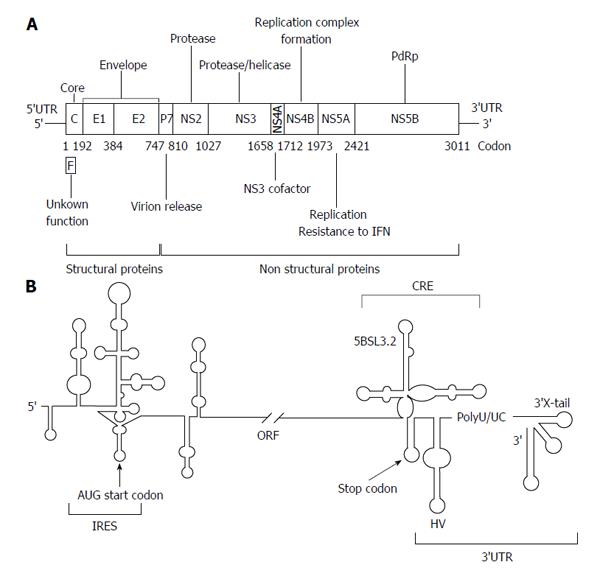
Figure 1 Genetic organization of the hepatitis C virus genomic RNA.
A: A schematic view of the hepatitis C virus (HCV) genome, showing the 5’ and 3’ untranslatable regions (UTRs) and the genes encoding for the different viral proteins. Numbers allude to codon positions; B: A detailed diagram of the secondary structure proposed for the 5’ and 3’ ends is pictured. The region required for internal ribosome entry site (IRES) activity is indicated. The 3’ end of the viral genomic RNA is organized into two well-defined structural elements: the cis-acting replicating element region containing the essential domain 5BSL3.2, and the 3’X-tail, separated by a hypervariable sequence (HV) and a polyU/UC tract. Start and stop translation codons, placed at positions 342 and 9371, respectively, are indicated by arrows. Numbers refer to the aminoacid positions according to the HCV Con1 isolate (GenBank accession number AJ238799). RdRp: RNA-dependent RNA polymerase; IFN: interferon; ORF: Open reading frame.
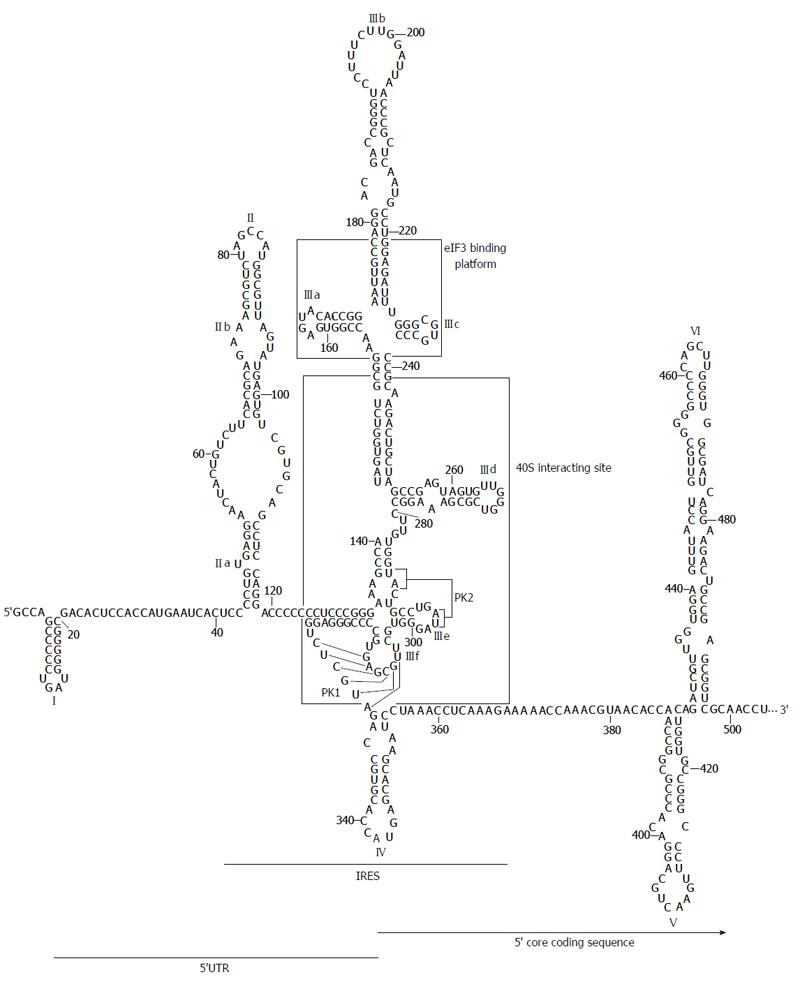
Figure 2 Secondary structure proposed for the hepatitis C virus internal ribosome entry site.
The 5’ untranslatable region (UTR) plus domains V and VI located at the core coding sequence are included. Minimum region for internal ribosome entry site (IRES) activity is depicted. Domains involved in the interaction with eIF3 factor and ribosomal subunit 40S are marked in boxes. Pseudoknot elements are indicated as PK1 and PK2. The translation start codon is shown in bold. Nucleotide numbering corresponds to hepatitis C virus con1 isolate.
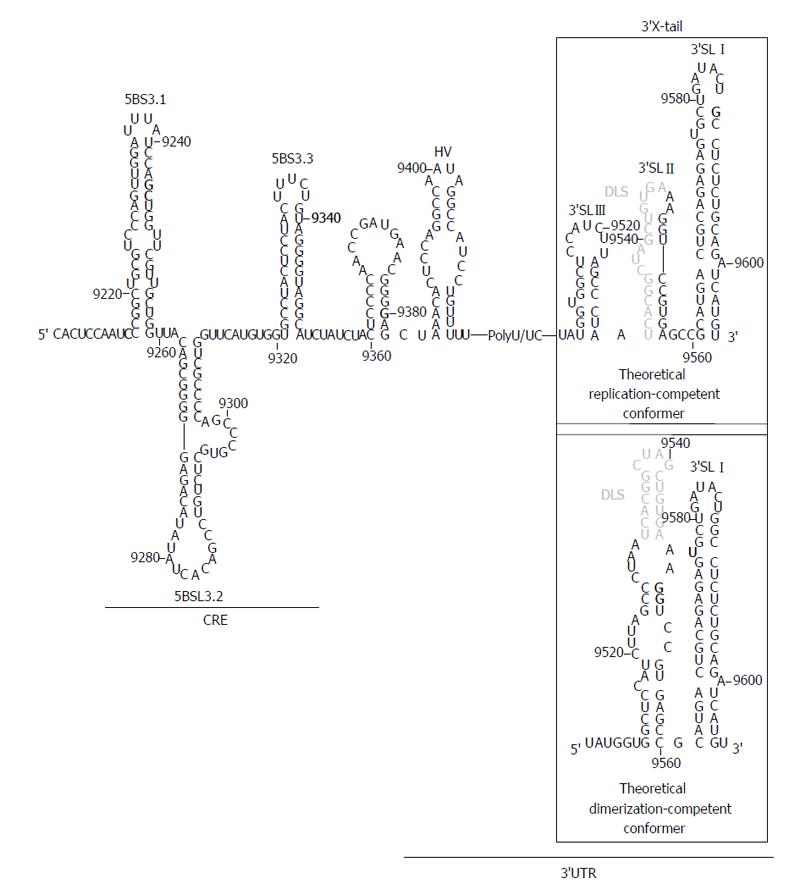
Figure 3 Theoretical folding of the 3’ end of the hepatitis C virus genomic RNA.
The figure shows the entire 3’ end containing the cis-acting replicating element (CRE) region plus the 3’ untranslatable region (UTR). The 3’X-tail folds into two different conformers with distinct functional roles. Dimer linkage sequence (DLS) is shown in grey. Translation stop codon in position 9371 is indicated in bold. Nucleotide numbering is as noted in Figure 2.
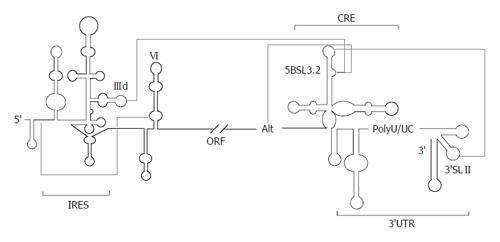
Figure 4 Long-range RNA-RNA interactions in the hepatitis C virus genome.
Detailed diagram of the interacting network in the genomic hepatitis C virus RNA. The minimum region for internal ribosome entry site (IRES) activity is marked. The 3’ end of the viral genome, containing both the cis-acting replicating element (CRE) and the 3’ untranslatable region (UTR), is included. Functional RNA domains involved in the establishment of long-distant contacts are indicated. Figure adapted from[5].
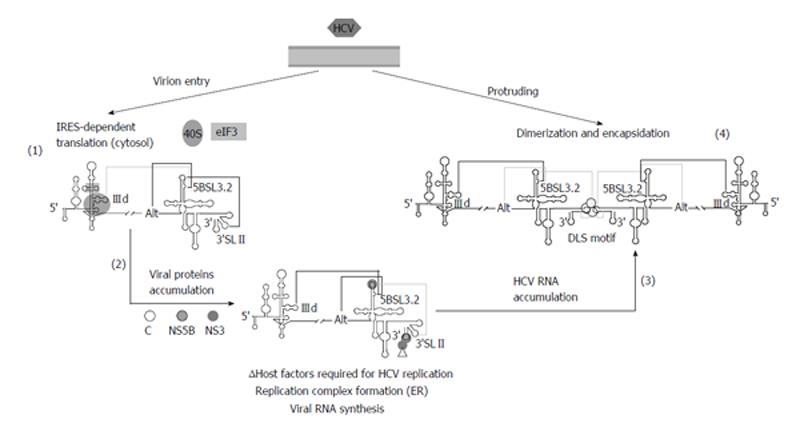
Figure 5 Proposed model for the participation of long-distant RNA-RNA interactions in the consecution of the hepatitis C virus infective cycle.
Virions penetrate inside the cell and the genomic RNA is released in the cytoplasm to initiate viral translation (1). The 40S ribosomal subunit binds to the IIId subdomain of the internal ribosome entry site (IRES) region and avoids its interaction with domain 5BSL3.2, thus favoring the contacts Alt-5BSL3.2-3’SLII. Once protein levels have surpassed a certain concentration (2), replication complexes are fixed on the surface of the endoplasmic reticulum (ER) to initiate the genomic RNA amplification. The recruitment of protein factors at the 3’X-tail would hide the 3’SLII domain. This would displace the interactions balance toward the IIId-5BSL3.2 contact, thus impeding efficient translation. Importantly, a considerable pool of molecules should alternatively display a preferred folding defined by the interaction Alt-5BSL3.2, which is indispensable for replication. The accumulation of newly naked viral genomes (3) could induce a replication-defective state by the acquisition of a favored conformation involving the interaction IIId-5BSL3.2, which would yield the dimer linkage sequence (DLS) exposed in an apical loop. The presence of the viral core chaperone protein would participate in the formation of dimeric genomes (4), which would be finally encapsidated, enveloped and released to the extracellular medium. Improbable contacts are indicated by grey lines at each step. Figure adapted from[31].
- Citation: Romero-López C, Berzal-Herranz A. Structure-function relationship in viral RNA genomes: The case of hepatitis C virus. World J Med Genet 2014; 4(2): 6-18
- URL: https://www.wjgnet.com/2220-3184/full/v4/i2/6.htm
- DOI: https://dx.doi.org/10.5496/wjmg.v4.i2.6