Published online Mar 6, 2020. doi: 10.12998/wjcc.v8.i5.986
Peer-review started: December 10, 2019
First decision: December 23, 2019
Revised: January 16, 2020
Accepted: January 24, 2020
Article in press: January 24, 2020
Published online: March 6, 2020
Primary intimal sarcoma of the pulmonary artery is a rare malignant tumor originating from the pulmonary artery, which has a low incidence rate and is easily misdiagnosed as pulmonary embolism. There is no standard protocol for the treatment of primary intimal sarcoma of the pulmonary artery.
This study reports a patient with primary intimal sarcoma of the pulmonary artery who was admitted to our hospital in 2017. The clinical characteristics, diagnosis, treatment and outcome of the patient were retrospectively analyzed. The patient was a Chinese Han male aged 44 years. He had three consecutive episodes of syncope, and was thus admitted to a local hospital. Computed tomography pulmonary angiography showed multiple lesions with abnormal densities in the pulmonary trunk, left pulmonary artery, mediastinum and pericardium, which were consistent with recurrence after tumor resection. He underwent surgery, and was pathologically diagnosed with intimal sarcoma of the pulmonary artery. He relapsed 3 mo after surgery, and apatinib was administered. His condition was stable after 4 mo, with tolerable and controllable adverse reactions. He subsequently died 19 mo after surgery.
Primary intimal sarcoma of the pulmonary artery has no specific clinical or imaging manifestations. The diagnosis of this disease depends on histopathology and immunohistochemistry, and has a poor clinical prognosis. Surgical treatment is currently a favorable option for primary intimal sarcoma of the pulmonary artery, and targeted therapy may provide new insights for the development of effective treatment methods.
Core tip: Primary intimal sarcoma of the pulmonary artery is a rare malignant tumor. Herein, we present the case of a 44-year-old man who showed multiple lesions with abnormal densities in the pulmonary trunk, left pulmonary artery, mediastinum and pericardium on computed tomography pulmonary angiography, which were consistent with disease recurrence. Three months after surgery, disease relapse was reported, and the patient was given apatinib. The patient’s condition was stable after 4 mo, with tolerable and controllable adverse reactions. The patient subsequently died 19 mo after surgery.
- Citation: Lu P, Yin BB. Misdiagnosis of primary intimal sarcoma of the pulmonary artery as chronic pulmonary embolism: A case report. World J Clin Cases 2020; 8(5): 986-994
- URL: https://www.wjgnet.com/2307-8960/full/v8/i5/986.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i5.986
Primary intimal sarcoma of the pulmonary artery is a rare malignant mesenchymal tumor occurring in the aortic system. The tumor originates from the vascular wall, which gradually grows inwards to block the lumen and outwards to invade the vascular wall, and/or detaches to form tumor thrombi that spread to surrounding or distant organs. Moreover, in the clinic, primary intimal sarcoma of the pulmonary artery has often been misdiagnosed as chronic pulmonary thromboembolism (CPTE), and the diagnosis is always difficult, therefore frequently leading to delayed treatment[1]. In this study, we report a patient who was first misdiagnosed with CPTE, and later pathologically diagnosed with primary intimal sarcoma of the pulmonary artery. The patient subsequently died. The relevant literature was also reviewed.
The patient was a Chinese Han male aged 44 years. On May 12, 2017, he was admitted to the Department of Cardiac Surgery, Qianfoshan Hospital, Shandong Province, China, due to recurrent transient syncope for 1 d.
The patient had transient loss of consciousness while working at home. He woke up after a few minutes, without speech and physical dysfunction. After three consecutive episodes of syncope that day, the patient was admitted to a local hospital, where Color Doppler sonography indicated tricuspid insufficiency and pulmonary hypertension (medium-to-severe). The patient was then transferred to our hospital for further diagnosis and treatment.
The patient has no history of past illness.
In terms of family history, the patient’s mother had hypertension, his father died due to pulmonary embolism, and his sister and brother were physically healthy. A family history of tumors was denied.
Physical examination indicated consciousness, engorgement of the jugular vein, the patient was capable of cooperating with physical examination, increased cardiac boundary, II-III/6 grade systolic murmurs under the xiphoid process, P2 hyperthyroidism, normal liver and spleen, and mild edema in the lower limbs.
No abnormalities in coagulation parameters were observed, with normal D-dimer and normal tumor indicators. No abnormalities were noted during routine blood tests, routine urine tests and urinary sediment examination, routine fecal tests and occult blood test, blood biochemistry, immune indices, and infection indices.
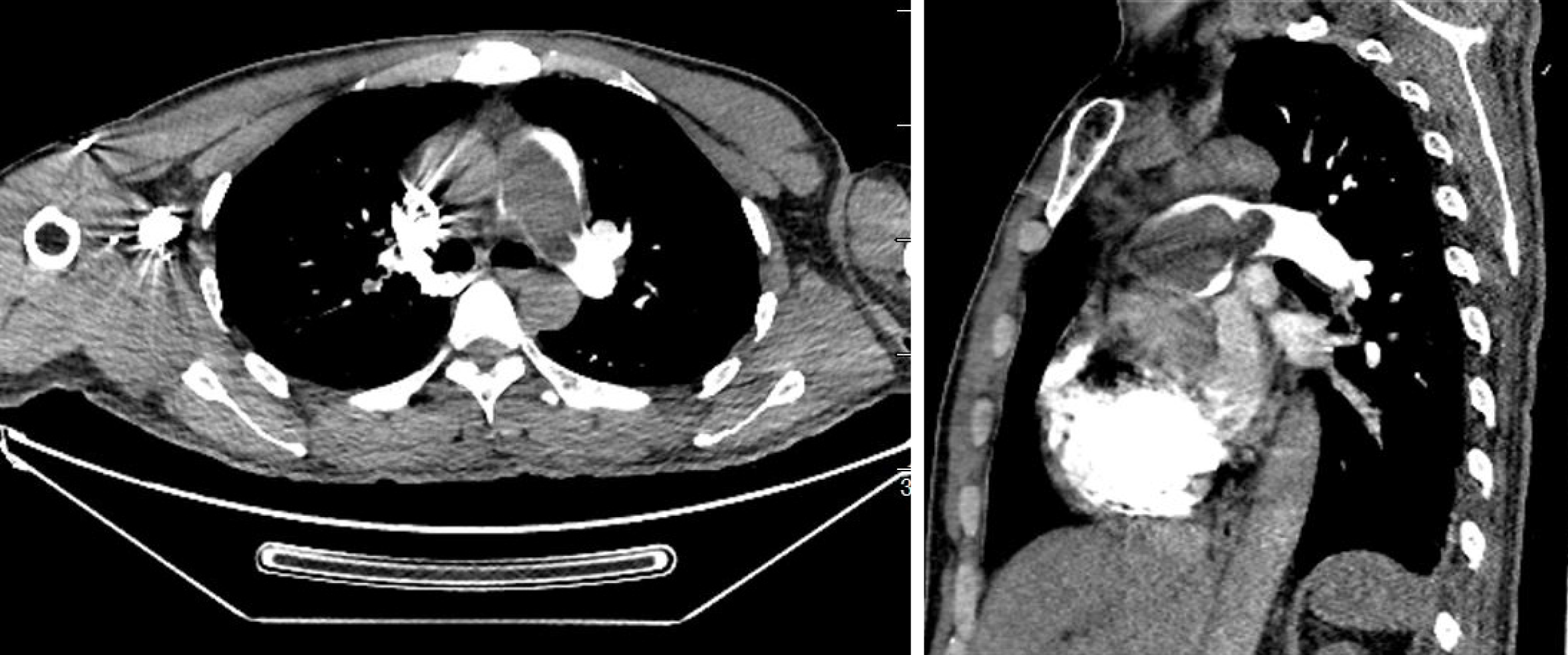
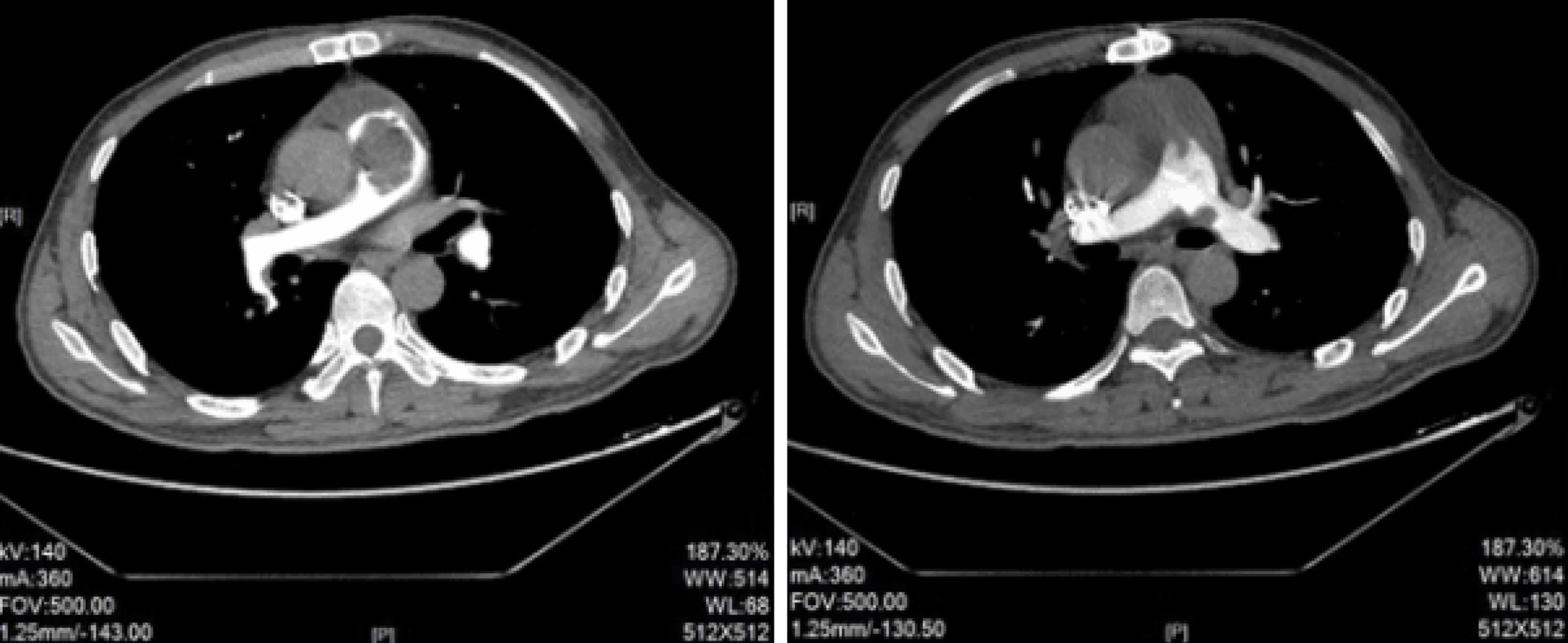
X-ray revealed reduced and sparse texture in the lung, full pulmonary artery, and right atrial enlargement, with a cardiothoracic ratio of 0.56. Electrocardiography (ECG) showed incomplete right bundle branch block. In addition, ECG revealed an irregular echogenic mass in the aortic-pulmonary junction, masses in the left and right pulmonary arteries, significantly enlarged right atrium, moderate-to-severe tricuspid insufficiency, and pulmonary hypertension (moderate), with a pulmonary artery pressure of 56 mmHg. Computed tomography pulmonary angiography (CTPA) examination showed visible low-density masses and filling defects (with a computed tomography value of approximately 50 HU) in the distal segment of the right ventricular outflow tract, main pulmonary artery, and left and right pulmonary arteries, as well as almost complete occlusion of the corresponding arterial lumen (Figure 1). Brain CT showed no obvious abnormalities.
Based on these findings, prior to surgery, the patient was diagnosed with CPTE, tricuspid insufficiency (moderate-to-severe), and pulmonary hypertension. The pulmonary artery was almost fully occluded, and the recurrent syncope was possibly related to the large amount of thrombosis in the lung.
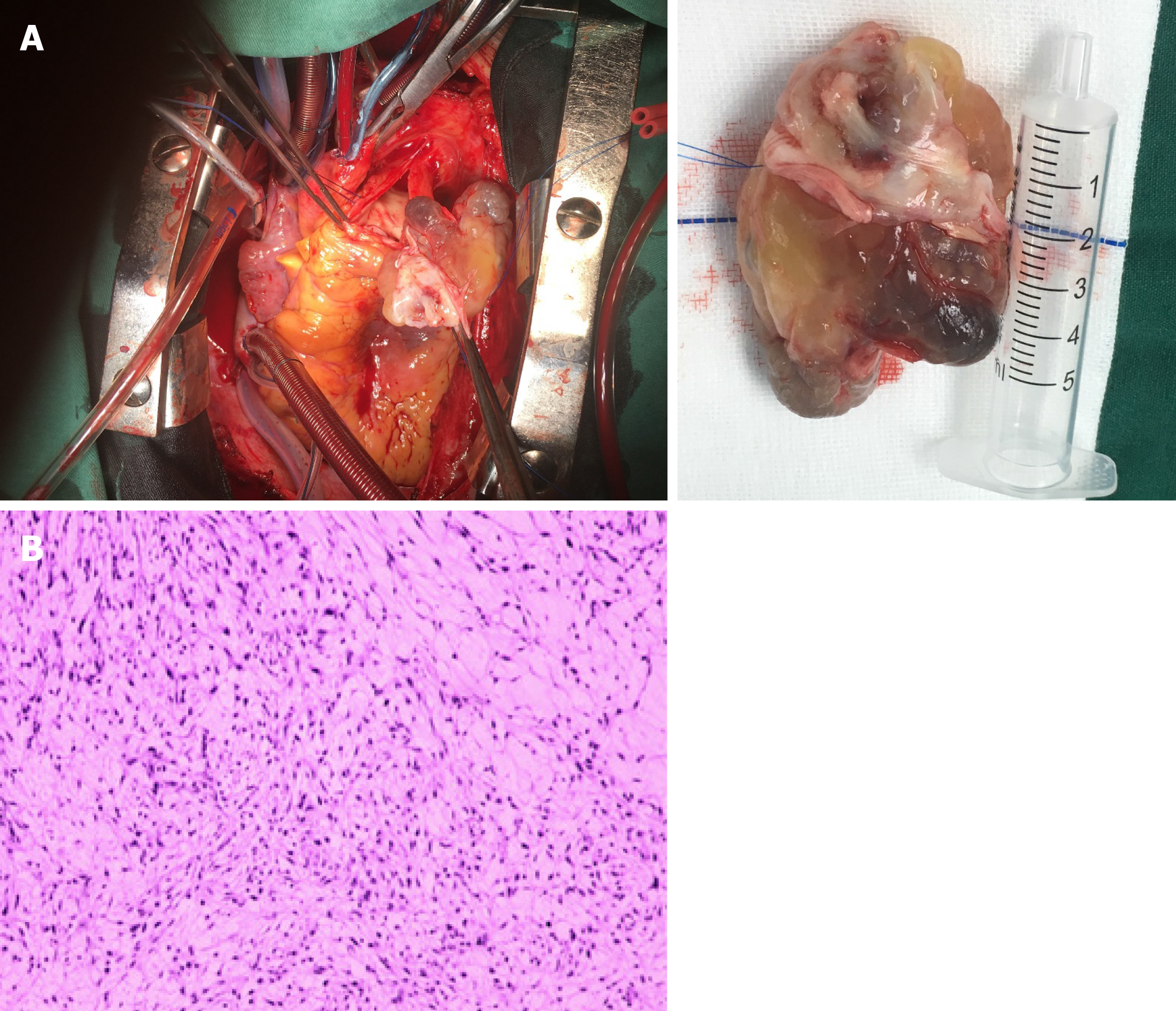
On May 16, 2017, the patient received surgical treatment, under general anesthesia and extracorporeal circulation (to avoid the risk of sudden death). During surgery, a median sternotomy was first performed. Extracorporeal circulation was established through the ascending aorta and the superior and inferior vena cava, and cold blood cardioplegia was perfused through the aortic root. The pulmonary artery was longitudinally cut via the anterior wall of the pulmonary artery. Through this incision, a huge tumor was observed in the pulmonary artery lumen (Figure 2A), which seemed to be semi-translucent, with a wide pedicle and intact adventitia. The pulmonary artery lumen was incompletely occluded, but showed severe stenosis (up to 90%). The pulmonary endarterium was carefully stripped, and the tumor was completely removed. The full-thickness of the pulmonary artery wall was resected from the pedicle island (0.5 cm × 0.5 cm). After washing, the longitudinal incision of the pulmonary artery was closed by a continuous reciprocating suture with 4/0 slide wire, followed by contraction of the tricuspid annulus using DeVega annuloplasty.
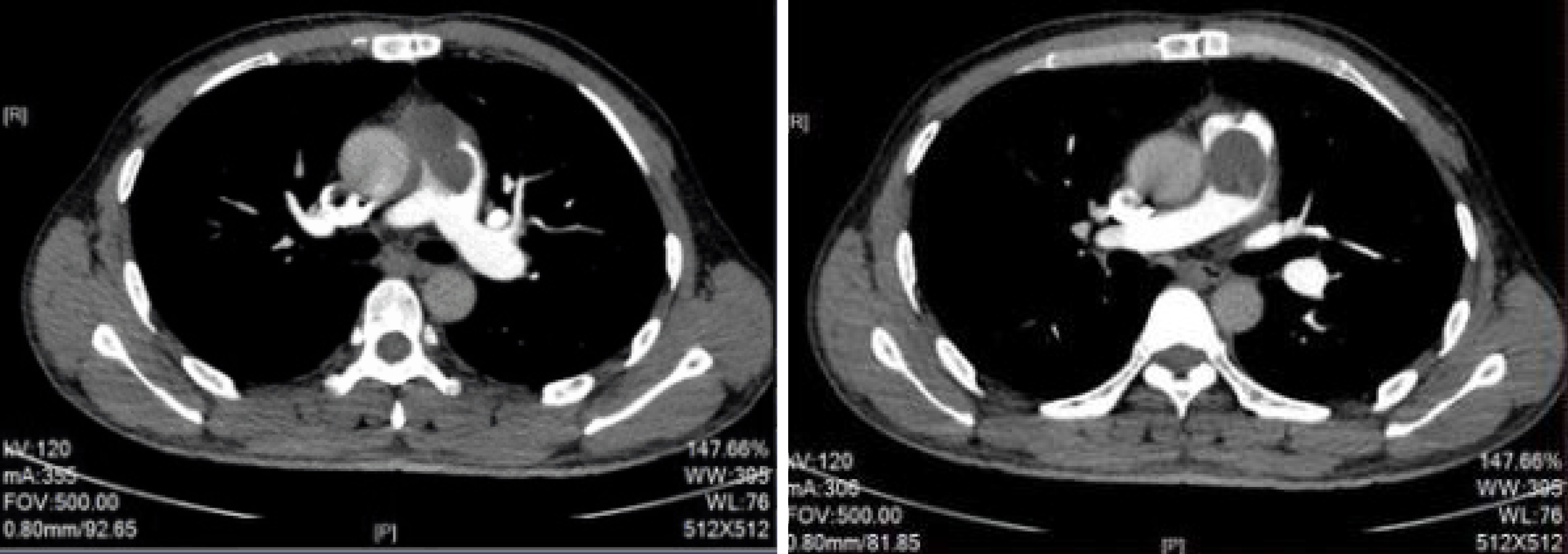
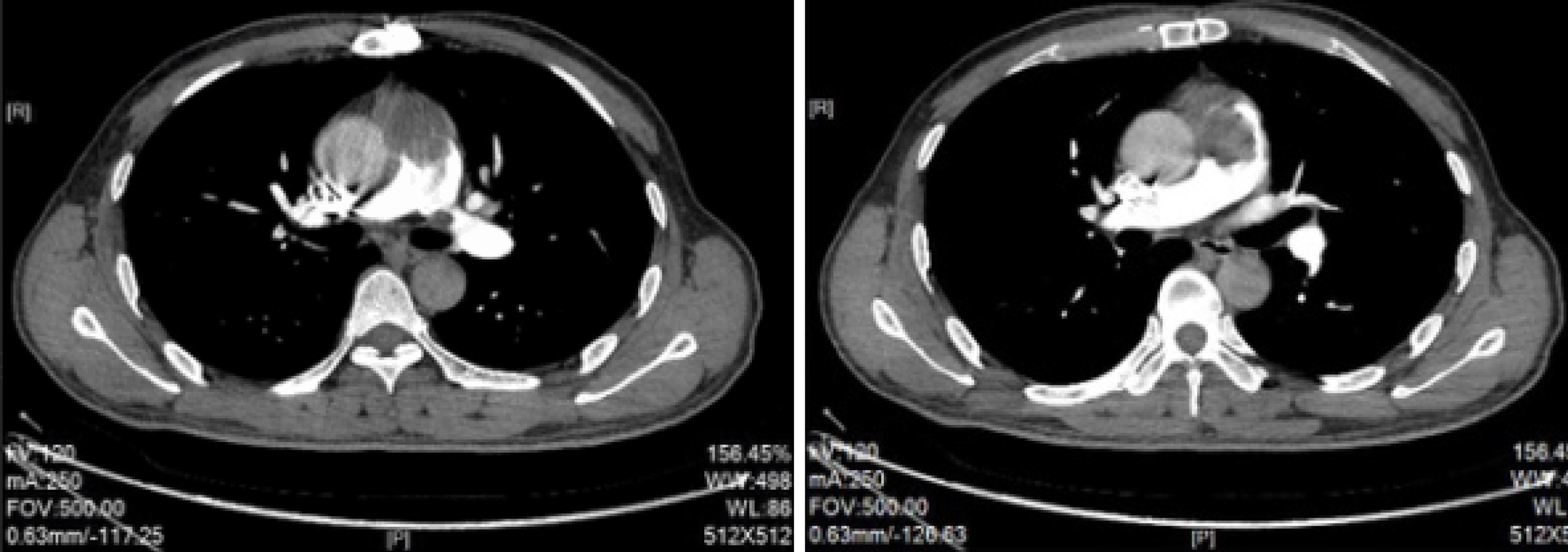
Postoperative pathology indicated a mucinous spindle cell tumor (Figure 2B), which was consistent with the diagnosis of intimal sarcoma of the pulmonary artery. Moreover, immunohistochemical staining showed smooth muscle actin (SMA) (+), FLI-1 (+), CD34 blood vessels (+), and broad-spectrum CK (-). The Ki-67 positive rate was approximately 40%. The patient was discharged 12 d after surgery, and no longer received treatment due to personal reasons. At the end of August 2017, pulmonary artery CTPA showed multiple lesions with abnormal densities in the pulmonary trunk, left pulmonary artery, mediastinum and pericardium (Figure 3), which were consistent with recurrence after tumor resection. In early September 2017, the patient was administered targeted drug therapy with oral apatinib (500 mg, qd), and developed tolerable adverse reactions. After two months of drug therapy, CTPA suggested multiple abnormalities in the pulmonary trunk, left pulmonary artery, left atrium and mediastinum (Figure 4). Some of the lesions were smaller compared with those measured previously, and the patient’s condition had improved. After another 2 mo of medication, CTPA revealed enlarged multiple abnormalities in the pulmonary trunk, left pulmonary artery, left atrium, mediastinum and left ventricle (Figure 5), indicating disease progression. The patient subsequently underwent chemotherapy with vinorelbine combined with cisplatin, gemcitabine and other regimens, during which time apatinib (250 mg, qd) was administered intermittently. However, a poor curative effect was observed. CT demonstrated that the pulmonary sarcoma had grown.
The patient died 19 mo after surgery.
Intimal sarcoma of the pulmonary artery is a very rare malignant mesenchymal tumor, and always occurs in the aortic wall of the pulmonary circulation. According to the World Health Organization Classification of Tumors, intimal sarcoma mainly originates from the blood vessel lumen, which then blocks the lumen and produces tumorous emboli, leading to serious clinical symptoms and even peripheral organ embolism[2]. The average age of disease onset is 50 years (ranging from 13 to 86 years)[3], and only one child of 2 years with this disease has been reported[4]. Pulmonary artery sarcoma is more commonly found in women, with a male to female ratio of 1:1.3[5]. However, Cox et al[6] showed no significant difference in disease incidence related to gender. Intimal sarcoma of the pulmonary artery mainly involves the proximal end of blood vessels, and is often located in the pulmonary trunk (80%), the right or left pulmonary artery (50%-70%), or both (40%). Moreover, approximately 20% of cases have extrathoracic malignant metastasis, involving the lungs, kidneys, lymph nodes, brain, and skin. In 1990, Kruger et al[7] reviewed a total of 93 cases with pulmonary artery sarcoma, and 60% of these cases were diagnosed by autopsy. In 1997, Cox et al[6] reported 42 cases of pulmonary artery sarcoma, and 90% were diagnosed before death. The actual incidence of intimal sarcoma is unclear, and may be underestimated due to its resemblance to CPTE[8]. Moreover, the etiology and risk factors of this tumor have not yet been fully elucidated. It has been shown that the CDK4 gene mutation may have a role in the pathogenesis of the disease[9].
Diagnosis of intimal sarcoma of the pulmonary artery is difficult and often delayed due to concealed and/or non-specific symptoms. Common symptoms include dyspnea, chest or back pain, cough, hemoptysis, weight loss, fatigue, syncope, and fever. In rare cases, embolism derived from a pulmonary valve sarcoma may have manifestations similar to chronic thromboembolic pulmonary hypertension[10]. Moreover, there may be systemic symptoms, such as weight loss, fever or anorexia, severe dyspnea, and right-sided heart failure[11]. Vascular symptoms are mainly the result of thrombosis or severe arterial stenosis, only when the lumen is compressed or invaded. In the present study, the patient had clinical symptoms very similar to those of CPTE. In general, a patient may be asymptomatic for a long time before symptoms appear, while the tumor progresses, gradually blocking the pulmonary artery. When the patient reports obvious symptoms, the disease has already progressed to the late stage, which causes great difficulties in disease diagnosis and treatment, often leading to misdiagnosis (as chronic embolic diseases).
The results of laboratory examinations are often non-specific, including an accelerated erythrocyte sedimentation rate, thrombocytopenia, erythrocytosis, and disseminated intravascular coagulation. ECG mainly shows pressure overload in the right ventricle, right ventricular hypertrophy, and T-wave variation in the ST segment, which are non-specific. Color Doppler echocardiography mainly shows right ventricular enlargement, pulmonary hypertension, and tricuspid regurgitation. In the present case, in addition to right ventricular enlargement and pulmonary hypertension, slight low-density shadows were also observed in the pulmonary trunk, and in the left and right branches, which increased the possibility of pulmonary embolism. Therefore, ultrasound examination is able to detect pulmonary artery obstruction in some patients; however, the cause is impossible to determine. Considering the high incidence of common clinical diseases, patients are often diagnosed with pulmonary embolism, while intimal sarcoma of the pulmonary artery would be excluded due to its low incidence.
CTPA is the main method used to detect pulmonary artery tumors, which can timely identify occupying lesions in the pulmonary artery, although the nature of the lesion is difficult to determine. According to clinical experience, if the pulmonary artery obstruction images show invasion into the lumen wall and extravascular adjacent structures, or the lesion involves the pulmonary valve and the right ventricular outflow tract (presented as lobulation and/or separation), the possibility of pulmonary artery tumor should be highly suspected[12]. In the case reported herein, the CT images were consistent with the above-mentioned manifestations. Moreover, pulmonary angiography can provide enough clues for the diagnosis of pulmonary artery sarcoma, typically manifested as an obstruction in the pulmonary trunk, reciprocating motion of the obstructive shadow or a small and thin distal end of the pulmonary artery, cutting off the surrounding blood vessels[13]. However, pulmonary angiography is prone to causing trauma and increases the risk of pulmonary emboli, and is thus not preferable in clinical application. Furthermore, medical imaging plays a critical role in the detection of pulmonary artery lesions. However, it is still difficult to distinguish tumor occlusion from pulmonary vascular embolism.
At present, autopsy and postoperative histopathological examination are considered the gold standard for the diagnosis of intimal sarcoma of the pulmonary artery, and the oncologic histological pattern under microscopy ranges from intermittent undifferentiated round cells to spiral-shaped spindle cells[14]. Histopathological examinations have revealed that the majority of primary intimal sarcomas of the pulmonary artery are poorly differentiated or undifferentiated malignant spindle cell sarcomas, with different characteristics related to fibroblasts or myofibroblasts. Moreover, there are marked differences in the atypia of neoplastic cells, mitotic figures, and necrosis among cases, in which obvious mucosal degeneration or epithelium-like neoplastic cells are observed in some tumors. Based on immunohistochemical staining, intimal sarcoma has diffuse expression of vimentin, and commonly includes SMA, CD34, osteopontin, and CD31[15]. Moreover, epithelium-derived markers, histiocyte-derived and neuroendocrine markers are all negatively expressed. Cases with the above-mentioned manifestations would be diagnosed with mesenchymal tumors. Some cases may have differentiations, such as leiomyosarcoma, angiosarcoma, and osteosarcoma. In the case presented herein, postoperative pathology indicated a mucus-like spindle cell tumor, and immunohistochemical staining revealed SMA (+), FLI-1 (+), and CD34 blood vessels (+), which were in accordance with the diagnosis of intimal sarcoma of the pulmonary artery.
At present, it is generally believed that surgical resection is a favorable treatment option for intimal sarcoma of the pulmonary artery, which can prolong the survival period[3]. It has been reported that after surgical resection, the survival period can range from a few weeks to more than 10 years[16]. However, there is no consensus on the impact of surgery combined with radiotherapy and chemotherapy, on patient overall survival rate[17]. Chemotherapy is an option for patients with focal sarcoma which cannot be resected or has relapsed[18]. Manso et al[19] reported 3 cases of primary pulmonary artery sarcoma, who underwent surgery and chemotherapy (anthracyclines and ifosfamide), with different outcomes. Palliative chemotherapy with anthracyclines and ifosfamide has been shown to be the typical treatment strategy for advanced diseases, with response rates of approximately 50%[19]. It has been shown that vinorelbine is a better option in terms of tolerance.
Growth, invasion and metastasis of malignant tumors are closely associated with tumor angiogenesis, and anti-angiogenesis represents an important treatment method. Apatinib mesylate is a vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitor, which has been independently developed in China. In the present study, the patient did not receive any treatment 3 mo after surgery. Thereafter, he started targeted therapy with apatinib following relapse, and the patient’s condition improved after drug administration for 2 mo. The patient’s condition was stable for 4 mo, and adverse effects were well tolerated during apatinib therapy. The reasons for selecting apatinib were as follows: (1) A pharmacodynamics study showed that apatinib can inhibit tumor angiogenesis by inhibiting the activity of VEGFR-2 tyrosine kinase and blocking signal transduction of VEGF after binding to the receptor[20]. Considering the mechanism of action of apatinib on tumor cells, it may have acceptable efficacy in the treatment of intimal sarcoma of the pulmonary artery; (2) There is no standard protocol for the treatment of primary intimal sarcoma of the pulmonary artery. At present, the feasibility of apatinib for the treatment of advanced sarcoma has been confirmed[21], exhibiting appropriate clinical application potential; and (3) As a highly effective and low-toxic anti-tumor angiogenesis drug, apatinib is highly convenient due to oral administration, which would be easily accepted by the patient.
Primary intimal sarcoma of the pulmonary artery is strongly invasive, and has a very poor prognosis and high relapse rate if treatment is delayed. Tumors may transfer to the brain, pancreas, adrenal glands, and lungs. The life expectancy after symptom onset ranges from 12 to 18 mo, while the 1- and 2-year survival rates are 22% and 7%, respectively. The median survival period for patients who are unable to undergo surgery due to disease progression (progressive right heart failure) is only 6 wk, which could be prolonged to up to 3 years in patients receiving surgery[8].
The incidence of intimal sarcoma of the pulmonary artery is low, and its clinical manifestations are similar to those of CPTE. Moreover, the treatment of intimal sarcoma of the pulmonary artery can be delayed due to missed diagnosis and misdiagnosis. Therefore, disease history and physical signs, characteristics on medical imaging and histopathological examination should be combined to achieve an early diagnosis and improve diagnostic accuracy, thereby improving disease prognosis. In particular, for patients with low D-dimer levels who are diagnosed with CPTE, the possibility of intimal sarcoma of the pulmonary artery should be considered. However, at present, there is still no standard protocol for the treatment of primary intimal sarcoma of the pulmonary artery; therefore, a more effective treatment strategy is required to further improve patient quality of life and prolong survival time. In this case report, our findings showed that apatinib had potential therapeutic value for primary intimal sarcoma of the pulmonary artery, which may provide new insight for disease diagnosis and treatment. The results reported herein should be further assessed in future in-depth studies.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Richardson WS, Schoenhagen P S-Editor: Zhang L L-Editor: Webster JR E-Editor: Xing YX
| 1. | Yamamoto K, Nozue T, Tsuchida M, Iwaki T, Nagamine H, Yasuda T, Kawase H, Matsushita K, Michishita I. Pulmonary embolism caused by intimal sarcoma of the pulmonary artery. Intern Med. 2012;51:3031-3034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Grazioli V, Vistarini N, Morsolini M, Klersy C, Orlandoni G, Dore R, D'Armini AM. Surgical treatment of primary pulmonary artery sarcoma. J Thorac Cardiovasc Surg. 2014;148:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Timmers L, Bové T, De Pauw M. Intimal sarcoma of the pulmonary artery: a report of two cases. Acta Cardiol. 2009;64:677-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Chappell T, Creech CB, Parra D, Strauss A, Scholl F, Whitney G. Presentation of pulmonary artery intimal sarcoma in an infant with a history of neonatal valvular pulmonic stenosis. Ann Thorac Surg. 2008;85:1092-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ozbek C, Emrecan B, Calli AO, Gurbuz A. Intimal sarcoma of the pulmonary artery with retrograde extension into the pulmonic valve and right ventricle. Tex Heart Inst J. 2007;34:119-121. [PubMed] [Cited in This Article: ] |
| 6. | Cox JE, Chiles C, Aquino SL, Savage P, Oaks T. Pulmonary artery sarcomas: a review of clinical and radiologic features. J Comput Assist Tomogr. 1997;21:750-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Kruger I, Borowski A, Horst M, de Vivie ER, Theissen P. Sympotoms, diagnosis, and therapy of primary sarcomas of the pulmonary artery. Thorac Cardiovasc Surg. 1990;38:91-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 140] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Dornas AP, Campos FT, Rezende CJ, Ribeiro CA, Amaral NF, Corrêa Rde A. Intimal sarcoma of the pulmonary artery: a differential diagnosis of chronic pulmonary thromboembolism. J Bras Pneumol. 2009;35:814-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Gao F, Liu QC, Wang M, Wang ZQ, Huang Y, Zhou XH, Zhang S. Novel mutation of the cyclin-dependent kinase 4 gene in a Chinese patient with intimal sarcoma of the pulmonary artery. Chin Med J (Engl). 2009;122:1107-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Scheidl S, Taghavi S, Reiter U, Tröster N, Kovacs G, Rienmüller R, Lang S, Klepetko W, Olschewski H. Intimal sarcoma of the pulmonary valve. Ann Thorac Surg. 2010;89:e25-e27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Halank M, Jakob C, Kolditz M, Hoeffken G, Kappert U, Ehninger G, Weise M. Intimal pulmonary artery sarcoma presenting as severe dyspnea and right heart insufficiency. Onkologie. 2010;33:313-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Wong HH, Gounaris I, McCormack A, Berman M, Davidson D, Horan G, Pepke-Zaba J, Jenkins D, Earl HM, Hatcher HM. Presentation and management of pulmonary artery sarcoma. Clin Sarcoma Res. 2015;5:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Alsoufi B, Slater M, Smith PP, Karamlou T, Mansoor A, Ravichandran P. Pulmonary artery sarcoma mimicking massive pulmonary embolus: a case report. Asian Cardiovasc Thorac Ann. 2006;14:e71-e73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Secondino S, Grazioli V, Valentino F, Pin M, Pagani A, Sciortino A, Klersy C, Callegari MG, Morbini P, Dore R, Paulli M, Pedrazzoli P, D'armini AM. Multimodal Approach of Pulmonary Artery Intimal Sarcoma: A Single-Institution Experience. Sarcoma. 2017;2017:7941432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Hirose T, Ishikawa N, Hamada K, Inagaki T, Kusumoto S, Shirai T, Okuda K, Ohnishi T, Kadokura M, Adachi M. A case of intimal sarcoma of the pulmonary artery treated with chemoradiotherapy. Intern Med. 2009;48:245-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Penel N, Taieb S, Ceugnart L, Dansin E, Hoguet D, Vanseymortier L, Lartigau E. Report of eight recent cases of locally advanced primary pulmonary artery sarcomas: failure of Doxorubicin-based chemotherapy. J Thorac Oncol. 2008;3:907-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Xu Y, Wang K, Geng Y, Shao Y, Yin Y. A case of intimal sarcoma of the pulmonary artery successfully treated with chemotherapy. Int J Clin Oncol. 2012;17:522-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Shah DK, Joyce LD, Grogan M, Aubry MC, Miller JA, Ding W, Haddock MG. Recurrent pulmonary intimal sarcoma involving the right ventricular outflow tract. Ann Thorac Surg. 2011;91:e41-e42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Manso L, Alvarez E, Quintela M, Cortes-Funes H, Hitt R. Primary pulmonary artery sarcoma: report of three cases and review of the literature. Clin Lung Cancer. 2007;8:277-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Roviello G, Ravelli A, Polom K, Petrioli R, Marano L, Marrelli D, Roviello F, Generali D. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett. 2016;372:187-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Zhu B, Li J, Xie Q, Diao L, Gai L, Yang W. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: An observational study. Cancer Biol Ther. 2018;19:198-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |