Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5104
Peer-review started: May 15, 2020
First decision: September 12, 2020
Revised: September 14, 2020
Accepted: September 23, 2020
Article in press: September 23, 2020
Published online: November 6, 2020
The current standard surgical treatment for non-metastatic upper urinary tract urothelial carcinoma (UTUC) is radical nephroureterectomy (RNU) with bladder cuff excision (BCE). Typically, BCE techniques are classified in one of the following three categories: An open technique described as intrasvesical incision of the bladder cuff, a transurethral incision of the bladder cuff (TUBC), and an extravesical incision of the bladder cuff (EVBC) method. Even though each of these management techniques are widely used, there is no consensus about which surgical intervention is superior, with the best oncologic outcomes.
To investigate the oncological outcomes of three BCE methods during RNU for primary UTUC patients.
We retrospectively analyzed the data of 248 primary UTUC patients, who underwent RNU with BCE between January 2004 to December 2018. Patients were analyzed according to each BCE method. Data extracted included patient demographics, perioperative parameters, and oncological outcomes. Statistical analyses were performed using chi-square and log-rank tests. The Cox proportional hazards regression model was utilized to identify independent predictors. P < 0.05 was considered statistically significant.
Of the 248 participants, 39.9% (n = 99) underwent intrasvesical incision of the bladder cuff, 38.7% (n = 96) EVBC, and 21.4% (n = 53) TUBC. At a median follow-up of 44.2 mo, bladder recurrence developed in 17.2%, 12.5%, and 13.2% of the cases, respectively. Cancer-specific deaths occurred in 11.1%, 5.2%, and 7.5% of patients, respectively. Kaplan-Meier survival curves with a log-rank test highlighted no significant differences in intravesical recurrence-free survival, cancer-specific survival, and overall survival among these approaches with P values of 0.987, 0.825, and 0.497, respectively. Multivariate analysis showed that the lower ureter location appears to have inferior intravesical recurrence-free survival (P = 0.042). However, cancer-specific survival and overall survival were independently influenced by tumor stage (hazard ratio [HR] = 8.439; 95% confidence interval: 2.424-29.377; P = 0.001) and lymph node status (HR = 14.343; 95%CI: 5.176-39.745; P < 0.001).
All three techniques had comparable outcomes; although, EVBC and TUBC are minimally invasive. While based upon rather limited data, these findings will support urologists in blending experience with evidence to inform patient choices. However, larger, rigorously designed, multicenter studies with long term outcomes are still required.
Core Tip: In this work, by focusing on a Chinese population, we intended to evaluated the oncological outcomes of three bladder cuff excision methods during radical nephroureterectomy for primary upper urinary tract urothelial carcinoma patients. Survival analysis suggests all three techniques had comparable outcomes, although, extravesical incision of the bladder cuff and transurethral incision of the bladder cuff are minimally invasive. We hope that current comparative knowledge can add further sophistication to the evidence base for this disease and intervention. Generating accurate estimations using long-term morbidity data benefits both clinicians and patients in terms of disease management and for shared decision-making.
- Citation: Lai SC, Wu PJ, Liu JY, Seery S, Liu SJ, Long XB, Liu M, Wang JY. Oncological impact of different distal ureter managements during radical nephroureterectomy for primary upper urinary tract urothelial carcinoma. World J Clin Cases 2020; 8(21): 5104-5115
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5104.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5104
Upper urinary tract urothelial carcinomas (UTUC) is a relatively uncommon disease, which accounts for only 5%-10% of all urothelial carcinomas[1-3]. The current standard surgical treatment for non-metastatic UTUC is radical nephroureterectomy (RNU) with bladder cuff excision (BCE) which consists of two separate procedures, i.e. the removal of the ipsilateral distal ureter and partial cystectomy along with the ureteral orifice[3,4]. Previous literature and the European Association of Urology guidelines purport that laparoscopic RNU has equivalent oncological efficacy compared to open RNU when adhering to strict oncological principles[1,5]. However, the safety and efficacy of the second step involved in the BCE method have been debated by urologists for a number of years[6].
Typically, methods are classified in one of following three categories: An open technique described as intrasvesical incision of the bladder cuff (IVBC), transurethral incision of the bladder cuff (TUBC), and extravesical incision of the bladder cuff (EVBC)[7]. Even though each of these management techniques are widely used, there is no consensus about which surgical intervention is superior, leading to the best oncologic outcomes[1,6]. In a previous related meta-analysis, we found that IVBC was more strongly associated with improved intravesical recurrence-free survival (IRFS) compared to EVBC and TUBC[8]. However, the findings from that study can be criticized because pooled analysis did not adjust for other important clinic-opathological parameters such as tumor multiplicity, location, stage, grade, and gender. Additionally, the prevalence, biological behavior, and UTUC prognosis differ among nationalities and ethnicities[1]. That study generated only tentative recommendations since most of the included studies were conducted in western populations, preventing generalizations to Asian and other populations.
As such, there is still a need to fill this knowledge gap. Thus, the aim of this study was to retrospectively collate data from a nationwide tertiary care center in mainland China, in order to investigate the oncological impact of the three, different BCE techniques on primary UTUC patients following RNU in this Asian population.
