Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1087
Peer-review started: October 21, 2019
First decision: November 11, 2019
Revised: November 13, 2019
Accepted: December 6, 2019
Article in press: December 6, 2019
Published online: March 26, 2020
The conventional implant approach involves flap elevation, which may result in increased soft tissue and bone loss and postoperative morbidity. The flapless surgical technique, aided by three-dimensional medical imaging equipment, is regarded as a possible alternative to the conventional approach to alleviate the above issues. Several studies have been performed regarding the role of flapless implant surgery. However, the results are inconsistent and there is no robust synthesis of long-term evidence to better inform surgeons regarding which type of surgical technique is more beneficial to the long-term prognosis of patients in need of implant insertion.
To compare the long-term clinical performance after flapless implant surgery to that after the conventional approach with flap elevation.
PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and grey literature databases were searched from inception to 23 September 2019. Randomised controlled trials (RCTs) and cohort studies comparing the long-term clinical performance after flapless implant surgery to that after the conventional approach over a follow-up of three years or more were included. Meta-analyses were conducted to estimate the odds ratios (ORs) or mean differences (MDs) and their 95% confidence intervals (CIs) between the long-term implant survival rate, marginal bone loss, and complication rate of the flapless and conventional groups. Subgroup analyses were carried out to account for the possible effects of the guided or free-hand method during flapless surgery.
Ten articles, including four RCTs and six cohort studies, satisfied the eligibility criteria and nine of them were included in the meta-analysis. There was no significant difference between the long-term implant survival rate [OR = 1.30, 95%CI (0.37, 4.54), P = 0.68], marginal bone loss [MD = 0.01, 95%CI (-0.42, 0.44), P = 0.97], and complication rate [OR = 1.44, 95%CI (0.77, 2.68), P = 0.25] after flapless implant surgery and the conventional approach. Moreover, subgroup analyses revealed that there was no statistically significant difference between the implant survival rate [guided: OR = 1.52, 95%CI (0.19, 12.35), P = 0.70]; free-hand: n = 1, could not be estimated), marginal bone loss [guided: MD = 0.22, 95%CI (-0.14, 0.59), P = 0.23; free-hand: MD = -0.27, 95%CI (-1.10, 0.57), P = 0.53], or complication rate [guided: OR = 1.16, 95%CI (0.52, 2.63), P = 0.71; free-hand: OR = 1.75, 95%CI (0.66, 4.63), P = 0.26] in the flapless and conventional groups either with use of the surgical guide or by the free-hand method.
The flapless surgery and conventional approach had comparable clinical performance over three years or more. The guided or free-hand technique does not significantly affect the long-term outcomes of flapless surgery.
Core tip: This is the first systematic review and meta-analysis to compare the long-term clinical performance after flapless implant surgery to that of the conventional approach with flap elevation over a follow-up of three years or more. Interestingly, we found that the flapless implant surgery and conventional approach had comparable implant survival rates, marginal bone loss, and complication rates over the follow-up period. Moreover, guided or free-hand implant insertion does not affect the long-term effects of flapless surgery. Thus, the flapless technique can be considered a promising alternative to the conventional implant approach without significantly compromising the long-term outcomes of implant treatment.
- Citation: Cai H, Liang X, Sun DY, Chen JY. Long-term clinical performance of flapless implant surgery compared to the conventional approach with flap elevation: A systematic review and meta-analysis. World J Clin Cases 2020; 8(6): 1087-1103
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1087.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1087
The long-term clinical performance of dental implant treatment can be affected by primary factors related to different surgical techniques and secondary factors associated with the onset of marginal bone loss[1]. The conventional approach to implant surgery involves flap elevation, which allows a direct access to implant sites, improves the control of placement angulation, and reduces the risk of bone fenestrations and dehiscences[2]. However, some studies revealed that this approach resulted in increased soft tissue and bone loss, postoperative morbidity (e.g., oedema and pain), and delayed recovery due to the increased surgical trauma and longer surgical time[3-6].
Thus, a flapless surgical technique has emerged and is regarded as a possible alternative to the conventional approach to alleviate the above issues. In the flapless approach, a minimal soft-tissue excision is performed to remove a small specific diameter of mucoperiosteum, which matches up with the diameter of the planned implant, without any flap elevation[7,8]. It has been reported that the flapless technique can significantly shorten the surgical time, preserve the peri-implant soft and hard tissue, and reduce the subsequent discomfort to patients[4-6,9]. The flapless surgery also avoids the detachment of periosteum, which provides the blood supply and osteogenic potential to peri-implant alveolar bone to maintain the level of marginal bone and overlying soft tissue[10,11].
Despite having potential advantages, the flapless surgery has generally been considered a “blind” procedure due to the difficulty in evaluating the contour and condition of underlying alveolar bone and anatomical areas. With the advent of new medical imaging equipment such as cone-beam computed tomography (CBCT), the remaining bone and critical anatomical structures in surgical sites can be accurately assessed from three-dimensional radiography, which facilitates the planning of implant insertion via the flapless approach[7,12]. Surgeons can then conduct a free-hand flapless surgery or a guided method via a prefabricated surgical guide that transfers the preoperative planning to the accurate implant positioning and insertion[13].
There is no consensus regarding the role of flapless implant surgery and whether the surgical guide during flapless surgery is beneficial. While one previous systematic review[14] suggested that the flapless technique significantly affected the implant survival rate, other studies[15,16] indicated that there was no difference between the flapless technique and the conventional approach with flap elevation in respect to either the survival rate or marginal bone loss of dental implants. Also, although the guided flapless surgery has been reported to be significantly more accurate than free-hand insertion[17], one literature review showed that the surgical guide did not demonstrate any advantages over the free-hand method[18]. To date, there is no robust synthesis of relevant long-term evidence to better inform surgeons regarding which type of surgical technique is more beneficial to the long-term prognosis of patients in need of implant insertion. Thus, the aim of the current systematic review is to compare the long-term clinical performance of flapless implant surgery to the conventional approach with flap elevation in edentulous patients over a follow-up of three years or more, and to synthesise relevant findings via meta-analysis and subgroup (i.e., guided flapless technique) analysis where appropriate.
This systematic review was performed in accordance with the Cochrane Handbook[19] and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[20]. The protocol of this systematic review was defined by HC, XL, and JC prior to the literature search.
The current systematic review was conducted to address the focused clinical question “How is the long-term clinical performance in edentulous patients after flapless implant surgery compared to the conventional approach with flap elevation over a follow-up of three years or more?” according to the following participant-intervention-comparison-outcomes-study framework: Participant (P): Edentulous patients in need of dental implant surgery; Intervention (I): Flapless implant surgery; Comparison (C): Conventional implant surgery with flap elevation; Outcomes (0): Long-term clinical performance after implant surgery over a follow-up of three years or more; Study design (S): Randomised controlled trials (RCTs), prospective or retrospective cohort studies.
Based on the participant-intervention-comparison-outcomes-study framework, studies were eligible for the current systematic review if they satisfied the following inclusion criteria: (1) Studies on edentulous patients who were in need of dental implant surgery; (2) Studies that compared at least two implant surgical techniques (i.e., flapless surgery versus the conventional approach with flap elevation) in the same study; (3) Studies with a minimum follow-up of three years after implant surgery; (4) Studies that included at least one measure of clinical performance (e.g., survival rate, marginal bone loss, and complication rate) of implant surgery; (5) Studies designed as RCTs, prospective or retrospective cohort studies; (6) Studies with an abstract and full text available; and (7) Studies published in English.
If a study met any of the following exclusion criteria, it was excluded from the study: (1) Duplicate publication; (2) Studies that evaluated only one implant surgical technique; and (3) In vitro, animal or cadaver studies, case reports, study protocols, technical notes, opinions, letters, and reviews.
PubMed, EMBASE, and Cochrane Central Register of Controlled Trials were systematically searched for articles published from inception to 23 September 2019 with no restrictions. The search strategy (Table 1) was developed for the PubMed search by combining both MeSH and free text words and adapted for other electronic databases.
| Search strategy | |
| 1 | “Dental Implantation”[Mesh] OR ((dent* OR oral* OR mouth* OR stomatology*) AND implant*) |
| 2 | flap* |
| 3 | “Randomized Controlled Trial”[Mesh] OR “Prospective Studies”[Mesh] OR “Retrospective Studies”[Mesh] OR random* OR control* OR prospective OR retrospective |
| 4 | 1 AND 2 AND 3 |
To ensure literature saturation, “grey” literature databases were searched to seek literature that had not been formally published[19]. The ClinicalTrials.gov and the International Clinical Trials Registry Platform were searched for unpublished reports of clinical trials; the ProQuest Dissertation Abstracts and Thesis database was searched to identify relevant dissertations and theses; the Conference Proceedings Citation Index-Science was searched via Web of Science to identify relevant trials reported in conference proceedings; in addition, the Open SINGLE and National Technical Information Service databases were also searched as supplements. Subsequently, a manual search was conducted based on the reference lists of selected studies and relevant reviews to identify studies that were not indexed in the above databases.
After removal of duplicates, the titles and abstracts of articles were screened independently by two reviewers (HC and JC) to exclude those with no relevance to this study. Full texts of all the relevant articles were retrieved and assessed according to the eligibility criteria by the same two independent reviewers (HC and JC). Any disagreement in study selection was resolved through discussion with a third assessor (XL). The agreement strength between the reviewers was assessed using Cohen’s kappa statistic.
Relevant information in eligible articles was extracted independently by two reviewers (HC and JC) and cross-checked by the same two reviewers (HC and JC) for accuracy. Data collected comprised: Authors; year of publication; study design; number, age range, and gender distribution of participants; number, type, system, size (diameter × length), and location of implants; tools for flapless surgery; free-hand or guided flapless surgery; prostheses; implant survival rate, marginal bone loss, complication rate, and other outcome measures; follow-up; and loss to follow-up. Disagreements during data collection were resolved by discussion and consultation with a third reviewer (XL).
Two reviewers (HC, JC) independently assessed the risk of bias for each eligible study. The Cochrane Collaboration’s tool for assessing risk of bias[19] was adopted to evaluate the quality of selected RCTs using the Review Manager version 5.3 software (The Cochrane Collaboration, Copenhagen, Denmark). According to this, the risk of bias of studies was estimated as “low”, “unclear”, or “high” for each item (i.e., random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias) in the tool. The methodological quality of each selected cohort study was rated using the Newcastle-Ottawa Scale[21,22]. The quality of cohort studies was scored with “stars” (*) in accordance with three major domains (i.e., selection, comparability, and outcome), where higher scores represented higher quality and less risk of bias.
The long-term clinical performance after the flapless implant technique was mainly assessed using the implant survival rate, marginal bone loss, and complication rate over a follow-up of three years or more. The events of implant failure/survival at the endpoint of follow-up were identified in accordance with the criteria specified in individual studies and the proportion of remaining implants in the oral cavity was calculated to establish the implant survival rate. Digital periapical radiographs were obtained in some studies immediately after implant placement and at follow-up visits. Peri-implant marginal bone loss (i.e., marginal bone resorption) over the follow-up was measured on the radiographs and generally reported with a mean and a standard deviation (SD), where the negative value was noted for bone gain. Marginal bone loss could also be calculated using the marginal bone levels at the baseline and follow-up visits[19]. The events of biological (i.e., peri-implant mucosa, peri-implantitis, or suppuration) and technical (i.e., abutment-screw fracture, abutment loosening, crown debonding, or crown chipping) complications recorded in some studies were also obtained to calculate the complication rate. Other measures, such as the changes in Pocket Probing Depth, Plaque Index, and Gingival Index over a follow-up of at least three years, were also used in some studies to describe the long-term clinical performance of patients after flapless and conventional implant surgery.
For an accurate estimate of treatment effects, data were pooled from individual studies with the same outcome measure to perform a meta-analysis. The implant survival and complication rates were treated as dichotomous data, where the numbers of events in two research groups (i.e., flapless and conventional groups) were collected and compared via odds ratios (ORs) and their 95% confidence intervals (CIs). For marginal bone loss, the means and SDs in two groups were obtained to estimate the mean differences (MDs) and their 95%CIs. Some studies reported the marginal bone loss in other data formats, such as means and 95%CIs, which were then converted into the desired format (i.e., means and SDs) to be synthesised in the analyses. In the meta-analyses, P < 0.05 was deemed significant.
Statistical heterogeneity across trials was investigated using the χ² test and I² statistics[23]. Heterogeneity was considered substantial if χ² exceeded the degree of freedom (df) and I² was greater than 50%[19]. In the case of substantial heterogeneity, a random-effects model was used to estimate the overall treatment effects; otherwise, analysis was performed using a fixed-effects model[24]. The Review Manager version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) was used for the analyses.
The publication bias across studies was investigated using the Egger’s test by Stata SE release15 (StataCorp LP, College Station, TX, USA)[25].
Sensitivity analyses were performed to determine if the findings of the current meta-analyses were dependent on any individual study, especially the retrospective study. The Stata SE release15 (StataCorp LP, College Station, TX, USA) was used for possible sensitivity analyses to explore the impact of removing each of the selected studies.
Moreover, to assess the correlation of clinical performance after flapless implant surgery with the use of the surgical guide, studies in the flapless group were stratified into “guided” and “free-hand” subgroups, which were defined by whether the flapless surgery was carried out with a surgical guide or not. Subgroup analyses were performed for each outcome (i.e., implant survival rate, marginal bone loss, and complications).
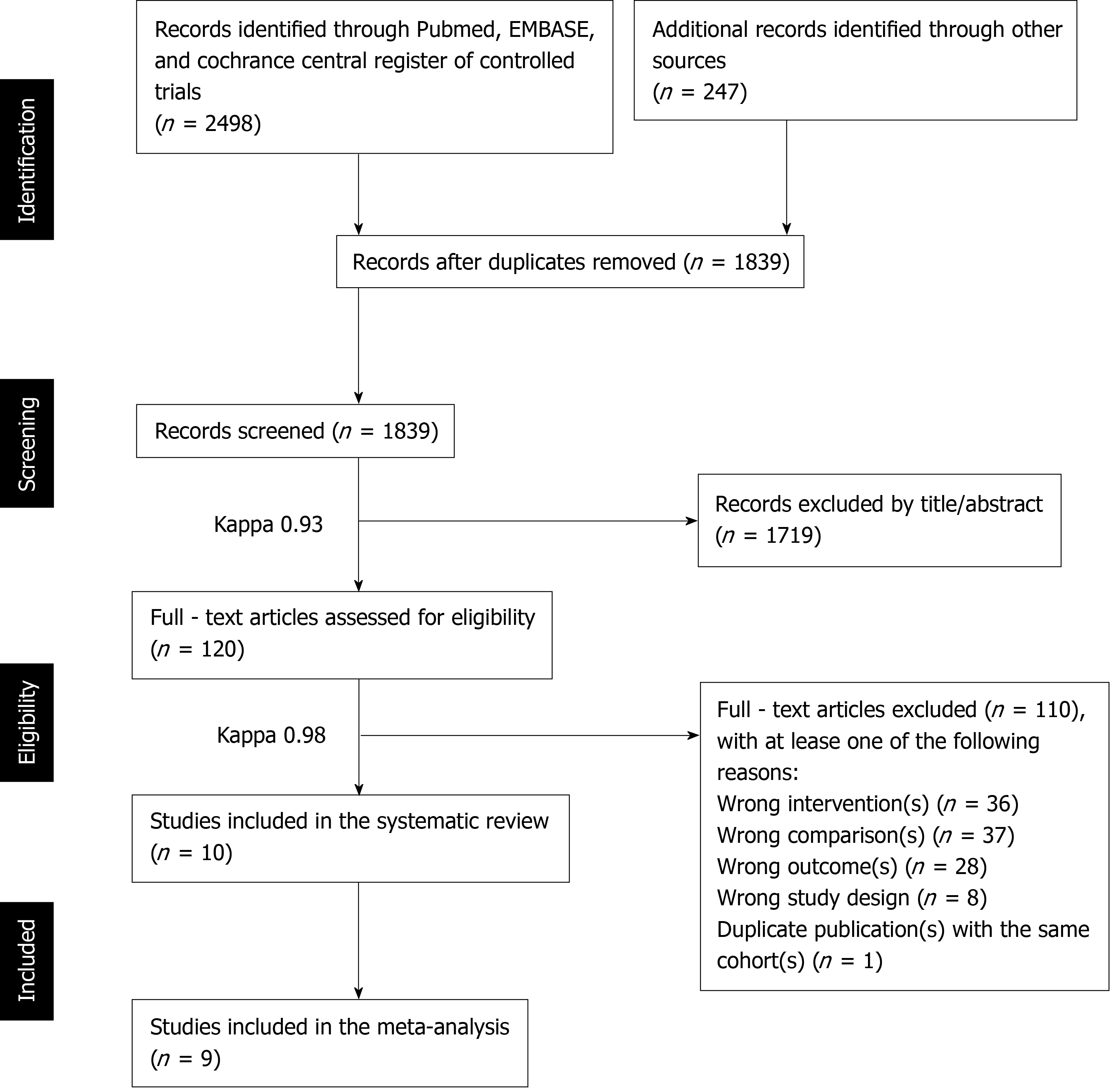
After the removal of duplicate publications, 1839 records were obtained via the electronic and manual search. The screening of titles and abstracts resulted in 120 full-text articles, where 110 articles were excluded as they did not meet the eligibility criteria. Therefore, a total of ten articles[4,26-34] based on independent cohorts comparing the long-term clinical performance after flapless implant surgery to the conventional approach were considered eligible studies for the current systematic review and nine of these studies[4,26,27,29-34] were selected for the meta-analysis. Details of the study selection process are outlined in Figure 1.
The Cohen’s kappa values for measuring the agreement strength between the two independent reviewers (HC and JC) during study selection were 0.93 in the initial screen of titles and abstracts and 0.98 in full-text assessment (Figure 1), indicating an “almost perfect” inter-agreement[35].
Table 2 summarises the main characteristics of the ten included studies. Four RCTs[4,26,27,29], five prospective studies[28,31-34], and one retrospective study[30] involving a total of 8607 participants and 20428 implants were included in the current systematic review and nine of them[4,26,27,29-34] were selected for the meta-analyses to compare the long-term implant survival rate, marginal bone loss, and complication rate after flapless implant surgery to those after the conventional approach.
| Ref. | Study design | Participants | Intervention | Outcomes | Follow-up | Loss (%)a | ||||||||||
| Number of patients | Mean (range) age | Gender | Number of implants | Implant type, system and size | Implant location | Guided or free-hand | Tools for flapless surgery | Prostheses | Implant survival rate (%) | Marginal bone loss (mean ± SD, mm) | Complication rate (%) | Other measures | ||||
| Bömicke et al[26], 2017 | RCT | 38 (F: 19; C: 19) | 53 (21-70) | Female: 22; Male: 16 | 38 (F: 19; C: 19) | One-piece (NobelDirect Groovy, Nobel Biocare) and two-piece (NobelReplace Tapered Groovy, Nobel Biocare), Ø 4.3-5.0 × 10.0 mm | Posterior mandible | Guided | Punch | Single-crown | F: 95%; C: 100% | F: 1.34 ± 1.19; C: 0.67±0.37 | F: 32%; C: 31% | Prosthesis failure; PPD; PI; GI | 3 yr | 3 (8%) |
| Cannizzaro et al[27], 2008 | RCT | 40 (F: 20; C: 20) | 39 (18-64) | Female: 21; Male: 19 | 108 (F: 52; C: 56) | Two-piece (Tapered SwissPlus, Zimmer Dental), Ø 3.7-4.8 × 10.0-14.0 mm | Maxilla and mandible | Guided | Drill | Single-crown | F: 100%; C: 100% | NR | F: 19%; C: 16% | Prosthesis failure; postoperative oedema and pain; analgesic consumption; implant stability quotient | 3 yr | 0 (0%) |
| Cooper et al[28], 2014 | PC | 113 (F: 68; C: 45) | 43 | Female: 66; Male: 47 | 113 (F: 68; C: 45) | Two-piece (OsseoSpeed, Dentsply Implants), Ø 3.5-5.0 × 11.0-17.0 mm | Maxilla except the molars | NR | NR | Single-crown | NR | NR | NR | Mucosal zenith position | 5 yr | 19 (17%) |
| Froum et al[29], 2017b | RCT | 60 (F: 30; C: 30) | NAb | Female: 35; Male: 25 | 60 (F: 30; C: 30) | One-piece (NobelDirect, Nobel Biocare), Ø 4.3-5.0 × 10+ mm | Maxilla and mandible | Guided | Punch | Single-crown | F: 100%; C: 100% | F: 0.36 ± 0.63; C: 0.23 ± 0.95 | NAc | PPD; BoP; interproximal papilla levels | 8.6 yr | 32 (53%) |
| Jesch et al[30], 2018 | RC | 7783 | NR | NR | 18945(F: 17517; C: 1428) | Two-piece (ANKYLOS, Dentsply Implants), Ø 3.5+ × ? mm | Maxilla and mandible | NR | Punch | Implant-support prostheses | F: 93%; C: 92% | NR | NR | None | 10 yr | 7018 (90%) |
| Maló et al[31], 2015 | PC | 41 (F: 20; C: 21) | 46 (19-79) | Female: 22; Male: 19 | 72 (F: 32; C: 40) | Two-piece (NobelSpeedy Groovy, Nobel Biocare), Ø 4.0 × 10.0-15.0 mm | Maxilla and mandible | Free-hand | Drill | Single-crown or fixed partial prostheses | F: 97%; C: 100% | F: 1.60 ± 1.22; C: 1.14 ± 0.49 | F: 29%; C: 11% | None | 3 yr | 8 (20%) |
| Naeini et al[32], 2018d | PC | 49 | 53 (20-79) | Female: 27; Male: 22 | 53 (F: 28; C: 25) | Two-piece (Brånemark system, Nobel Biocare), Ø 3.3-5.0 × 7.0-18.0 mm | Maxilla and mandible | Free-hand | Drill | Single-crown | F: 100%; C: 100% | F: -0.89 ± 0.96; C: 0.49 ± 1.12 | F: 5 (22); C: 4 (15) | PI; BI; PPD | 6-9 yr | 13 (27%) |
| Oliva et al[33], 2010 | PC | 378 | 48 (19-80) | Female: 227; Male: 151 | 831 (F: 323; C: 508) | One-piece (CeraRoot type 11, 12, 14, 16, and 21, Oral Iceberg), Ø 3.5-4.8 × ? mm | Maxilla and mandible | Guided | NR | Implant-support prostheses | F: 99%; C: 93% | NR | NR | None | 3.4 yr | 0 (0%) |
| Pisoni et al[4], 2016 | RCT | 45 | 61 | Female: 6; Male:39 | 76 (F: 42; C: 34) | Two-piece (SLA standard, Straumann), Ø 4.1 × ? mm | Posterior maxilla and mandible | Guided | Punch | Implant-support prostheses | F: 100%; C: 100% | F: 0.20 ± 0.76; C: 0.17 ± 0.94 | NR | None | 3 yr | 5 (11%) |
| Prati et al[34], 2016 | PC | 60 | 56 (25-72) | Female: 26; Male:34 | 132 (F: 66; C: 66) | Two-piece (PrimaConnex, Keystone Dental), Ø 3.5-5.0 × ? mm | Maxilla and mandible | Free-hand | Drill | Single-crown | F: 97%; C: 98% | F: 1.22 ± 0.87; C: 1.23 ± 0.88 | F: 0%; C: 0% | PI; SBI; BoP; Microtopographic observations | 3 yr | 0 (0%) |
The participants had a mean age of 49 years (range 18-80 years) and there were more female participants (n = 452) than males (n = 372). However, two studies[29,30] did not report the details of age at baseline, and the gender distribution was not reported in one study[30]. One-piece[26,29,33] or two-piece implants[4,26-28,30-32,34] with various systems, diameters (range 3.5-5.0 mm), and lengths (range 7.0-18.0 mm) were used in the selected studies. Implants were then placed in one specific arch (maxilla or mandible)[26,28] or both arches (maxilla and mandible)[4,27,29-34] to support single crowns or other prostheses. Surgical guides (e.g., templates or stents) were used in five studies[4,26,27,29,33] to control the trajectory and depth of implant insertion, and three studies[31,32,34] used free-hand flapless surgery, which indicated a greater demand. Different tools for flapless surgery were introduced in the selected studies. Punches were used in four studies[4,26,29,30] and drills were used in four other studies[27,31,32,34] to prepare the implant recipient sites with a minimal surgical intervention. Most studies reported the details of implant survival[4,26,27,29-34], marginal bone loss[4,26,29,31,32,34], and complications[26,27,31,32,34]. The postoperative oedema and pain[27], analgesic consumption[27], implant stability quotient[27], microtopographic observations[34], prosthesis failure[26,27], and various peri-implantitis related indices[26,28,29,32,34] were also measured in some studies. Only the studies with a long follow-up of at least three years (range 3-10 years) were included in the current systematic review. Three studies[28-30,32] even had a follow-up period of more than five years, although the loss to follow-up rate in these studies increased to more than 20%.
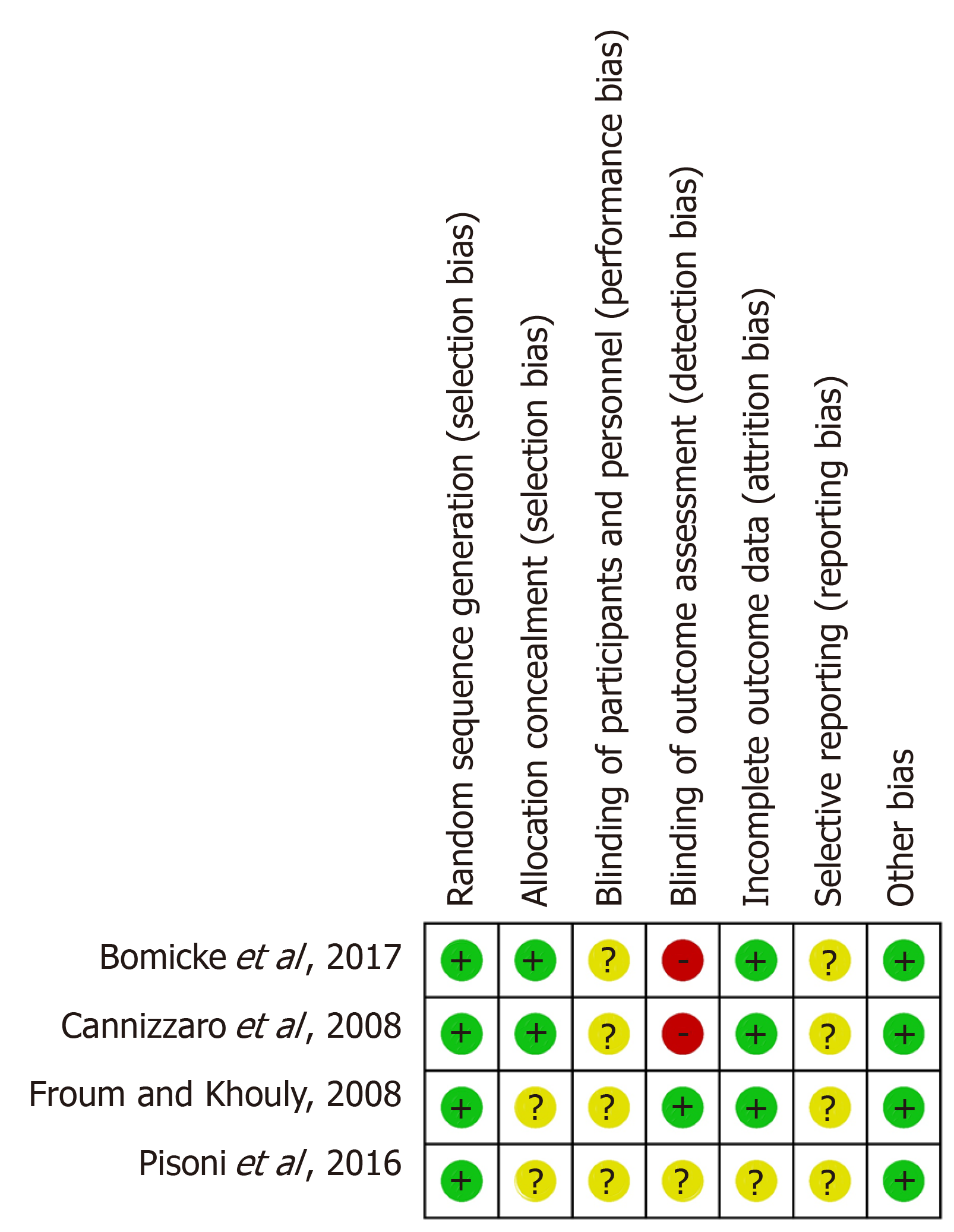
None of the included RCTs showed an overall low risk of bias. Two studies[26,27] (50%) were evaluated to have an unclear risk of bias and two studies[4,29] (50%) were shown to have a high risk of bias (Figure 2). All the studies[4,26,27,29] (100%) provided details of the randomisation sequence generation process; however, two studies[4,29] (50%) did not give sufficient information regarding the concealment of patients’ allocation. The blinding of participants and personnel was not explicitly stated in all the RCTs[4,26,27,29] (100%) and two studies[26,27] (50%) did not blind the assessors during the measurement of outcomes. Only one RCT[4] (25%) did not provide specific reasons for missing data, which resulted in an unclear risk of bias. With regard to reporting bias, no protocols were available for any of the selected RCTs (0%) to pre-specify the study outcomes. All the selected RCTs[4,26,27,29] appeared to be free from other potential sources of bias.
Table 3 summarises the reviewers’ judgments on the risk of bias items and quality for each selected non-randomised study using the Newcastle-Ottawa quality assessment scale. The overall quality of the selected non-randomised studies reached an average of 6.5 stars, which was acceptable as no study had less than five stars. Among these, three studies[28,31,33] were found to have a low risk of bias (seven to eight stars), and three studies[30,32,34] were judged to have moderate risk of bias (five to six stars). Most studies[30,32-34] (67%) did not reach the comparability of cohorts by adjusting the results for important factors and none of the selected studies (0%) took into account the additional factors, which resulted in an absence of stars and a decrease in study quality. There was also a lack of representativeness of exposed cohorts[32,34], adequate cohorts over follow-up[30,32], or demonstration that outcome of interest was not present at baseline[30] in some studies.
| Study | Coding Manual for Cohort Studies | Newcastle-Ottawa Scale |
| Cooper et al[28], 2014 | Selection | |
| (1) Representativeness of the exposed cohort | * | |
| (2) Selection of the non-exposed cohort | * | |
| (3) Ascertainment of exposure | * | |
| (4) Demonstration that outcome of interest was not present at start of study | * | |
| Comparability | ||
| (1) Comparability of cohorts on the basis of the design or analysis | * | |
| Outcome | ||
| (1) Assessment of outcome | * | |
| (2) Was follow-up long enough for outcomes to occur | * | |
| (3) Adequacy of follow-up of cohorts | * | |
| Total Scale | ******** (8) | |
| Jesch et al[30], 2018 | Selection | |
| (1) Representativeness of the exposed cohort | * | |
| (2) Selection of the non-exposed cohort | * | |
| (3) Ascertainment of exposure | * | |
| (4) Demonstration that outcome of interest was not present at start of study | - | |
| Comparability | ||
| (1) Comparability of cohorts on the basis of the design or analysis | - | |
| Outcome | ||
| (1) Assessment of outcome | * | |
| (2) Was follow-up long enough for outcomes to occur | * | |
| (3) Adequacy of follow-up of cohorts | - | |
| Total Scale | ***** (5) | |
| Maló et al[31], 2015 | Selection | |
| (1) Representativeness of the exposed cohort | * | |
| (2) Selection of the non-exposed cohort | * | |
| (3) Ascertainment of exposure | * | |
| (4) Demonstration that outcome of interest was not present at start of study | * | |
| Comparability | ||
| (1) Comparability of cohorts on the basis of the design or analysis | * | |
| Outcome | ||
| (1) Assessment of outcome | * | |
| (2) Was follow-up long enough for outcomes to occur | * | |
| (3) Adequacy of follow-up of cohorts | * | |
| Total Scale | ******** (8) | |
| Naeini et al[32], 2018 | Selection | |
| (1) Representativeness of the exposed cohort | - | |
| (2) Selection of the non-exposed cohort | * | |
| (3) Ascertainment of exposure | * | |
| (4) Demonstration that outcome of interest was not present at start of study | * | |
| Comparability | ||
| (1) Comparability of cohorts on the basis of the design or analysis | - | |
| Outcome | ||
| (1) Assessment of outcome | * | |
| (2) Was follow-up long enough for outcomes to occur | * | |
| (3) Adequacy of follow-up of cohorts | - | |
| Total Scale | ***** (5) | |
| Oliva et al[33], 2010 | Selection | |
| (1) Representativeness of the exposed cohort | * | |
| (2) Selection of the non-exposed cohort | * | |
| (3) Ascertainment of exposure | * | |
| (4) Demonstration that outcome of interest was not present at start of study | * | |
| Comparability | ||
| (1) Comparability of cohorts on the basis of the design or analysis | - | |
| Outcome | ||
| (1) Assessment of outcome | * | |
| (2) Was follow-up long enough for outcomes to occur | * | |
| (3) Adequacy of follow-up of cohorts | * | |
| Total Scale | ******* (7) | |
| Prati et al[34], 2016 | Selection | |
| (1) Representativeness of the exposed cohort | - | |
| (2) Selection of the non-exposed cohort | * | |
| (3) Ascertainment of exposure | * | |
| (4) Demonstration that outcome of interest was not present at start of study | * | |
| Comparability | ||
| (1) Comparability of cohorts on the basis of the design or analysis | - | |
| Outcome | ||
| (1) Assessment of outcome | * | |
| (2) Was follow-up long enough for outcomes to occur | * | |
| (3) Adequacy of follow-up of cohorts | * | |
| Total Scale | ****** (6) | |
There was no consensus regarding whether the long-term clinical performance of flapless implant surgery was comparable to that of the conventional approach. Four studies[26,30,31,34] observed that there was no difference between the flapless and conventional approaches regarding the implant survival rate, and four other studies[4,27,29,32] even showed a 100% implant survival rate in either the flapless or the conventional group. However, the results in one study[33] also indicated that more implants survived after conventional surgery with flap elevation over a follow-up of 3.4 years.
Two studies[26,27] indicated that more bone loss and less implant stability were observed after flapless surgery than after the conventional approach. Four studies[4,29,31,34] suggested that there was a comparable effect on marginal bone between the flapless and conventional groups, and one study[32] even reported much better preservation of marginal bone in the flapless group than the conventional group.
Four studies[26,27,31,32] found that the complication rates in the two groups were comparable and one study[34] indicated that no postoperative infection, dysesthesia, perforation, or medical complication was observed in each group during the follow-up. However, patients in the flapless group were also found to have significantly less postoperative oedema and pain[27], less analgesic consumption[27], and increased peri-implant mucosal tissue dimension[28] than those in the conventional group after flap elevation.
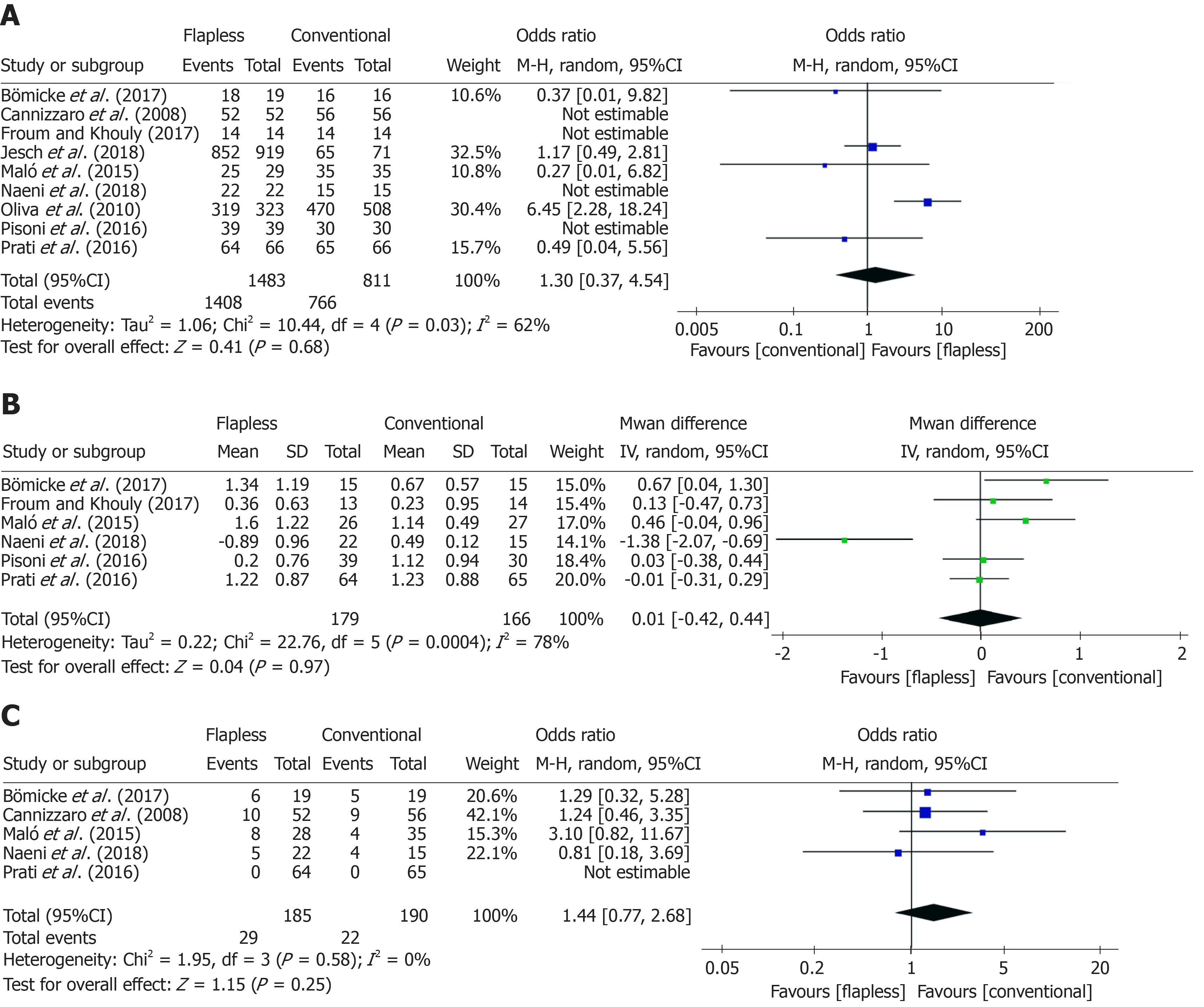
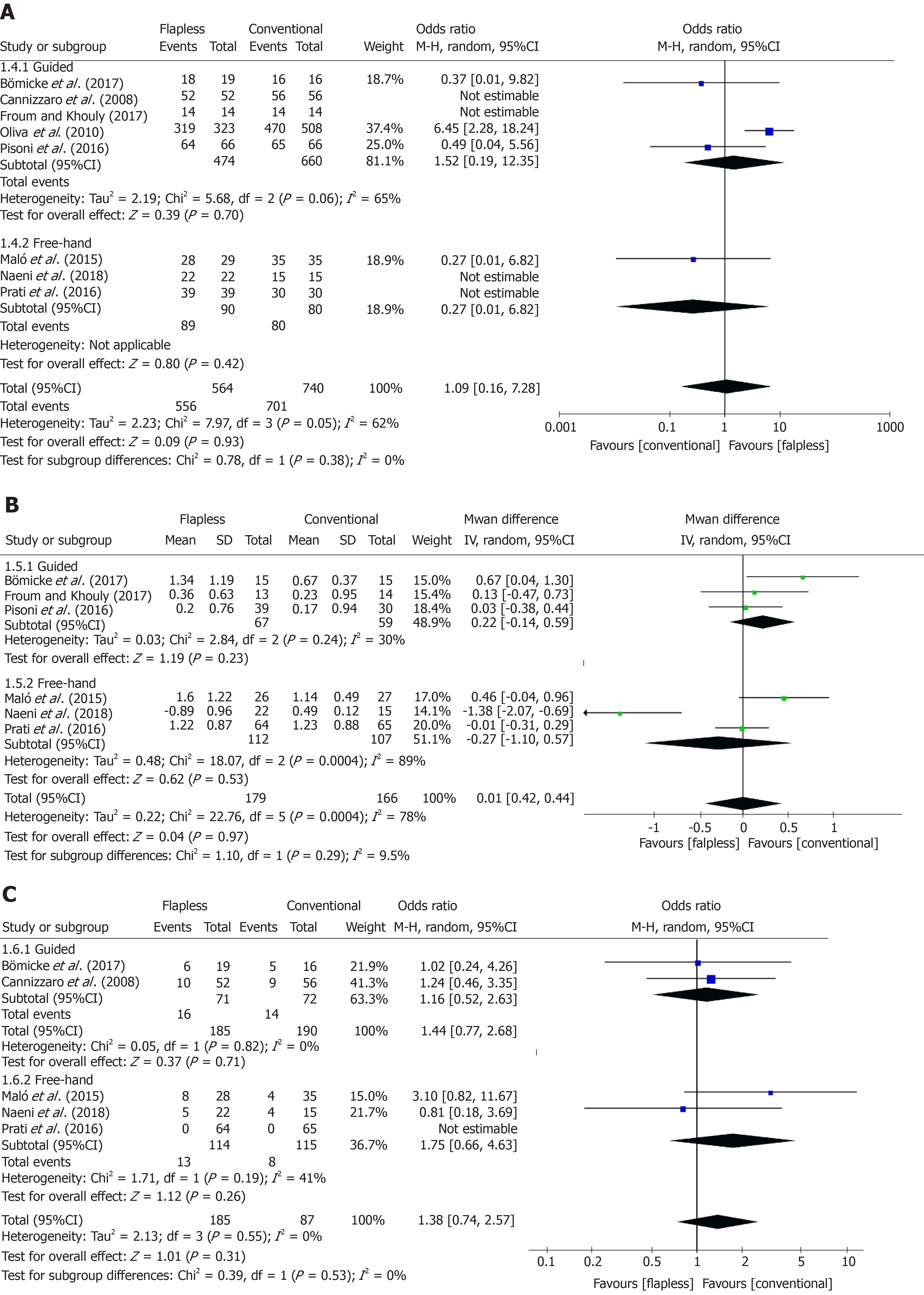
For the comparison of implant survival rates between flapless and conventional surgery, nine studies[4,26,27,29-34] with 2174 implants were included in the meta-analysis. As substantial heterogeneity (χ² = 10.44 > df = 4, I² = 62% > 50%, P = 0.03) was observed across the studies, a random-effects model was carried out. No significant difference was found between the flapless group and conventional group [flapless vs conventional: OR = 1.30, 95%CI (0.37, 4.54), P = 0.68] regarding the implant survival rate over the follow-up period of three years or more (Figure 3A).
Six studies[4,26,29,31,32,34] with 345 implants compared the marginal bone loss between the two surgical approaches. Among these studies, considerable heterogeneity (χ² = 22.76 > df = 5, I² = 78% > 50%, P < 0.001) was observed, and a random-effects model was employed. After meta-analysis, no significant difference in the long-term marginal bone loss around implants was found after flapless surgery compared to conventional surgery (flapless vs conventional: MD = 0.01, 95%CI (-0.42, 0.44), P = 0.97) (Figure 3B).
With regard to the complication rate, five studies[26,27,31,32,34] with 375 implants reported the details of complication events after the two implant surgeries. Little heterogeneity (χ² = 1.95 > df = 3, I² = 0% < 50%, P = 0.58) was found across the studies, hence, a fixed-effects model was performed. It was revealed that there was no significant difference between the flapless and conventional surgery [flapless vs conventional: OR = 1.44, 95%CI (0.77, 2.68), P = 0.25] regarding the complications after a follow-up period of at least three years (Figure 3C).
Publication bias was investigated in the meta-analyses using Egger’s test. It was demonstrated that there was no significant evidence of publication bias among the included studies in each of the three meta-analyses (flapless vs. conventional: survival: coefficient = -1.15, 95%CI (−57.22, 54.91), P = 0.837; marginal boss loss: coefficient = -0.83, 95%CI (−9.86, 8.21), P = 0.812; complications: coefficient = -0.08, 95%CI (−12.64, 12.49), P = 0.982). However, the relevant results were reported for reference only due to the limited number (n < 10) of included studies in each meta-analysis.
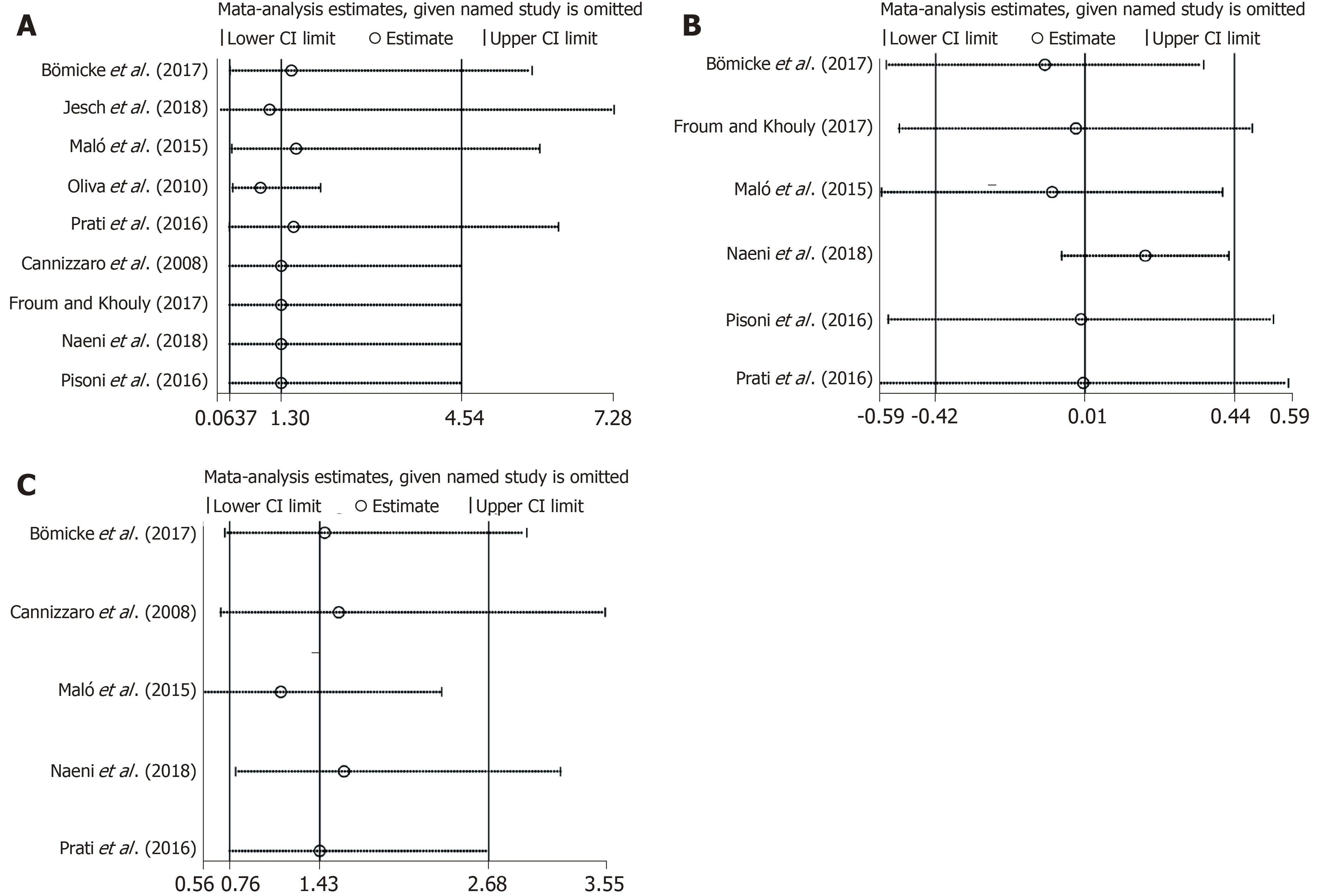
Sensitivity analyses were performed. The overall results and conclusions of the meta-analyses were not affected by the exclusion or inclusion of individual studies (Figure 4A-C). Therefore, the results of this review can be considered with a degree of certainty.
Subgroup analyses were carried out to estimate the long-term difference between flapless surgery and the conventional approach in guided and free-hand flapless subgroups, respectively. With regard to the implant survival rate, no difference was observed between guided flapless surgery and the conventional approach [guided vs conventional: OR = 1.52, 95%CI (0.19, 12.35), P = 0.70]; however, the difference between the free-hand flapless subgroup and the conventional group could not be estimated due to insufficient included studies and rare failure events in two studies[32,34] (Figure 5A). No significant difference in marginal bone loss between the flapless and conventional implant surgery was detected either with the use of a surgical guide [guided vs conventional: MD = 0.22, 95%CI (-0.14, 0.59), P = 0.23] or in the free-hand subgroup [free-hand vs conventional: MD = -0.27, 95%CI (-1.10, 0.57), P = 0.53] (Figure 5B). The complication rate over the long-term follow-up period, was not significantly different between the two surgical techniques (flapless and conventional) in the guided flapless subgroup [guided vs conventional: OR = 1.16, 95%CI (0.52, 2.63), P = 0.71) or the free-hand flapless subgroup [free-hand vs conventional: OR = 1.75, 95%CI (0.66, 4.63), P = 0.26] (Figure 5C).
The current systematic review and meta-analysis is the first study to compare the long-term clinical performance of flapless implant surgery to the conventional approach with flap elevation in edentulous patients over a follow-up period of at least three years. Evidence from a total of 8607 participants and 20428 implants indicated that there was no significant difference between the implant survival rate, marginal bone loss, and complication rate after flapless implant surgery and the conventional approach with flap elevation. Moreover, there was no significant difference between the flapless and conventional approaches either with the use of a surgical guide or by a free-hand method over the follow-up period of three years or more.
Earlier reviews[14-16] provided controversial conclusions regarding the flapless implant technique compared with the conventional approach with flap elevation. Some studies[16] showed similar implant survival rates between the flapless and conventional approaches, which is consistent with the current findings, while another study[14] suggested that implants placed without flap elevation had a decreased survival rate than those with flap elevation. It should be noted that none of these studies focused on long-term observations. As the authors sought to eliminate the influence of studies that followed participants for a limited time, only studies with a minimum follow-up of three years were eligible for this systematic review to synthesise more robust data.
Conventional implant surgery leads to direct access to the recipient sites during implant placement. However, the “blind” flapless technique aided by preoperative three-dimensional planning can also lead to the appropriate positioning and angulation of implant placement[7,12]. Moreover, the flapless procedure can avoid detachment of the periosteum to even preserve the peri-implant soft and bone tissue[10,11]. These benefits may contribute to comparable long-term clinical performance between flapless and conventional implant surgery. Thus, the flapless technique can be considered a promising alternative to the conventional implant approach without significantly compromising the long-term outcomes of implant treatment. Additionally, although peri-implant bone gain was rarely observed in both the flapless and conventional groups, flapless surgery can guarantee minor discomfort (e.g., oedema and pain) for patients during postoperative recovery[27].
The current findings also showed that guided or free-hand implant insertion did not affect the long-term effects of flapless surgery when compared to the conventional approach with flap elevation. The results were similar to those in a previous review[15], where no difference was found in the implant survival rate after use of the free-hand flapless technique or guided method with or without three-dimensional planning compared to conventional implant surgery. In contrast, Nickenig et al[17] suggested that the guided flapless technique was significantly more accurate than free-hand surgery. These controversial statements can be explained by the different experience of surgeons in individual studies. Thus, skilled and experienced surgeons are generally recommended during the free-hand flapless surgery to control the angulation of implant insertion in a very precise way[36-38]. In addition, the cost-effectiveness of the guided implant technique still requires further discussion.
Flapless surgery is now typically advocated, as it is in line with the progress towards more minimally invasive surgery in general medicine. However, not all cases are suitable for flapless implant surgery due to the bone and soft tissue associated preconditions. It is generally accepted that patients with a sufficient amount of alveolar bone and keratinised tissue (i.e., a minimum of 6 mm in width) can be treated by flapless implant surgery[30,31]. Otherwise, mucoperiosteal flaps should be elevated as required for implant placement. Further criteria for patient allocation to flapless surgery should include the surgical sites should be free from remnants or soft tissue concavities after tooth extraction. Surgeons need to be aware of and finely balance the risks and benefits related to the flapless technique. Moreover, preoperative CBCT analysis is recommended in either flapless implant surgery or the conventional approach for ideal treatment planning.
The findings of the current study need to be interpreted with caution due to its limitations. As this study focused on the long-term clinical performance of implants, the eligibility criteria then became very strict and the number of included studies was limited. Consequently, strong evidence on this clinical question could not be obtained, and the validity of the current analysis could be compromised to some extent. Further high-quality RCTs with a long-term follow-up are needed for a more robust assessment. In addition, one retrospective study[30] was included in the analysis, which could be another limitation of the study. The nature of a retrospective design inherently results in flaws; for instance, it might result in a false positive association between the flapless technique and the long-term success of implant insertion[39]. Moreover, it should be noted that the loss to follow-up rates in three studies[29,30,32] were more than 20% over the follow-up period of more than five years. This could pose a threat to the validity of the evidence because the patients who left these studies might have a different clinical performance than those who completed the study[40]. As the study design and loss to follow-up rate may affect the validity of evidence, sensitivity analyses were then performed for each included study, especially the retrospective study, to assess their possible impact on current findings. No significant difference in the overall results was detected after the exclusion or inclusion of any individual studies, which indicated that the conclusions of this review seem to be reliable and worth considering.
In conclusion, this overview of selected studies indicated that flapless implant surgery and the conventional approach with flap elevation have comparable effects regarding the long-term implant survival rate, marginal bone loss, and complication rate over a follow-up of three years or more. Moreover, the guided or free-hand implant insertion does not significantly affect the long-term effects of flapless surgery when compared to the conventional approach. Hence, the flapless technique is considered a promising alternative to the conventional implant approach in patients with appropriate alveolar bone and soft tissue conditions, without significantly compromising the long-term outcomes of implant treatment. However, surgeons' experience and relevant cost-effectiveness should be considered regarding the option of a surgical guide or free-hand method in flapless surgery. Further high-quality RCTs with long-term follow-up are needed for a more robust assessment.
Conventional implant surgery involves flap elevation, which may result in increased postoperative discomfort and morbidity. The flapless surgical technique, aided by three-dimensional medical imaging equipment, is regarded as a possible alternative to the conventional approach to alleviate the above issues. However, previous results regarding the role of flapless implant surgery are inconsistent and there is still concern regarding the long-term clinical performance of the flapless surgical technique. To date, no meta-analysis or systematic review comparing the long-term clinical performance of the flapless surgical technique to the conventional approach have been published.
The long-term clinical performance of dental implant treatment can be affected by different surgical techniques. Thus, it is important to compare the long-term outcomes of flapless implant surgery to those of the conventional approach over a follow-up of three years or more. A better insight into this topic would help inform surgeons regarding which type of surgical technique is more beneficial to the long-term prognosis of patients in need of implant insertion.
To compare the long-term clinical performance after flapless implant surgery to that after the conventional approach with flap elevation.
This was a systematic review and meta-analysis. The protocol of this study was defined by the authors prior to the literature search. Nine electronic databases were systematically searched from inception to September 23, 2019. A manual search was also carried out to identify studies that were not indexed in the above databases. Randomised controlled trials (RCTs) and cohort studies comparing the long-term clinical performance after flapless implant surgery to the conventional approach over a follow-up of three years or more were included in the current systematic review. The risk of bias in selected RCTs and cohort studies was assessed using the Cochrane Collaboration’s tool for assessing risk of bias and the Newcastle-Ottawa Scale, respectively. Meta-analyses were conducted to estimate the odds ratios (ORs) or mean differences (MDs) and their 95% confidence intervals (CIs) for the implant survival rate, marginal bone loss, and complication rate of the flapless and conventional groups. Sensitivity analyses were performed to determine if the findings of the current meta-analyses were dependent on any individual study. Moreover, subgroup analyses were carried out to account for the possible effects of the guided or free-hand method during flapless surgery.
Of 1839 records, ten articles (i.e., four RCTs and six cohort studies) involving a total of 8607 participants and 20428 implants satisfied the eligibility criteria and nine of them (i.e., four RCTs and five cohort studies) were included in the meta-analysis. Two RCTs (50%) were evaluated to have an unclear risk of bias and the other two RCTs (50%) were found to have a high risk of bias. Three cohort studies had a low risk of bias, and the other three cohort studies were judged to have a moderate risk of bias. After meta-analyses, there was no significant difference between the long-term implant survival rate [OR = 1.30, 95%CI (0.37, 4.54), P = 0.68], marginal bone loss [MD = 0.01, 95%CI (-0.42, 0.44), P = 0.97], and complication rate [OR = 1.44, 95%CI (0.77, 2.68), P = 0.25] between the flapless implant surgery group and the conventional approach group. The overall results and conclusions of the meta-analyses were not affected by the exclusion or inclusion of individual studies. Moreover, subgroup analyses revealed that there was no statistically significant difference between the implant survival rate [guided: OR = 1.52, 95%CI (0.19, 12.35), P = 0.70; free-hand: n = 1, could not be estimated], marginal bone loss [guided: MD = 0.22, 95%CI (-0.14, 0.59), P = 0.23; free-hand: MD = -0.27, 95%CI (-1.10, 0.57), P = 0.53], or complication rate [guided: OR = 1.16, 95%CI (0.52, 2.63), P = 0.71; free-hand: OR = 1.75, 95%CI (0.66, 4.63), P = 0.26] in the flapless and conventional groups either with the use of a surgical guide or by the free-hand method.
These findings indicated that flapless surgery and the conventional approach have comparable clinical performance over a long-term follow-up of three years or more. The guided or free-hand technique does not significantly affect the long-term effects of flapless surgery. Hence, the flapless technique is considered a promising alternative to the conventional implant approach without significantly compromising the long-term outcomes of implant treatment.
The overall results of long-term clinical performance after flapless implant surgery are acceptable. These findings provide surgeons with evidence-based practical insight that the flapless technique can be considered an alternative to the conventional implant approach in patients with appropriate alveolar bone and soft tissue conditions. Although evidence from the study suggests that guided or free-hand implant insertion does not significantly affect the long-term outcomes of flapless implant surgery, surgeons' experience and relevant cost-effectiveness should be considered regarding the option of a surgical guide or free-hand method in flapless surgery. Further high-quality RCTs with a long-term follow-up are needed for a more robust assessment.
The authors would like to thank Dr Ying He from the Centre for Statistics in Medicine (University of Oxford, United Kingdom) for her kind help in reviewing the statistical methods and techniques mentioned in the manuscript.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Araby YA S-Editor: Dou Y L-Editor: Webster JR E-Editor: Liu MY
| 1. | Chrcanovic BR, Albrektsson T, Wennerberg A. Reasons for failures of oral implants. J Oral Rehabil. 2014;41:443-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 2. | Cannizzaro G, Felice P, Leone M, Checchi V, Esposito M. Flapless versus open flap implant surgery in partially edentulous patients subjected to immediate loading: 1-year results from a split-mouth randomised controlled trial. Eur J Oral Implantol. 2011;4:177-188. [PubMed] [Cited in This Article: ] |
| 3. | De Bruyn H, Atashkadeh M, Cosyn J, van de Velde T. Clinical outcome and bone preservation of single TiUnite™ implants installed with flapless or flap surgery. Clin Implant Dent Relat Res. 2011;13:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Pisoni L, Ordesi P, Siervo P, Bianchi AE, Persia M, Siervo S. Flapless Versus Traditional Dental Implant Surgery: Long-Term Evaluation of Crestal Bone Resorption. J Oral Maxillofac Surg. 2016;74:1354-1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Nkenke E, Eitner S, Radespiel-Tröger M, Vairaktaris E, Neukam FW, Fenner M. Patient-centred outcomes comparing transmucosal implant placement with an open approach in the maxilla: a prospective, non-randomized pilot study. Clin Oral Implants Res. 2007;18:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 6. | Fortin T, Bosson JL, Isidori M, Blanchet E. Effect of flapless surgery on pain experienced in implant placement using an image-guided system. Int J Oral Maxillofac Implants. 2006;21:298-304. [PubMed] [Cited in This Article: ] |
| 7. | Laverty DP, Buglass J, Patel A. Flapless dental implant surgery and use of cone beam computer tomography guided surgery. Br Dent J. 2018;224:601-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Brodala N. Flapless surgery and its effect on dental implant outcomes. Int J Oral Maxillofac Implants. 2009;24 Suppl:118-125. [PubMed] [Cited in This Article: ] |
| 9. | Arisan V, Karabuda CZ, Ozdemir T. Implant surgery using bone- and mucosa-supported stereolithographic guides in totally edentulous jaws: surgical and post-operative outcomes of computer-aided vs. standard techniques. Clin Oral Implants Res. 2010;21:980-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Lazić Z, Golubović M, Marković A, Šćepanović M, Mišić T, Vlahović Z. Immunohistochemical analysis of blood vessels in peri-implant mucosa: a comparison between mini-incision flapless and flap surgeries in domestic pigs. Clin Oral Implants Res. 2015;26:775-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Maier FM. Initial Crestal Bone Loss After Implant Placement with Flapped or Flapless Surgery-A Prospective Cohort Study. Int J Oral Maxillofac Implants. 2016;31:876-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Mozzo P, Procacci C, Tacconi A, Martini PT, Andreis IA. A new volumetric CT machine for dental imaging based on the cone-beam technique: preliminary results. Eur Radiol. 1998;8:1558-1564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 711] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 13. | van Steenberghe D, Glauser R, Blombäck U, Andersson M, Schutyser F, Pettersson A, Wendelhag I. A computed tomographic scan-derived customized surgical template and fixed prosthesis for flapless surgery and immediate loading of implants in fully edentulous maxillae: a prospective multicenter study. Clin Implant Dent Relat Res. 2005;7 Suppl 1:S111-S120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Chrcanovic BR, Albrektsson T, Wennerberg A. Flapless versus conventional flapped dental implant surgery: a meta-analysis. PLoS One. 2014;9:e100624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Lemos CAA, Verri FR, Cruz RS, Gomes JML, Dos Santos DM, Goiato MC, Pellizzer EP. Comparison between flapless and open-flap implant placement: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Lin GH, Chan HL, Bashutski JD, Oh TJ, Wang HL. The effect of flapless surgery on implant survival and marginal bone level: a systematic review and meta-analysis. J Periodontol. 2014;85:e91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Nickenig HJ, Wichmann M, Hamel J, Schlegel KA, Eitner S. Evaluation of the difference in accuracy between implant placement by virtual planning data and surgical guide templates versus the conventional free-hand method - a combined in vivo - in vitro technique using cone-beam CT (Part II). J Craniomaxillofac Surg. 2010;38:488-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Voulgarakis A, Strub JR, Att W. Outcomes of implants placed with three different flapless surgical procedures: a systematic review. Int J Oral Maxillofac Surg. 2014;43:476-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Cited in This Article: ] |
| 20. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18463] [Cited by in F6Publishing: 16685] [Article Influence: 1112.3] [Reference Citation Analysis (0)] |
| 21. | Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute, 2016. [Cited in This Article: ] |
| 22. | Hartling L, Hamm M, Milne A, Vandermeer B, Santaguida PL, Ansari M, Tsertsvadze A, Hempel S, Shekelle P, Dryden DM. Validity and Inter-Rater Reliability Testing of Quality Assessment Instruments. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. [PubMed] [Cited in This Article: ] |
| 23. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21630] [Cited by in F6Publishing: 23095] [Article Influence: 1049.8] [Reference Citation Analysis (0)] |
| 24. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26739] [Cited by in F6Publishing: 28561] [Article Influence: 751.6] [Reference Citation Analysis (0)] |
| 25. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34245] [Cited by in F6Publishing: 36164] [Article Influence: 1339.4] [Reference Citation Analysis (1)] |
| 26. | Bömicke W, Gabbert O, Koob A, Krisam J, Rammelsberg P. Comparison of immediately loaded flapless-placed one-piece implants and flapped-placed conventionally loaded two-piece implants, both fitted with all-ceramic single crowns, in the posterior mandible: 3-year results from a randomised controlled pilot trial. Eur J Oral Implantol. 2017;10:179-195. [PubMed] [Cited in This Article: ] |
| 27. | Cannizzaro G, Leone M, Consolo U, Ferri V, Esposito M. Immediate functional loading of implants placed with flapless surgery versus conventional implants in partially edentulous patients: a 3-year randomized controlled clinical trial. Int J Oral Maxillofac Implants. 2008;23:867-875. [PubMed] [Cited in This Article: ] |
| 28. | Cooper LF, Reside GJ, Raes F, Garriga JS, Tarrida LG, Wiltfang J, Kern M, De Bruyn H. Immediate provisionalization of dental implants placed in healed alveolar ridges and extraction sockets: a 5-year prospective evaluation. Int J Oral Maxillofac Implants. 2014;29:709-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Froum SJ, Khouly I. Survival Rates and Bone and Soft Tissue Level Changes Around One-Piece Dental Implants Placed with a Flapless or Flap Protocol: 8.5-Year Results. Int J Periodontics Restorative Dent. 2017;37:327-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Jesch P, Jesch W, Bruckmoser E, Krebs M, Kladek T, Seemann R. An up to 17-year follow-up retrospective analysis of a minimally invasive, flapless approach: 18 945 implants in 7783 patients. Clin Implant Dent Relat Res. 2018;20:393-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Maló P, de Araújo Nobre M, Lopes A. Three-Year Outcome of Fixed Partial Rehabilitations Supported by Implants Inserted with Flap or Flapless Surgical Techniques. J Prosthodont. 2016;25:357-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Naeini EN, Dierens M, Atashkadeh M, De Bruyn H. Long-term clinical outcome of single implants inserted flaplessly or conventionally. Clin Implant Dent Relat Res. 2018;20:829-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Oliva J, Oliva X, Oliva JD. Five-year success rate of 831 consecutively placed Zirconia dental implants in humans: a comparison of three different rough surfaces. Int J Oral Maxillofac Implants. 2010;25:336-344. [PubMed] [Cited in This Article: ] |
| 34. | Prati C, Zamparini F, Scialabba VS, Gatto MR, Piattelli A, Montebugnoli L, Gandolfi MG. A 3-Year Prospective Cohort Study on 132 Calcium Phosphate-Blasted Implants: Flap vs Flapless Technique. Int J Oral Maxillofac Implants. 2016;31:413-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43944] [Cited by in F6Publishing: 38850] [Article Influence: 826.6] [Reference Citation Analysis (0)] |
| 36. | Wang F, Huang W, Zhang Z, Wang H, Monje A, Wu Y. Minimally invasive flapless vs. flapped approach for single implant placement: a 2-year randomized controlled clinical trial. Clin Oral Implants Res. 2017;28:757-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Jeong SM, Choi BH, Kim J, Xuan F, Lee DH, Mo DY, Lee CU. A 1-year prospective clinical study of soft tissue conditions and marginal bone changes around dental implants after flapless implant surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Sunitha RV, Sapthagiri E. Flapless implant surgery: a 2-year follow-up study of 40 implants. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e237-e243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58:635-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 698] [Cited by in F6Publishing: 702] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 40. | Dettori JR. Loss to follow-up. Evid Based Spine Care J. 2011;2:7-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |