Published online Aug 6, 2019. doi: 10.12998/wjcc.v7.i15.2065
Peer-review started: March 4, 2019
First decision: May 31, 2019
Revised: June 17, 2019
Accepted: June 26, 2019
Article in press: June 27, 2019
Published online: August 6, 2019
We present the case of a 72-year-old female patient with gallbladder cancer (GBC) who developed in situ recurrence and liver metastases 9 mo after irreversible electroporation ablation and oral tegafur (a fluoropyrimidine derivative) chemotherapy, which failed to control the progression of the disease. The patient further developed metastases in the lymph nodes around the head of the pancreas. The patient had severe anemia, requiring weekly blood transfusions. The gallbladder tumor invaded the descending part of the duodenum, causing intestinal leakage and hepatic colonic adhesion.
The patient refused other treatments and began daily hydrogen inhalation therapy. After 1 mo of treatment, the gallbladder and liver tumors continued to progress, and intestinal obstruction occurred. After continuous hydrogen therapy and symptomatic treatments including gastrointestinal decompression and intravenous nutrition support, the intestinal obstruction was gradually relieved. Three months after hydrogen therapy, the metastases in the abdominal cavity gradually reduced in size, her anemia and hypoalbuminemia were corrected, lymphocyte and tumor marker levels returned to normal, and the patient was able to resume normal life.
This is the first report of an efficacy and safety study about hydrogen therapy in patient with metastatic GBC and a critical general condition, who has remained stable for more than 4 months.
Core tip: This is the first report of an efficacy and safety study about hydrogen therapy in patient with metastatic gallbladder cancer and a critical general condition, who has remained stable for more than 4 mo.
- Citation: Chen JB, Pan ZB, Du DM, Qian W, Ma YY, Mu F, Xu KC. Hydrogen gas therapy induced shrinkage of metastatic gallbladder cancer: A case report. World J Clin Cases 2019; 7(15): 2065-2074
- URL: https://www.wjgnet.com/2307-8960/full/v7/i15/2065.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i15.2065
Gallbladder cancer (GBC) is the sixth most common gastrointestinal cancer and the sixth highest cause of cancer-related deaths in China[1]. Despite improvements in the diagnosis and treatment of GBC, the majority of the patients are usually confirmed as having metastatic GBC (mGBC)[2,3]. Due to the high incidence of lymph node or distant metastasis, the 5-year overall survival (OS) rate for GBC is only 5%[3,4]. In mGBC patients who are not candidates for surgery, the National Comprehensive Cancer Network (NCCN) guidelines reco-mmend the use of biliary drainage, chemotherapy, clinical trials, and the best supportive care available. At present, there are few alternative options, and new and more effective methods are urgently required.
In patients with cancer, the treatment strategy, outcome, and prognosis are strongly dependent on immune status. CD8+ T cells become exhausted by persistent stimu-lation by tumor antigens, resulting in cessation of proliferation, cytokine production, and immune function[5,6]. The preventive and therapeutic effects of hydrogen in various diseases, including cancer, have been investigated in several studies[7-9]. Hydrogen was recently reported to stimulate peroxisome proliferatoractivated receptor γ coactivator 1α[10], which enhances mitochondrial function[11] and thus, may rescue exhausted CD8+ T cells. In an advanced study of the effects of inhaled hydrogen gas in 55 patients with stage IV colorectal cancer[12], Akagi et al[12] identified accumulation of terminal programmed cell death 1 (PD1)+ CD8+ T cells as a significant index for poor prognosis, with reduction in terminal PD1+ CD8+ T cells and accumulation of terminal PD1- CD8+ T cells following hydrogen gas treatment found to be significantly associated with improved progression-free survival and OS.
Here, we present the case of a 72-year old Chinese woman with stage IIIA GBC who was treated with irreversible electroporation (IRE) and oral tegafur (a fluoropyrimidine derivative) chemotherapy. Primary recurrence and liver metastases were identified 9 mo later. Obvious shrinkage of the patient’s recurrent and metastatic lesions was observed with continuous application of a hydrogen oxygen nebulizer, and a clinically objective response lasting for more than 4 mo was achieved. The patient remained disease-free at the time of writing this report.
We present the case of a 72-year-old female GBC patient, who was admitted to our hospital due to chest tightness in September 2018.
After examination, GBC was found to recur in situ, invading the duodenal descending part and causing intestinal fistula and hepatic colon adhesion. In addition, multiple metastases of the liver, lymph node metastasis around the head of the pancreas, severe anemia requiring weekly blood transfusion, and symptoms of heart failure were noted.
In December 2017, the 72-year old Chinese patient underwent IRE following diagnosis as GBC (T3N1M0 stage IIIA) based on computed tomography (CT) imaging and pathological findings of GBC (8.0 cm × 3.9 cm) with multiple hilar lymph node invasion (max 4.7 cm × 3.6 cm). She had elevated levels of a variety of serum tumor markers, including CA19-9 (2556 U/mL) and CEA (607.6 ng/mL).
After IRE ablation of the gallbladder and hilar lymph nodes, oral tegafur (20 mg bid) chemotherapy was administered for 9 mo. CT re-examination in April 2018 showed liquefactive necrosis at the ablation site. During the treatment period, the patient received intermittent red blood cell (RBC) infusions as supportive care for repeated episodes of anemia.
Accompanied by a progressive increase in pain in the upper right abdomen, a large area of adhesion between the gallbladder and the descending and horizontal segments of the duodenum was found in addition to compression of the inferior vena cava (October 6, 2018).
She had elevated levels of a variety of serum tumor markers, including CA19-9 (88.18 U/mL), AFP (14.11 IU/mL), and CEA (39.68 ng/mL) in September, 2018.
After 2 wk of blood transfusions and anti-infective treatment, CT examination again revealed a bladder tumor (6.3 cm × 4.9 cm), with an adjacent descending duodenal fistula, multiple spotted high-density lesions in the liver parenchyma, and dilatation and gas accumulation in the intrahepatic and extrahepatic bile ducts. Enlarged lymph nodes (2.7 cm × 2.1 cm) were visible around the pancreatic head.
Metastatic GBC, severe anemia, infection, and gallbladder-duodenal fistula.
The hydrogen oxygen nebulizer (AMS-H-01, Asclepius Meditec, Shanghai, China) generates 3 L/min hydrogen gas by water electrolysis. As measured by gas chromatography, the gas generated consisted of 67% hydrogen and 33% oxygen. Using a special mask, the patient continued to inhale hydrogen for 3-6 h a day at rest, with no interruption even after the obvious relief of symptoms.
Due to the long-term difficulty in reversing the anemia in the early stage of hydrogen inhalation therapy, the patient received weekly (1-2) blood infusions, and her RBC and hemoglobin levels were tested regularly. Daily anti-infection treatment was administered for the gallbladder-duodenal fistula, and white blood cell counts were measured regularly. After 1 mo of hydrogen aspiration therapy, the patient presented with intestinal obstruction, which was treated by gastrointestinal decompression and intravenous nutrition. Analgesics and sedatives were prescribed for pain and insomnia, respectively.
Due to the lack of currently available conventional treatments combined with the patient’s poor condition requiring continuous supportive care, hydrogen gas monotherapy was started on October 24, 2018 (Table 1). At the time of writing this report, the patient still continues to receive daily hydrogen therapy.
| Date | Therapy | |
| 2017 | December | (A) Symptoms of upper right quadrant distension pain and discomfort were aggravated; (B) Color doppler ultrasound: gallbladder tumor, local liver invasion, and multiple enlarged lymph nodes; (C) Gallbladder tumor aspiration biopsy: gallbladder cancer |
| 2018 | January 5 | (A) Irreversible electroporation ablation of the gallbladder and hilar lymph nodes; (B) Started oral 5-FU (20 mg bid); (C) Infusion of red blood cells for anemia |
| April | CT examination: gallbladder tumors and hilar lymph nodes showed liquefactive necrosis after treatment | |
| September 20–October 8 | (A) Routine blood tests and tumor markers; (B) CT examination: gallbladder tumor invaded the descending duodenum; inferior vena cava was compressed; (C) Infusion of red blood cells for anemia | |
| October 24 | (A) CT examination: gallbladder tumor enlarged, multiple spotted metastases found in the liver, and multiple lymph nodes around the pancreatic head; (B) Started hydrogen gas therapy | |
| November 26 | (A) CT examination: gallbladder and intrahepatic tumors enlarged, duodenal bowel obstruction; (B) Gastrointestinal decompression and intravenous nutrition support | |
| December 10–24 | (A) Routine blood tests and tumor markers; (B) Gastric tube removed, the patient gradually began taking semi-liquid food, and her spirit, appetite, and sleep were good | |
| 2019 | January 8–11 | (A) Routine blood tests and tumor markers; (B) CT examination: gallbladder and intrahepatic tumors, lymph nodes around the pancreatic head all shrank significantly, and obstruction was markedly relieved |
According to the Common Terminology Criteria for Adverse Events version 3.0 (National Cancer Institute, Bethesda, MD, United States), adverse events were classified and graded every week for at least 2 mo after the treatment was started[13]. Liver function was evaluated based on the levels of alanine transaminase, aspartate transaminase, total bilirubin, and gamma-glutamyl transpeptidase on multiple occasions during hydrogen treatment. Bone marrow hematopoietic function was evaluated based on peripheral blood erythrocyte counts and hemoglobin levels. Inflammation caused by infection was assessed based on white blood cell counts.
The curative effects of the treatment were evaluated according to several aspects of the patient’s general condition. Since patients cannot eat normally, serum total protein and albumin levels were monitored after intravenous nutrition. Due to visceral adhesion, intestinal fistula and obstruction, degrees of pain and relief in the upper abdomen were recorded continuously. Absolute lymphocyte counts and serum tumor markers were measured to reflect changes in immune function and tumor activity.
Changes in CT tumor imaging were monitored to evaluate the curative effect of hydrogen gas therapy. According to the RECIST 1.1 guidelines[14], therapeutic effects are categorized as a complete response (CR), characterized as disappearance of tumor detection in all target lesions; partial response (PR), total reduction in the diameter of the target lesions ≥ 30%; stable disease (SD), tumor regression failing to reach PR or progressive disease (PD); or PD, defined as total progression of the tumor diameter ≥ 20%.
Hydrogen gas inhalation was started on October 24, 2018. Within 1 mo of treatment, the patient’s condition was relatively stable, and her blood indexes, infection status, and degree of pain gradually improved. Subsequently, intestinal obstruction occurred at the adhesion site between the gallbladder and duodenum; this was relieved after 1 mo of gastrointestinal decompression and intravenous nutrition. The patient insisted on regular hydrogen therapy throughout the course of this treatment.
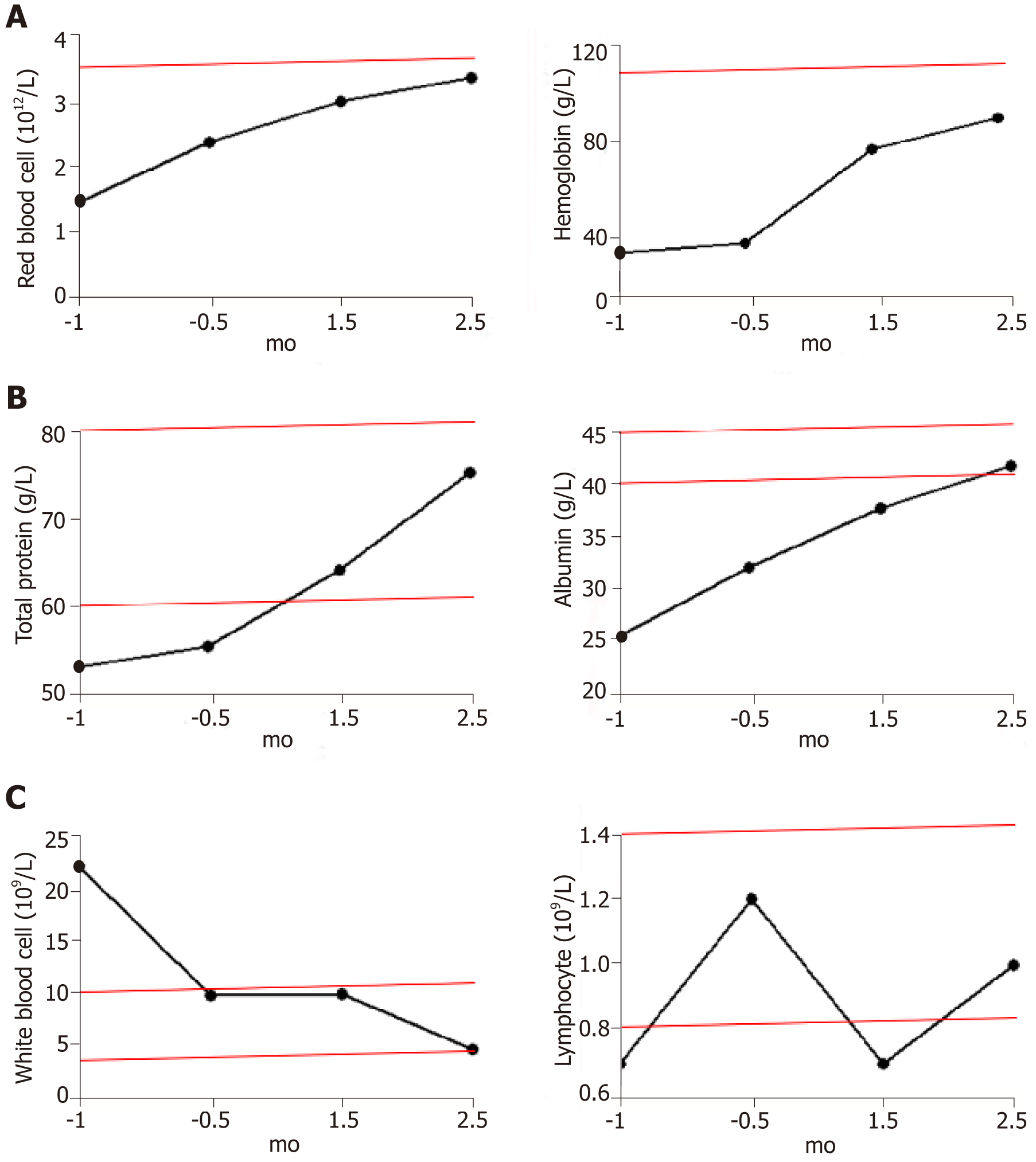
The most common adverse reactions recorded included drowsiness or agitation, and no other adverse events were observed. The patient’s transaminase levels remained normal throughout the treatment. Occasionally her bilirubin levels exceeded the reference range, but returned to normal after short-term use of hepatoprotective drugs. Despite regular blood transfusions, there was little change in the patient’s red blood cell counts and hemoglobin levels during the two weeks before hydrogen treatment was initiated. Both indexes continued to increase after hydrogen therapy and blood transfusions, and both reached the lower limit of the reference range after 2.5 mo of treatment (Figure 1A). The frequency of transfusions was decreased gradually after hydrogen therapy and stopped completely after 2 mo of treatment.
Within 1 mo prior to treatment, the patient had a high inflammatory response to infection and a high white blood cell count (22.1 × 109/L). After anti-infection treatment, the white blood cell count was restored to the upper limit of the reference range (9.8 × 109/L), with no further reduction. After 1.5 mo of hydrogen treatment (with no further anti-infection treatment), the leukocyte count decreased to 4.7 × 109/L (Figure 1C left).
Serum total protein levels returned to normal shortly after the start of hydrogen therapy and continued to rise. Because of the intestinal obstruction, the patient received intravenous nutrition for 1-2 mo after treatment. Within 2 wk of the removal of the gastric tube, the patient returned to a normal diet and the total protein remained at the upper limit of the reference range. Serum albumin levels returned to normal after 2 mo of hydrogen therapy and continued to rise (Figure 1B).
Prior to hydrogen therapy, extensive adhesion and compression of gallbladder tumors caused severe pain in the upper abdomen that required daily pain medication; this was stopped when the pain gradually diminished after 2 wk of hydrogen treatment. Subsequent pain-induced administration of analgesics was not necessary, even during ileus.
T and NK cells are the major populations of lymphocytes that mediate anti-tumor immunity; therefore, the total number of lymphocytes is positively correlated with the immune function of patients. Before tumor recurrence and metastasis, the total number of lymphocytes continued to rise (1.2 × 109/L), indicating an increase in immune function. However, the total number of lymphocytes declined rapidly with tumor progression, to significantly below the reference range (0.8-4 × 109/L), reaching a minimum (0.7 × 109/L) during intestinal obstruction (1.5 mo after hydrogen therapy). The lymphocyte numbers then rebounded, reaching 1 × 109/L after 2.5 mo of hydrogen treatment (Figure 1C right).
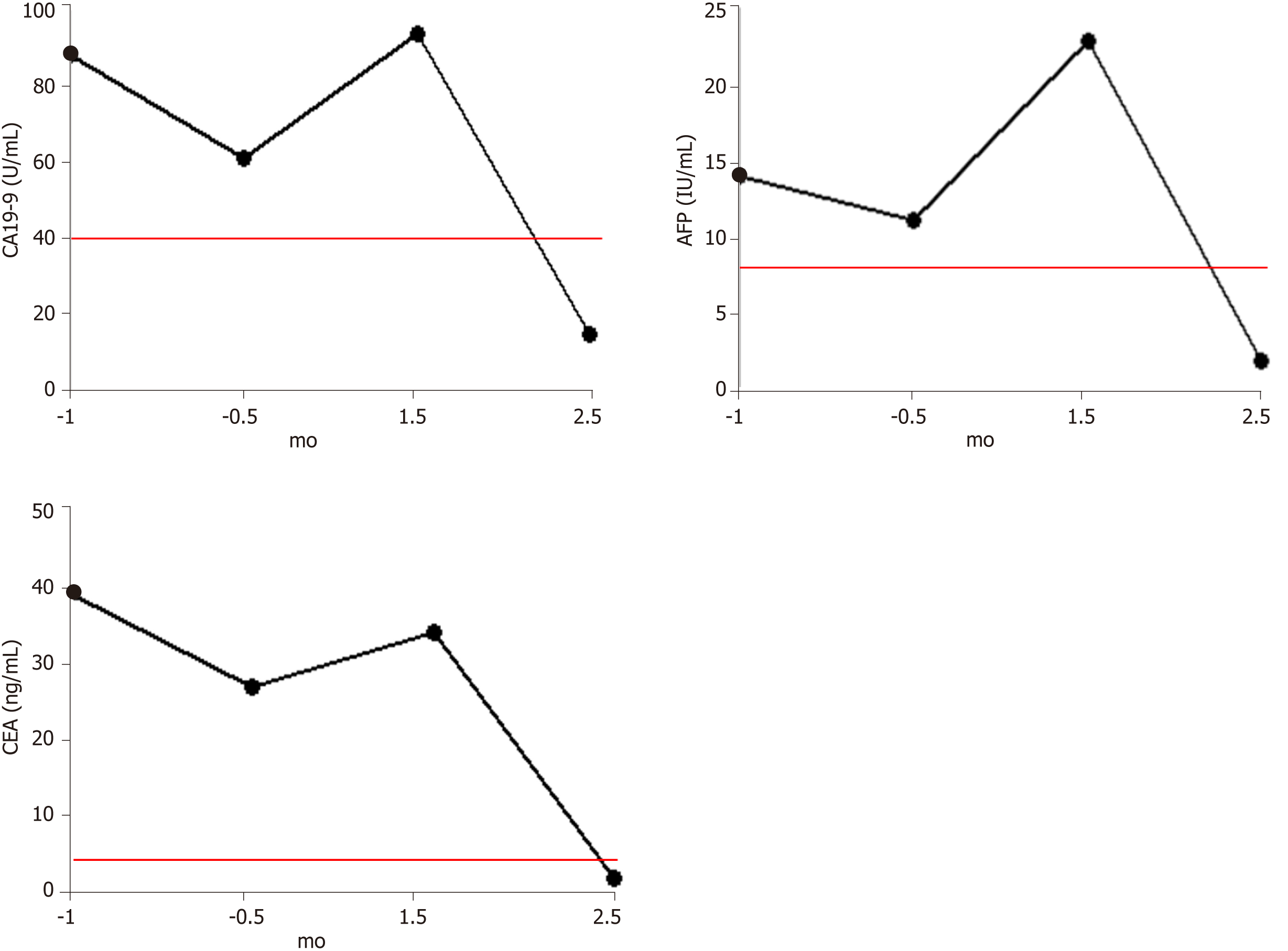
Before the detection of tumor recurrence (September 20, 2018), all three tumor markers were significantly higher than the reference range, but all showed a slight decline, which may be related to the compensation of immune function before treatment failure. After identification of tumor recurrence and metastasis, all three markers began to increase rapidly. This was consistent with the rapid growth of the tumor, which caused obstruction. After 1.5 mo of hydrogen therapy, the levels of all three markers began to decrease rapidly, falling into the reference ranges after 2.5 mo (Figure 2).
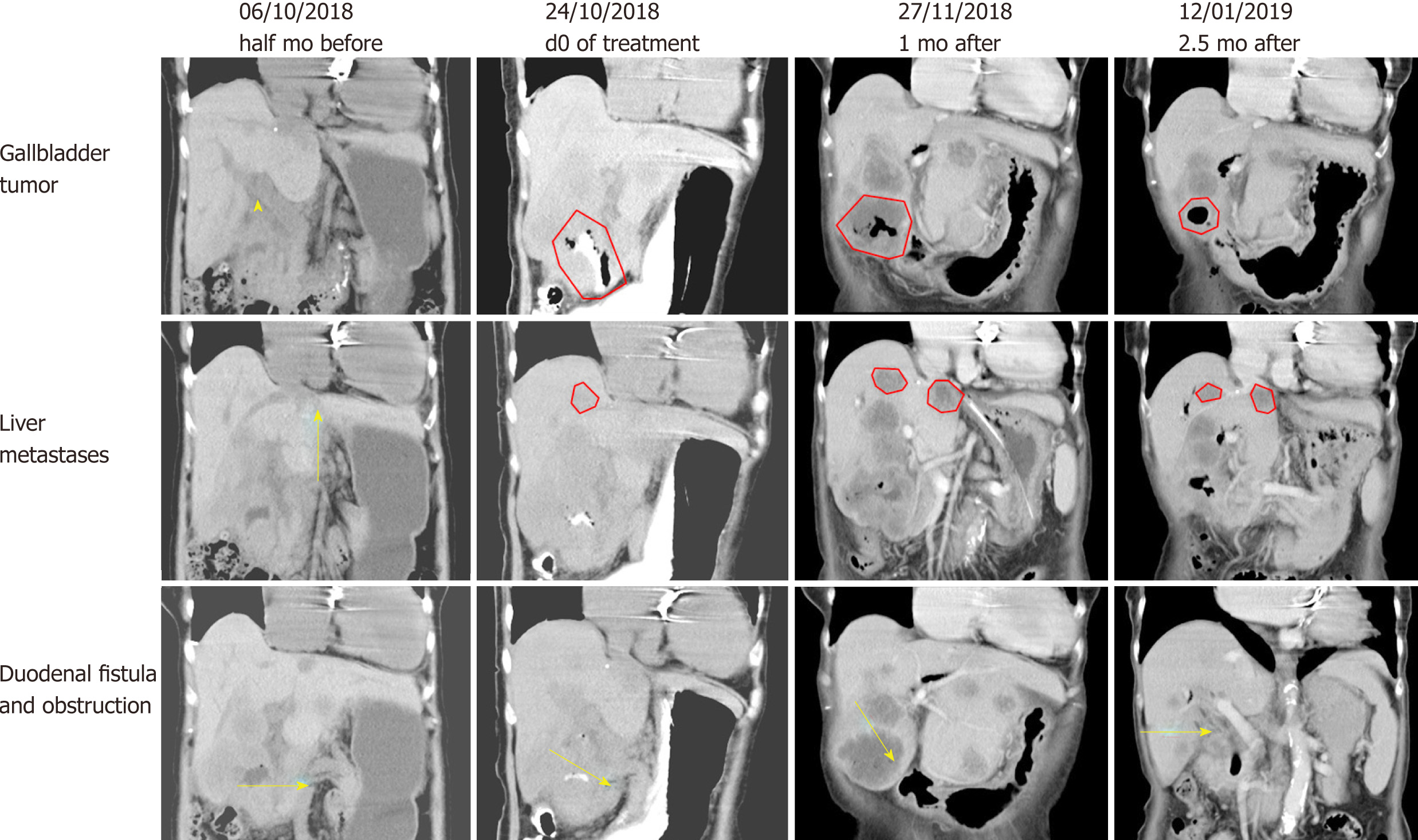
Before hydrogen gas treatment, the sum of the tumor diameters was 12.2 cm; 1 mo after treatment, the sum was 13.1 cm (37% increase, PD); and 2.5 mo after treatment, the sum was 7.5 cm (6% increase, SD) (Figure 3 and Table 2). At the end of February 2019, no tumor recurrence was detected (very similar to the CT examination at 2.5 mo; images not shown). To date, the patient appears completely well (Karnofsky score 100) and has resumed a normal diet and exercise.
| Gallbladder | Liver metastases | Lymph node around the pancreas head | Sum of the tumor diameters | |
| Pretreatment | 6.3 cm × 4.9 cm | 3.2 cm × 2.6 cm | 2.7 cm × 2.1 cm | 12.2 cm |
| 1 mo after treatment | 6.7 cm × 6.3 cm | 4.2 cm × 4.0 cm (large) 3.6 cm × 3.2 cm (small) | 2.2 cm × 1.8 cm | 16.7 cm |
| 2.5 mo after treatment | 3.7 cm × 3.6 cm | 4.0 cm × 3.3 cm (large) 3.2 cm × 2.8 cm (small) | 2.0 cm × 1.6 cm | 12.9 cm |
In GBC patients who are not candidates for surgery, the NCCN guidelines recommend the use of chemotherapy and best supportive care. Due to the large size of tumor in this patient (stage IIIA), the gallbladder function was completely lost with widespread adhesions to the hilum of the liver. Therefore, it was necessary to choose an ablation method that causes minimal damage to the extrahepatic bile duct system in reducing the tumor size. IRE is a novel ablation technology that utilizes short pulses of high voltage electrical energy to induce tissue necrosis. This technique has many advantages, including short ablation time, preservation of the internal structure of vital organs, and lack of the heat/cold-sink effect[15]. We have previously verified the safety and efficacy of gallbladder ablation in a rabbit model[16]. Although not yet included in the NCCN guidelines, IRE has the potential to be a superior alternative to other ablation techniques for the removal of tumors situated near the gallbladder[17-19].
IRE, combined with oral chemotherapy, effectively controlled the tumors in the gallbladder and the hilar part of the liver, and re-examination after 3 mo of treatment showed that the tumor was basically necrotic. However, for this patient with advanced GBC, palliative treatment could only delay the disease, with continued slow progression of the tumor. In the re-examination after 9 mo of treatment, recurrence of the gallbladder tumor, duodenum fistula, liver metastases, and lymph node metastases around the head of pancreas as well as severe anemia were detected, which indicated that the disease had entered a stage of rapid and life-threatening progression in a short period of time. Best supportive care was the only option at this timepoint, although in general, it is not possible to reverse the progression of such tumors.
Molecular hydrogen, or H2, can selectively neutralize hydroxyl radicals, but not other reactive oxygen species (ROS)[20]. Hydrogen has been used to treat various states associated with oxidative stress, including trauma[21], neurodegenerative disease[22], inflammatory disease[23], metabolic syndrome[24], adverse reactions to chemotherapy[25], and radiation injury[26]. In addition to the apparent improvement in the immune system[12], there is much evidence that hydrogen directly kills different types of tumors, including cutaneous squamous cell carcinoma[27], leukemia[28], tongue cancer[29,30], and colon cancer[8]. Since there has been no report on the upper limit of hydrogen use, this therapy might be a very safe method.
At the same time as the delivery of optimal supportive care, the patient was enrolled in this clinical study (24 October 2018) with the intention of improving clinical symptoms. One month after the start of hydrogen therapy, the disease continued to progress, and the levels of tumor markers (including CA19-9, AFP, and CEA) continued to rise, although the patient’s general status and blood indexes (including total protein, albumin, multiple blood cells count, hemoglobin content, etc.) showed continuous improvement, so the patient persisted with the hydrogen treatment. Gradually, the levels of multiple tumor markers began to decline, and multiple hematological indicators continued to improve. The patient underwent gastric tube removal on December 24, and gradually began eating semi-liquid food, with significant improvement in spirit, appetite, and sleep. Subsequent CT examination also confirmed relief of the obstruction, with significant shrinkage of tumors at multiple sites. The reason for the patient’s extraordinary recovery, apart from symptomatic treatment, is probably because the persistence of hydrogen therapy; this therapy not only mediates rapid improvement in the physical condition of the patient, but also significantly reduces tumor marker levels, increases lymphocyte counts, and even induces tumor shrinkage.
At present, there is only a temporal connection between hydrogen and improvement of this patient’s cancer. The proposed mechanism underlying the anti-cancer properties of hydrogen may involve: (1) Elimination of the high levels of ROS produced by cancer cells, thus inhibiting the proliferation, invasion, and migration of cancer cells[31]; (2) Downregulation of inflammatory factors, elimination of chronic inflammation, and modification of the microenvironment[32]; (3) Protection of mitochondria, which maintains energy generation and help correct hypoxia, thereby transforming the cardiovascular, endocrine, and nervous systems[11]; and (4) Restoration of the function of exhausted cytotoxic T cells, and enhancement of systemic anti-cancer effects[33]. The detailed mechanism remains to be fully elucidated in further follow-up studies, and indications for advanced cancer therapy require verification in studies with greater numbers of patients.
This is the first report on the rehabilitation of an advanced GBC patient with a critical general condition, whose disease has gradually improved and has survived for more than 4 mo.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Young C S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11444] [Cited by in F6Publishing: 12502] [Article Influence: 1562.8] [Reference Citation Analysis (0)] |
| 2. | Shi JS, Wang JS, Liu G, Yu YL, Lu Y, Jiao XY, Yang YJ, Li GC, Han Y. Early diagnosis of primary gallbladder carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:273-275. [PubMed] [Cited in This Article: ] |
| 3. | Hueman MT, Vollmer CM, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16:2101-2115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 420] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 5. | Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2815] [Cited by in F6Publishing: 2980] [Article Influence: 156.8] [Reference Citation Analysis (0)] |
| 6. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2481] [Cited by in F6Publishing: 2693] [Article Influence: 207.2] [Reference Citation Analysis (0)] |
| 7. | Ge L, Yang M, Yang NN, Yin XX, Song WG. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget. 2017;8:102653-102673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Runtuwene J, Amitani H, Amitani M, Asakawa A, Cheng KC, Inui A. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ. 2015;3:e859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 2018;104:788-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Kamimura N, Ichimiya H, Iuchi K, Ohta S. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1α to enhance fatty acid metabolism. NPJ Aging Mech Dis. 2016;2:16008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 839] [Cited by in F6Publishing: 858] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 12. | Akagi J, Baba H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep. 2019;41:301-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP, Bruner DW, Cleeland CS, Sloan JA, Chilukuri R, Baumgartner P, Denicoff A, St Germain D, O'Mara AM, Chen A, Kelaghan J, Bennett AV, Sit L, Rogak L, Barz A, Paul DB, Schrag D. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 598] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 14. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15860] [Cited by in F6Publishing: 19012] [Article Influence: 1267.5] [Reference Citation Analysis (1)] |
| 15. | Wagstaff PG, Buijs M, van den Bos W, de Bruin DM, Zondervan PJ, de la Rosette JJ, Laguna Pes MP. Irreversible electroporation: state of the art. Onco Targets Ther. 2016;9:2437-2446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Zeng J, Qin Z, Zhou L, Fang G, Chen J, Li J, Niu L, Liang B, Xu K. Comparison between cryoablation and irreversible electroporation of rabbit livers at a location close to the gallbladder. Radiol Oncol. 2017;51:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Chen X, Ren Z, Zhu T, Zhang X, Peng Z, Xie H, Zhou L, Yin S, Sun J, Zheng S. Electric Ablation with Irreversible Electroporation (IRE) in Vital Hepatic Structures and Follow-up Investigation. Sci Rep. 2015;5:16233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Herwald SE, Chen JH, Arellano RS. Irreversible Electroporation for Treatment of Hepatocellular Carcinoma Adjacent to the Gallbladder. J Vasc Interv Radiol. 2016;27:1093-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Siddiqui IA, Kirks RC, Latouche EL, DeWitt MR, Swet JH, Baker EH, Vrochides D, Iannitti DA, Davalos RV, McKillop IH. High-Frequency Irreversible Electroporation: Safety and Efficacy of Next-Generation Irreversible Electroporation Adjacent to Critical Hepatic Structures. Surg Innov. 2017;24:276-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1487] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 21. | Ji X, Tian Y, Xie K, Liu W, Qu Y, Fei Z. Protective effects of hydrogen-rich saline in a rat model of traumatic brain injury via reducing oxidative stress. J Surg Res. 2012;178:e9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Chen T, Tao Y, Yan W, Yang G, Chen X, Cao R, Zhang L, Xue J, Zhang Z. Protective effects of hydrogen-rich saline against N-methyl-N-nitrosourea-induced photoreceptor degeneration. Exp Eye Res. 2016;148:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Ren JD, Ma J, Hou J, Xiao WJ, Jin WH, Wu J, Fan KH. Hydrogen-rich saline inhibits NLRP3 inflammasome activation and attenuates experimental acute pancreatitis in mice. Mediators Inflamm. 2014;2014:930894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Kikkawa YS, Nakagawa T, Taniguchi M, Ito J. Hydrogen protects auditory hair cells from cisplatin-induced free radicals. Neurosci Lett. 2014;579:125-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Watanabe S, Fujita M, Ishihara M, Tachibana S, Yamamoto Y, Kaji T, Kawauchi T, Kanatani Y. Protective effect of inhalation of hydrogen gas on radiation-induced dermatitis and skin injury in rats. J Radiat Res. 2014;55:1107-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 190] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 28. | Roberts BJ, Fife WP, Corbett TH, Schabel FM. Response of five established solid transplantable mouse tumors and one mouse leukemia to hyperbaric hydrogen. Cancer Treat Rep. 1978;62:1077-1079. [PubMed] [Cited in This Article: ] |
| 29. | Saitoh Y, Okayasu H, Xiao L, Harata Y, Miwa N. Neutral pH hydrogen-enriched electrolyzed water achieves tumor-preferential clonal growth inhibition over normal cells and tumor invasion inhibition concurrently with intracellular oxidant repression. Oncol Res. 2008;17:247-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Saitoh Y, Yoshimura Y, Nakano K, Miwa N. Platinum nanocolloid-supplemented hydrogendissolved water inhibits growth of human tongue carcinoma cells preferentially over normal cells. Exp Oncol. 2009;31:156-162. [PubMed] [Cited in This Article: ] |
| 31. | Yang Y, Zhu Y, Xi X. Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species. Oncol Lett. 2018;16:2771-2776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Huang L. Molecular hydrogen: a therapeutic antioxidant and beyond. Med Gas Res. 2016;6:219-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Hu Z, Wu B, Meng F, Zhou Z, Lu H, Zhao H. Impact of molecular hydrogen treatments on the innate immune activity and survival of zebrafish (Danio rerio) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2017;67:554-560. [PubMed] [DOI] [Cited in This Article: ] |