This monocentric, retrospective cohort study was approved by the institutional research ethics committee of our hospital (2019BJYYEC-237-01), and all procedures were conducted in accordance with Helsinki Declaration principles.
We retrospectively analyzed the prospectively collected data of patients with primary UTUC, who underwent RNU with BCE in our hospital from January 2004 to December 2018. Patients who had previous or concomitant bladder cancer, systemic metastasis at presentation, bilateral synchronous UTUC, or prior cystectomy, ureterectomy, or nephrectomy were excluded. In addition, patients whose follow-up period was less than 12 mo were also excluded, with exceptions for those who, within this time frame, encountered tumor recurrence or in the case of death.
RNU was performed either openly or laparoscopically at the discretion of the surgeon. The distal bladder cuff was removed using either an IVBC, EVBC, or TUBC approach.
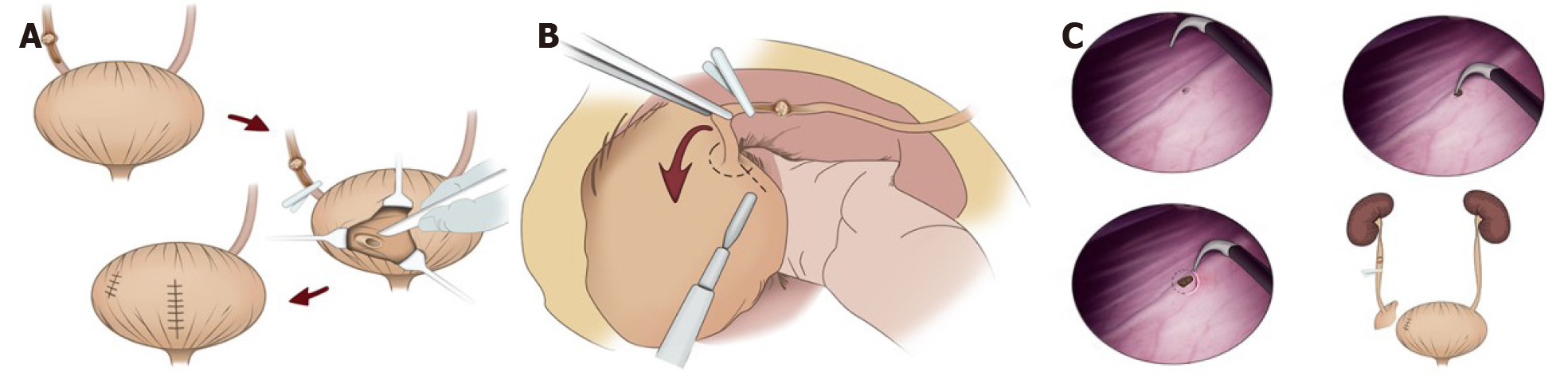
The intravesical method is performed via a lower quadrant or midline incision after radical nephroureterectomy. This procedure involves creating an anterior cystotomy around the intramural ureter and ureteric orifice while confirming the contralateral ureteral orifice. Then, the nephroureterectomy specimen with the bladder cuff is removed en bloc, and the anterior cystotomy and residual defect is closed in two layers (Figure 1A).
In the extravesical technique on the other hand, the ureter is ligated or clamped early using a Ham-Lock, below the level of the tumor, and then the intramural ureter is completely dissected with the bladder cuff approximately 1 cm around the ureteral orifice, extravesically after radical nephroureterectomy (Figure 1B).
The endoscopic approach is performed prior to nephroureterectomy. During this step, the ipsilateral ureteral orifice is endoscopically electrocauterized and a circumferential 10-mm cuff of bladder mucosa around the ureteral orifice is incised down to the perivesical fat using a resectoscope (Figure 1C). Then the patient is repositioned and the ureter below the distal border of the tumor is also ligated before performing nephrectomy.
None of the included patients received neo-adjuvant chemotherapy or radiotherapy, while adjuvant chemotherapy was administered at the clinicians’ discretion based on tumor stage and overall health status as well as patient preference.
Tumors are generally staged according to the 2004 Union for International Cancer Control tumor node metastasis classification. Tumor grade is determined according to the 2004 World Health Organization classification.
Postoperative follow-up generally includes interval history and physical examination, routine blood analysis, urine cytology, chest radiography, and computed tomography urograms. Cystoscopic evaluation and urinary cytology are performed every 3 mo for the first 2 years following RNU, and every 6 mo thereafter until 5 years. Thereafter check-ups take place annually. Additional imaging such as chest computed tomography (CT), bone scans, or position emission tomography/CT examination are also performed when clinically required.
Oncological outcome parameters were assessed for IVR, as well as other recurrences (i.e. operative field which includes ureteric stump, retroperitoneum or renal fossa, regional lymph nodes, contralateral recurrences, and/or distant metastasis), disease-specific mortality, and deaths related to other causes. Other demographic and clinicopathologic data including patient age, gender, American society of anesthesiologists scores, smoking status, distal ureter management approach, tumor side, tumor location, tumor stage, tumor grade, and lymph nodal status were systematically recorded.
Data were collated using Excel 2013 (Microsoft Corporation, Redmond, WA, United States) and all statistical analyses were performed with SPSS (version 20, IBM, Armonk, NY, United States). Fisher’s exact and chi-square tests were used to assess associations between clinicopathological variables and the three procedures. Survival curves were generated using the Kaplan-Meier method, and the log rank test was used to compare oncological outcomes such as IRFS, cancer-specific survival (CSS), and overall survival (OS). Multivariate survival analysis was assessed using the Cox proportional hazard regression model (a forward likelihood ratio-based model) to evaluate the risk of several independent factors. Statistical significance was determined around the standard threshold, P < 0.05.
A total of 248 patients were included, of which 49.2% (n = 122) were men. The remaining 50.8% (n = 126) were women. All participants underwent RNU for UTUC and met the predefined eligibility criteria. The median age was 69 years, ranging from 32 to 88 years. The most common symptom was gross hematuria (68.1%) and flank pain occurred in approximately 20% of cases. Among these patients, 39.9% (n = 99) underwent IVBC, 38.7% (n = 96) received EVBC, and 21.4% (n = 53) utilized TUBC. Detailed demographics and clinicopathological characteristics including patient age, gender, American society of anesthesiologists score, gross hematuria history, lymph node status, tumor side, location, stage, and grade of the entire populations are provided in Table 1.
| Variable | All patients (n = 248) | IVBC group (n = 99) | EVBC group (n = 96) | TVBC group (n = 53) | P value |
| Age | |||||
| < 70 yr | 128 (51.6) | 51 (51.5) | 56 (58.3) | 21 (39.6) | 0.09 |
| ≥ 70 yr | 120 (48.4) | 48 (48.5) | 40 (41.7) | 32 (60.4) | |
| ASA | |||||
| I | 23 (9.3) | 9 (9.1) | 10 (10.4) | 4 (7.5) | 0.07 |
| II | 167 (67.3) | 68 (68.7) | 70 (72.9) | 29 (54.7) | |
| III | 58 (23.4) | 22 (22.2) | 16 (16.7) | 20 (37.7) | |
| Gender | |||||
| Male | 122 (49.2) | 52 (52.5) | 46 (47.9) | 24 (45.3) | 0.66 |
| Female | 126 (50.8) | 47 (47.5) | 50 (52.1) | 29 (54.7) | |
| Smoke | |||||
| No | 204 (82.3) | 79 (79.8) | 83 (86.5) | 42 (79.2) | 0.39 |
| Yes | 44 (17.7) | 20 (20.2) | 13 (13.5) | 11 (20.8) | |
| Gross hematuria | |||||
| No | 79 (31.9) | 31 (31.3) | 28 (29.2) | 20 (37.7) | 0.56 |
| Yes | 169 (68.1) | 68 (68.7) | 68 (70.8) | 33 (62.3) | |
| Urinary cytology | |||||
| Negative | 77 (31.0) | 28 (28.3) | 34 (35.4) | 15 (28.3) | 0.50 |
| Positive | 171 (69.0) | 71(71.7) | 62 (64.6) | 38 (71.7) | |
| Tumor side | |||||
| Left | 134 (54.0) | 50 (50.5) | 52 (54.2) | 32 (60.4) | 0.51 |
| Right | 114 (46.0) | 49 (49.5) | 44 (45.8) | 21 (39.6) | |
| Tumor location | |||||
| Renal pelvis | 111 (44.8) | 37 (37.4) | 42 (43.8) | 32 (60.4) | 0.03 |
| Upper and middle ureter | 66 (26.6) | 25 (25.3) | 25 (26.0) | 16 (30.2) | |
| Lower ureter | 49 (19.8) | 26 (26.3) | 20 (20.8) | 3 (5.7) | |
| Multiple | 22 (8.9) | 11 (11.1) | 9 (9.4) | 2 (3.8) | |
| pT stage | |||||
| pT0-pTa-pT1-pTis | 69 (27.8) | 27 (27.3) | 23 (24.0) | 19 (35.8) | 0.17 |
| pT2 | 69 (27.8) | 31 (31.3) | 22 (22.9) | 16 (30.2) | |
| pT3 | 107 (43.1) | 39 (39.4) | 51 (53.1) | 17 (32.1) | |
| pT4 | 3 (1.2) | 2 (2.0) | 0 (0.0) | 1 (1.9) | |
| LN status | |||||
| LN-negative | 238 (96.0) | 94 (94.9) | 93 (96.9) | 51(96.2) | 0.787 |
| LN-positive | 10 (4.0) | 5 (5.1) | 3 (3.1) | 2 (3.8) | |
| Tumor grade | |||||
| Low | 29 (11.7) | 14 (14.1) | 9 (9.4) | 6 (11.3) | 0.58 |
| High | 221 (88.3) | 85 (85.9) | 87 (90.6) | 47 (88.7) | |
| LVI | |||||
| Negative | 223 (89.9) | 92 (92.9) | 84 (87.5) | 47 (88.7) | 0.43 |
| Positive | 25 (10.1) | 7 (7.1) | 12 (12.5) | 6 (11.3) | |
| Intravesical chemotherapy | |||||
| No | 171 (69.0) | 75 (75.8) | 66 (68.8) | 30 (56.6) | 0.05 |
| Yes | 77 (31.0) | 24 (24.2) | 30 (31.2) | 2 3 (43.4) | |
| Adjuvant therapy | |||||
| No | 218 (87.9) | 90 (90.9) | 80 (83.3) | 48 (90.6) | 0.21 |
| Yes | 30 (12.1) | 9 (9.1) | 16 (16.7) | 5 (9.4) |
All patients received successful operations with negative surgical margins. None of the patients received neo-adjuvant chemotherapy, while 30 patients had previously received adjuvant chemotherapy with or without radiotherapy for advanced stage carcinomas. After surgery, 25 patients received a single dose of intravesical instillation and 52 received at least four doses of chemotherapy weekly.
With a median follow-up of 44.2 mo (ranging from 3.4 to 172.1 mo), 76.6% (n = 190) of patients were alive and disease-free. Thirty-one (i.e. 12.5%) patients died, of which 8.1% (n = 20) deaths were attributed to UTUC, whereas 11 died from other causes. 19.4% (n = 48) of the patients had some form of disease recurrence during follow-up, of which 14.5% (n = 36) had recurrence within the bladder.
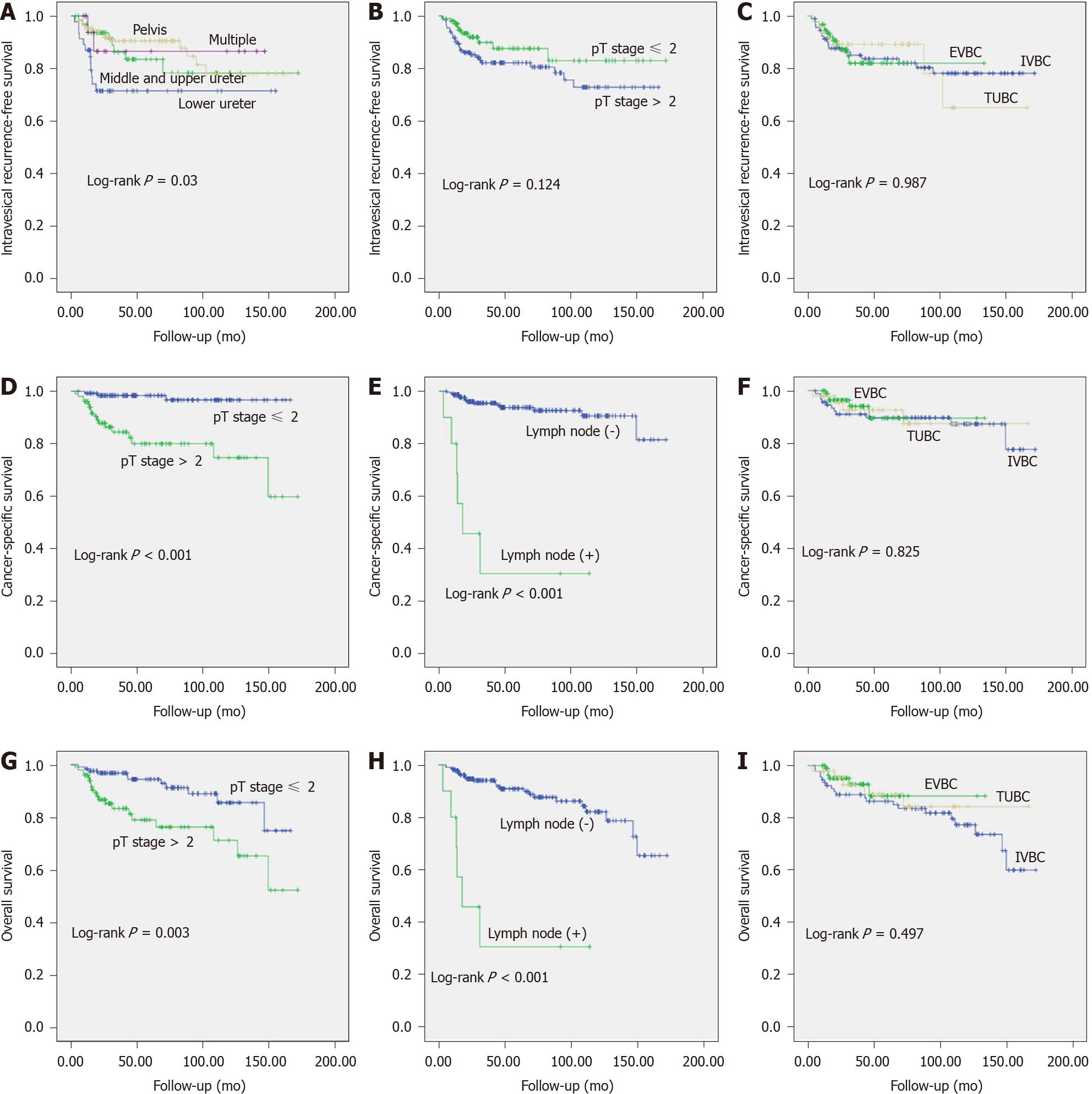
To analyze the factors associated with the oncology outcomes, univariate and multivariate Cox regression analyses were conducted (Table 2). Kaplan-Meier analysis suggested that patients with lower ureter tumor location had significantly higher IVR (P = 0.042; Figure 2A), while the tumor stage and BCE methods appeared to have no influence on IRFS (all P > 0.05; Figure 2B and C).
| Variable | IRFS | CSS | OS | |||||||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Age < 70 yr vs age ≥ 70 yr | 0.799 | 0.412-1.550 | 0.507 | - | 0.968 | 0.398-2.353 | 0.943 | 1.340 | 0.656-2.737 | 0.421 | ||||||||
| Male vs female | 0.535 | 0.273-1.047 | 0.068 | - | 0.820 | 0.339-1.986 | 0.661 | 0.644 | 0.315-1.319 | 0.229 | ||||||||
| BCE methods | 0.987 | - | 0.826 | 0.503 | ||||||||||||||
| IVBC | 1 | Reference | - | - | 1 | Reference | - | 1 | Reference | - | ||||||||
| EVBC | 1.032 | 0.474-2.246 | 0.937 | 0.707 | 0.235-2.128 | 0.537 | 0.587 | 0.223-1.542 | 0.280 | |||||||||
| TUBC | 0.954 | 0.392-2.323 | 0.917 | 0.879 | 0.276-2.797 | 0.828 | 0.695 | 0.256-1.884 | 0.474 | |||||||||
| Tumor location | 0.042 | 0.042 | 0.564 | 0.161 | ||||||||||||||
| Lower ureter | 1 | Reference | - | 1 | Reference | - | 1 | Reference | - | 1 | Reference | - | ||||||
| Middle and upper ureter | 0.421 | 0.177-1.002 | 0.051 | 0.421 | 0.177-1.002 | 0.051 | 0.930 | 0.295-2.933 | 0.901 | 1.148 | 0.451-2.920 | 0.772 | ||||||
| Renal pelvis or calyx | 0.339 | 0.153-0.750 | 0.008 | 0.339 | 0.153-0.750 | 0.008 | 0.505 | 0.160-1.598 | 0.245 | 0.466 | 0.176-1.230 | 0.123 | ||||||
| Multiple | 0.315 | 0.007-1.416 | 0.132 | 0.315 | 0.007-1.416 | 0.132 | 0.459 | 0.053-3.973 | 0.479 | 0.500 | 0.102-2.462 | 0.394 | ||||||
| pT ≤ 2 vs pT > 2 | 0.567 | 0.273-1.178 | 0.128 | 9.156 | 2.673-31.358 | 0.000 | 8.439 | 2.424-29.377 | 0.001 | 2.970 | 1.417-6.225 | 0.004 | 2.891 | 1.364-6.128 | 0.006 | |||
| LN (-/x) vs LN (+) | 0.901 | 0.123-6.586 | 0.918 | 16.793 | 6.328-44.568 | 0.000 | 14.343 | 5.176-39.745 | < 0.001 | 9.917 | 3.995-24.621 | 0.000 | 9.473 | 3.75-23.926 | < 0.001 | |||
| Tumor grade | 0.988 | 0.383-2.547 | 0.980 | 25.367 | 0.083-7755.852 | 0.268 | 0.981 | 0.341-2.826 | 0.972 | |||||||||
| LV (-) vs LV (+) | 0.611 | 0.146-2.552 | 0.499 | 2.933 | 0.969-8.881 | 0.057 | 2.385 | 0.907-6.273 | 0.078 | |||||||||
| Urinary cytology | 0.993 | 0.478-2.064 | 0.986 | 1.474 | 0.492-4.419 | 0.488 | 1.446 | 0.592-3.532 | 0.418 | |||||||||
| Intravesical chemotherapy (yes vs no) | 1.023 | 0.503-2.081 | 0.950 | 0.606 | 0.202-1.816 | 0.371 | 0.852 | 0.380-1.909 | 0.697 | |||||||||
Regarding CSS, pathological tumor stage and lymph node status were identified as significant predictors by univariate analysis (Figure 2D and E). Similarly, multivariate analysis also identified tumor stage i.e. ≤ T2 vs > T2 (hazard ratio [HR] = 8.439; 95% confidence interval (CI), 2.424-29.377; P = 0.001) and lymph node status i.e. negative vs positive (HR: 14.343; 95%CI: 5.176-39.745; P < 0.001) as independent predictors of CSS, even when adjusting for other important demographic and clinicopathological parameters such as age, gender, tumor location, tumor grade, and lymphovascular invasion. Nevertheless, the BCE methods evaluated here with additional variables did not appear to have little impact on CSS (all P > 0.05; Figure 2F, Table 2).
Likewise, Kaplan-Meier analysis suggested that the aforementioned two factors were significantly associated with OS (all P < 0.05; Figure 2G and H). By multivariate analysis, high tumor stage (≤ T2 vs > T2) had an HR = 2.891 and 95%CI: 1.364-6.128 (P = 0.006) and positive lymph node status i.e. negative vs positive with an HR = 9.473 and 95%CI: 3.75-23.926 (P < 0.001) have poorer OS. However, the BCE method evaluated here with additional variables did not appear to be associated with CSS and OS, all having P values below the predetermined threshold (i.e. P > 0.05). Please see Figure 2I and Table 2 for more details.
Although RNU with en bloc resection of the ipsilateral bladder cuff is the standard surgical practice for patients with non-metastatic UTUC, the optimal BCE approach remains controversial[1,6,9]. To the best of our knowledge, open removal of the distal ureter is traditionally used as the standard comparison for other techniques, because this not only guarantees complete resection of the distal ureter but also does do not damage the contralateral ureteric orifice[7,10]. While this approach is associated with a lower IVR rate, it has been criticized because of the significant postoperative complication and morbidity such as larger wounds, increased analgesic requirements and prolonged periods of hospitalization. While minimally invasive technology and equipment are under development, endoscopic and laparoscopic methods for resecting the distal ureter and bladder cuff have been proposed to complement RNU because these techniques reduce the number of abdominal incisions[1,7]. Although these techniques continue to be widely used, current evidence does not overwhelmingly favor one technique over the others regarding oncological safety[6,7].
We compared the oncological outcomes of three different techniques with a comparatively large cohort, over an adequate follow-up period in our hospital. Findings were generally consistent with the results of several previous studies, which found that minimally invasive technologies (i.e. EVBC and TUBC) are not inferior in terms of oncological outcomes when compared to IVBC. Li et al[11] performed a large sample size retrospective study with 301 patients who underwent BCE with either IVBC, EVBC or the TUBC technique. They found that the three techniques had equivalent oncological outcomes including IVR, distant metastasis, and CSS. In a similar study that compared the efficacy of TUBC and IVBC across a sample of 138 patients researchers found no significant differences in RFS and CSS between the two methods[12]. Likewise, the specific BCE method adopted during laparoscopic RNU appears to be associated with a higher risk of tumor recurrence and metastases. For example, Allard et al[13] conducted a retrospective review of all laparoscopic RNU performed within their center over a 10 year period. Their results further suggest that these three methods are oncologically valid with similar recurrence and metastases rates; however, conclusions are not consistent across all studies.
Xylinas et al[10] conducted a multi-center study involving 2681 patients whom underwent RNU for UTUC between 1987 and 2007. Evidence from their study suggests that the TUBC technique is associated with a higher incidence of IVR (HR: 1.74, P = 0.01). Equally controversial, the results from the Kapoor study found inferior RFS for patients undergoing TUBC compared to IVBC (HR: 1.488, P = 0.0424)[14]. These inconsistencies may manifest through several factors. For example, one potential criticism may be that the sample size in TUBC groups in both studies mentioned were relatively small, representing only 12.0% (98/820) and 3.2% (85/2681) of the total sample, respectively. These groups were also very different in terms of primary tumor location, tumor multifocality, pathologic staging and grade, which logically reduces the generalizability of findings. Some of the studies also included patients with bladder tumor history which is likely to have greatly influenced IVR outcomes. In fact, even though the multicenter study involved dozens of international clinics and urology departments which would generally yield strong evidence, there were technical discrepancies regarding BCE methods within groups[6]. Moreover, there is likely to have been variability in surgical skill and surgeons’ experience which greatly influences outcomes.
Our study was designed to overcome some of the more obvious limitations identified in the foundational research mentioned. All three methods analyzed were performed by experienced surgeons using the same surgical technique in one hospital. These control measures are likely to have reduced encroaching study biases. We found the IVR rate reached 15%, which is comparatively lower than the aforementioned related studies. One reason for this may be that patients with contamination/previous bladder tumor history were excluded according to our study protocol. Another reason for the lower IVR rate may be that the transurethral management techniques in our hospital are not identical to other techniques administered in the related foundational research. As a matter of course, during TUBC, we not only coagulated the ureteric orifice but also ligated the ureter below the distal border of the tumor prior to renal manipulation. Evidence and indeed surgical experience shows that early ligation of the ureter using a Ham-Lock provides superior closure of the ipsilateral collecting system which reduces the likelihood of urine leakage and prevents tumor seeding, thereby minimizing the postoperative risk of local tumor recurrence[15,16]. Furthermore, some of our sample had received at least one dose of prophylactic intravesical instillation postoperatively which can decrease tumor recurrence either[17,18].
Evidence around independent risk factors for IVR in primary UTUC across the Chinese population involved in this study appears to verify previously published data which suggests that tumor lesions involving lower ureter are independently associated with increased IVR[19,20]. Tumor cell implantation may be the mechanism which increases recurrence risk which is currently estimated to be between 16%-58% in the ureteric remnant considering the proximity of the primary tumor to the resection site[13]. This infers tumors located in the lower ureter can easily invade bladder mucosa, and patients with distal ureter carcinoma may have residual microscopic tumor cells even after radical surgical interventions[19]. In our study, even though IVR after RNU did not appear to have a direct impact on survival across the sample, the relapse rate suggests urothelial carcinoma continue to proliferate. This appears to necessitate further adjuvant prophylactic intravesical instillation and continued close postoperative surveillance[21].
Previous research has found that tumor stage and lymph node status are the most important predictors of CSS and OS in patients with UTUC[1,3,22], and our findings appear to confirm this. Higher tumor stages are certainly associated with more adverse oncologic outcomes which necessitates raising public awareness of the early signs of this potentially life changing disease. While beyond the remit of this study, we would encourage complementary qualitative research into the experiential elements involved in healthcare seeking behaviors related to this disease. While adjuvant therapies such as radiotherapy and chemotherapy may significantly improve overall survival[1,22,23], we must balance the system to reduce perceived social stigma and encourage early identification which will improve overall outcomes[23].
Our study had several limitations. The main limitation is inherent to retrospective study designs and analyses. Potential biases such as selection bias, information bias, and other confounding factors cannot be ignored. However, it is difficult to randomize patients to different surgical approaches due to its rareness, and clinicians have a duty to inform patients to make their own choices. In addition, postoperative adjuvant intravesical instillation may lower the IVR rate, thereby influencing the effects observed in our study. Even though the multivariate analysis was performed to adjust for possible confounding, caution must be given when drawing conclusions. Therefore, further well-designed studies with an increased number of cases and a longer follow-up time are warranted.
EVBC and TUBC appear to have equivalent oncologic outcomes to IVBC for treating UTUC when adhering to strict oncological principles, although these methods have the advantage of being minimally invasive. Selecting which of these methods to implement is dependent upon a surgeon’s experience, patient’s individual characteristics, and imparting the best evidence to inform patient choices. This study adds to the evidence base and will support urologists; however, larger, rigorously designed, multicenter studies with long term outcomes are still required.
Upper urinary tract urothelial carcinomas (UTUC) is a relatively uncommon disease accounting for only 5%-10% of all urothelial carcinomas. The current standard surgical treatment is radical nephroureterectomy (RNU) with bladder cuff excision (BCE), which consists of two separate procedures, i.e. the removal of the ipsilateral distal ureter and partial cystectomy along with the ureteral orifice. Guidelines purport that laparoscopic RNU has equivalent oncological efficacy compared to open RNU when adhering to strict oncological principles. Although, the safety and efficacy of the second step involved in the BCE method, has been debated by urologists for a number of years.
There is currently no consensus about which management technique is superior for treating primary UTUC. We previously found that intrasvesical incision of the bladder cuff (IVBC) is more strongly associated with improved intravesical recurrence-free survival, compared to EVBC and TUBC. Although, the findings can be criticized because pooled analysis did not adjust for other important clinic-opathological parameters, such as tumor multiplicity, location, stage, grade, gender.
To retrospectively collate data from a nationwide tertiary care center in mainland China, in order to investigate the oncological impact of the three, different BCE techniques on primary UTUC patients following RNU across this Asian population.
Data from 248 primary UTUC patients who underwent RNU with BCE between January 2004 to December 2018 were retrospectively analyzed. Patients were analyzed according to each BCE methods. Data extracted included patient demographics, perioperative parameters and oncological outcomes. Statistical analyses were performed using chi-square and log-rank tests. The Cox proportional hazards regression model was utilized to identify independent predictors. P < 0.05 was considered statistically significant.
Of the 248 participants, 39.9% (n = 99) underwent IVBC, 38.7% (n = 96) EVBC, and 21.4% (n = 53) TUBC. At a median follow-up of 44.2 mo, bladder recurrence developed in 17.2%, 12.5%, and 13.2% of the cases, respectively. Cancer specific deaths occurred in 11.1%, 5.2%, and 7.5%, respectively. Kaplan-Meier survival curves with a log-rank test highlighted no significant differences in intravesical recurrence-free survival, cancer-specific survival, and overall survival among these approaches with P values of 0.987, 0.825 and 0.497, respectively. Under multivariate analysis, the lower ureter location appears to have inferior intravesical recurrence-free survival (P = 0.042). However, cancer-specific survival and overall survival were independently influenced by tumor stage (HR: 8.439; 95%CI: 2.424-29.377; P = 0.001) and lymph node status (HR: 14.343; 95%CI: 5.176-39.745; P < 0.001).
EVBC and TUBC appear to have equivalent oncologic outcomes to IVBC for treating UTUC when adhering to strict oncological principles, although these methods have the advantage of being minimally invasive. Selecting which of these methods to implement is dependent upon a surgeon’s experience, patient’s individual characteristics and imparting best evidence to inform patient choices.
This study adds to the evidence-base and will support urologists; however, larger, rigorously designed, multicenter studies with long-term outcomes are still required.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Patel HR S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH
| 1. | Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Gontero P, Van Rhijn BWG, Mostafid AH, Palou J, Shariat SF. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol. 2018;73:111-122. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523-1525. [PubMed] [Cited in This Article: ] |
| 3. | Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG; Upper Tract Urothelial Carcinoma CollaborationThe Upper Tract Urothelial Carcinoma Collaboration. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224-1233. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Rouprêt M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, Babjuk M, Oosterlinck W. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59:584-594. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Ni S, Tao W, Chen Q, Liu L, Jiang H, Hu H, Han R, Wang C. Laparoscopic vs open nephroureterectomy for the treatment of upper urinary tract urothelial carcinoma: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2012;61:1142-1153. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Lee SM, McKay A, Grimes N, Umez-Eronini N, Aboumarzouk OM. Distal Ureter Management During Nephroureterectomy: Evidence from a Systematic Review and Cumulative Analysis. J Endourol. 2019;33:263-273. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Steinberg JR, Matin SF. Laparoscopic radical nephroureterectomy: dilemma of the distal ureter. Curr Opin Urol. 2004;14:61-65. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Lai S, Guo R, Seery S, Wu P, Liu J, Zhang Y, Zhu S, Li X, Liu M, Wang J. Assessing the impact of different distal ureter management techniques during radical nephroureterectomy for primary upper urinary tract urothelial carcinoma on oncological outcomes: A systematic review and meta-analysis. Int J Surg. 2020;75:165-173. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Phé V, Cussenot O, Bitker MO, Rouprêt M. Does the surgical technique for management of the distal ureter influence the outcome after nephroureterectomy? BJU Int. 2011;108:130-138. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Xylinas E, Rink M, Cha EK, Clozel T, Lee RK, Fajkovic H, Comploj E, Novara G, Margulis V, Raman JD, Lotan Y, Kassouf W, Fritsche HM, Weizer A, Martinez-Salamanca JI, Matsumoto K, Zigeuner R, Pycha A, Scherr DS, Seitz C, Walton T, Trinh QD, Karakiewicz PI, Matin S, Montorsi F, Zerbib M, Shariat SF; Upper Tract Urothelial Carcinoma Collaboration. Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2014;65:210-217. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Li WM, Shen JT, Li CC, Ke HL, Wei YC, Wu WJ, Chou YH, Huang CH. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol. 2010;57:963-969. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Walton TJ, Sherwood BT, Parkinson RJ, Obakponovwe O, Thomas SA, Taylor MC, England RC, Lemberger RJ. Comparative outcomes following endoscopic ureteral detachment and formal bladder cuff excision in open nephroureterectomy for upper urinary tract transitional cell carcinoma. J Urol. 2009;181:532-539. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Allard CB, Alamri A, Dason S, Farrokhyar F, Matsumoto ED, Kapoor A. The method of bladder cuff excision during laparoscopic radical nephroureterectomy does not affect oncologic outcomes in upper tract urothelial carcinoma. World J Urol. 2013;31:175-181. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kapoor A, Dason S, Allard CB, Shayegan B, Lacombe L, Rendon R, Jacobsen NE, Fairey A, Izawa J, Black P, Tanguay S, Chin J, So A, Lattouf JB, Bell D, Saad F, Drachenberg D, Cagiannos I, Fradet Y, Alamri A, Kassouf W. The impact of method of distal ureter management during radical nephroureterectomy on tumour recurrence. Can Urol Assoc J. 2014;8:E845-E852. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Gill IS, Soble JJ, Miller SD, Sung GT. A novel technique for management of the en bloc bladder cuff and distal ureter during laparoscopic nephroureterectomy. J Urol. 1999;161:430-434. [PubMed] [Cited in This Article: ] |
| 16. | Kurzer E, Leveillee RJ, Bird VG. Combining hand assisted laparoscopic nephroureterectomy with cystoscopic circumferential excision of the distal ureter without primary closure of the bladder cuff--is it safe? J Urol. 2006;175:63-7; discussion 67. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Wu P, Zhu G, Wei D, Liu S, Walsh K, Li D, Harron U, Wang X, Ma H, Wan B, Sun L, Yang Z, Wang J. Prophylactic intravesical chemotherapy decreases bladder tumor recurrence after nephroureterectomy for primary upper tract urothelial carcinoma: A systematic review and meta-analysis. J BUON. 2015;20:1229-1238. [PubMed] [Cited in This Article: ] |
| 18. | Wu WJ, Ke HL, Yang YH, Li CC, Chou YH, Huang CH. Should patients with primary upper urinary tract cancer receive prophylactic intravesical chemotherapy after nephroureterectomy? J Urol. 2010;183:56-61. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Luo HL, Kang CH, Chen YT, Chuang YC, Cheng YT, Lee WC, Chiang PH. Oncological impact of endoscopic bladder cuff management during nephroureterectomy varies according to upper urinary tract tumor location. Int J Urol. 2014;21:366-369. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Zigeuner RE, Hutterer G, Chromecki T, Rehak P, Langner C. Bladder tumour development after urothelial carcinoma of the upper urinary tract is related to primary tumour location. BJU Int. 2006;98:1181-1186. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | O'Brien T, Ray E, Singh R, Coker B, Beard R; British Association of Urological Surgeons Section of Oncology. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol. 2011;60:703-710. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Chromecki TF, Bensalah K, Remzi M, Verhoest G, Cha EK, Scherr DS, Novara G, Karakiewicz PI, Shariat SF. Prognostic factors for upper urinary tract urothelial carcinoma. Nat Rev Urol. 2011;8:440-447. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Hasan MN, Rouprêt M, Keeley F, Cracco C, Jones R, Straub M, Traxer O, Osther PJS, Brehmer M. Consultation on UTUC, Stockholm 2018 aspects of risk stratification: long-term results and follow-up. World J Urol. 2019;37:2289-2296. [PubMed] [DOI] [Cited in This Article: ] |