Published online Sep 6, 2018. doi: 10.12998/wjcc.v6.i9.284
Peer-review started: February 27, 2018
First decision: March 30, 2018
Revised: June 27, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: September 6, 2018
Here, we report a rare case of primary gastrointestinal amyloidosis in a stable condition after being followed up for three years. The patient was admitted to the hospital in 2014. Tests showed decreased levels of hemoglobin and ferritin. Transoral and transanal enteroscopy showed multiple nodular protuberances in the esophagus, ileum, colon and rectum. Endoscopic ultrasonography indicated the nodular protuberances stemmed from the submucosa and partially invaded the intrinsic myometrium. Pathological examinations found multiple small nodules in the submucosa and dyed structures, which were positive for special Congo red dyeing. After treatment with oral iron supplements, the levels of hemoglobin and ferritin became normal. It is concluded that the patient represents a case of primary gastrointestinal amyloidosis with multiple nodular protuberances in the digestive tract with controllable moderate abdominal discomfort and anemia and a benign course. Enteroscopy and endoscopic ultrasonography play an important role in the diagnosis of primary gastrointestinal amyloidosis.
Core tip: A 48-year-old woman was admitted with moderate abdominal discomfort for two years and decreased hemoglobin and ferritin levels. Transoral and transanal enteroscopy showed multiple nodular protuberances in the digestive tract. Endoscopic ultrasonography indicated the nodular protuberances stemmed from the submucosa and partially invaded the intrinsic myometrium. Pathological examinations showed the nodules were positive for special Congo red dyeing. It is concluded that the patient represents a case of primary gastrointestinal amyloidosis with multiple nodular protuberances in the digestive tract with controllable symptoms and a benign course during three-year follow-up. Enteroscopy and endoscopic ultrasonography play an important role in the diagnosis.
- Citation: Liu YP, Jiang WW, Chen GX, Li YQ. Case report and review of the literature of primary gastrointestinal amyloidosis diagnosed with enteroscopy and endoscopic ultrasonography. World J Clin Cases 2018; 6(9): 284-290
- URL: https://www.wjgnet.com/2307-8960/full/v6/i9/284.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i9.284
Amyloidosis is a rare disorder characterized by the extracellular deposition of an abnormal fibrillar protein. The pathogenesis is due to abnormal protein folding, which leads to the formation of a fibrous structure and disruption of tissue function. These misfolded proteins are deposited in the extracellular space of tissues. The excessive accumulation of these misfolded proteins leads to various degrees of organ dysfunction[1,2]. The disease is named amyloidosis because the color reaction of the accumulated substances to iodine and sulfuric acid is similar to that of starch to the same reagents, and the name has been used to date[3,4]. Epidemiological investigations in Latin America and Europe found that the incidence of amyloidosis is lower than ten cases per million people, which is considered a rare clinical disease[5-7]. The probability of involvement of the digestive system for amyloidosis is 70%-80%, and the lesions can occur throughout the gastrointestinal tract from the mouth to the rectum[8].
Currently, the prognosis of amyloidosis is primarily associated with a high mortality rate due to the progressive nature, especially with heart failure[9]. We encountered a case of primary amyloidosis in the digestive tract, which was diagnosed with enteroscopy and endoscopic ultrasonography. After a close follow-up of three years, it was considered a rare and unique case with a benign nature. Therefore, we wish to share our experience and opinions regarding diagnosing and treating such a case.
A 48-year-old woman was admitted into Yantai Affiliated Hospital of Binzhou Medical University on December 7, 2014 for the first time with a history of moderate daily “abdominal discomfort for two years”. She had a history of cesarean section. She had no unhealthy diet habits. Her family members had no gastrointestinal diseases, except that her father had a history of “gastric cancer” with surgical resection. Gastroscopy and colonoscopy in the outpatient department of the hospital reported multiple nodular protuberances in the esophagus, colon and rectum.
After admission, a comprehensive physical examination demonstrated upper abdominal tenderness and moderate anemia. The laboratory tests showed low plasma levels of hemoglobin (84 g/L, normal range 110-150 g/L) and ferritin (1.2 ng/mL, normal range 10-291 ng/mL) and normal values for urine and stool routines. Specifically, the levels of the only urine parameters Bence Jones protein and blood parameters such as digestive tract tumor markers, blood glucose, liver and kidney function, myocardial enzymes, coagulation function, thyroid function, Streptococcus hemolysin O, rheumatoid factor, erythrocyte sedimentation rate, C reactive protein, and β2 microglobulin, were all within normal ranges. Electrocardiogram, computed tomography and ultrasound in the chest and abdomen showed no abnormalities.
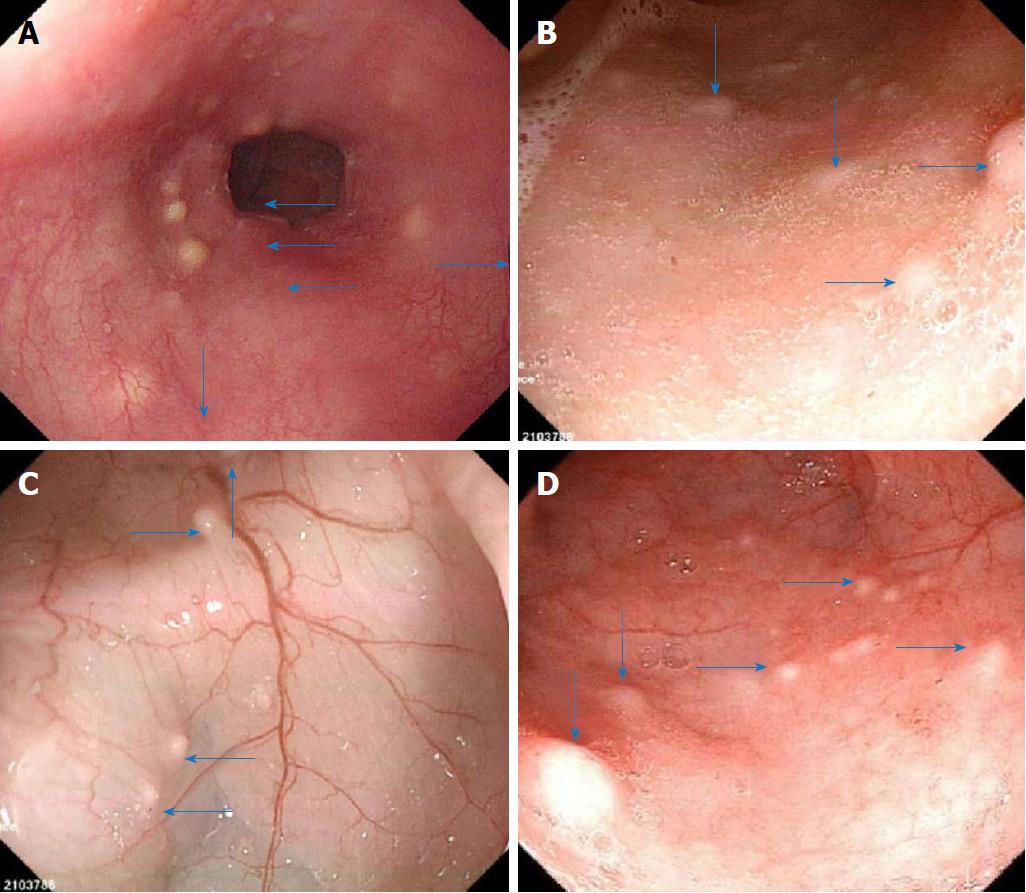
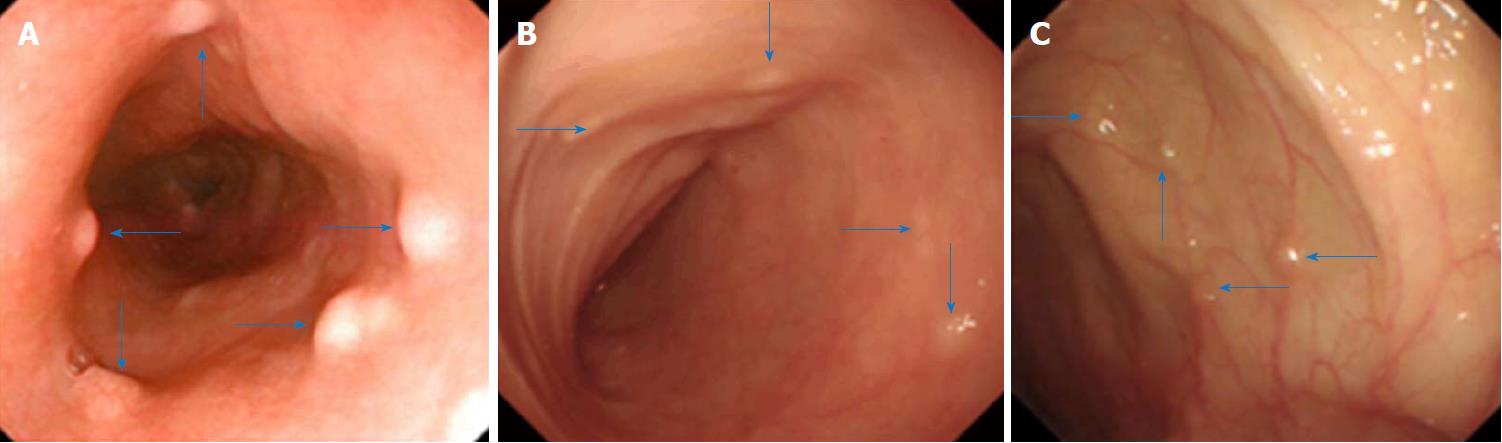
We conducted enteroscopy and endoscopic ultrasonography in the patient. Enteroscopy through the mouth and the anus performed on December 11, 2014 showed many yellowish nodular protuberances with diameters of approximately 0.2-0.5 cm in the esophagus, ileum, colon and rectum (Figure 1). The mucosa of the stomach, duodenum and jejunum appeared to be normal.
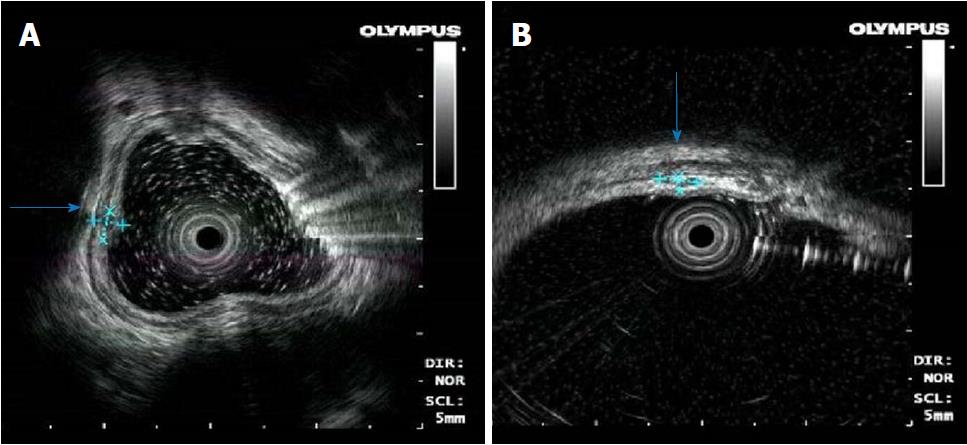
Endoscopic ultrasonography was obtained on December 8, 2014. The nodular protuberances of the esophagus stemmed from hypoechoic lesions of the submucosa, and the layer of the origin was clear (Figure 2A). The nodular protuberances of the colon stemmed from the submucosa and partially invaded the intrinsic myometrium with a hypoechoic structure, which is a partly hypoechoic fusion and inhomogeneous echo (Figure 2B).
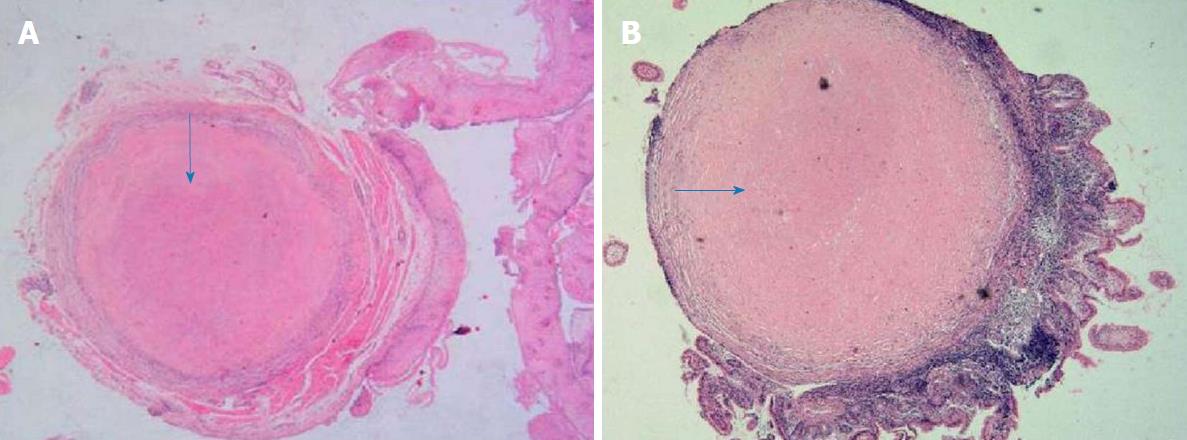
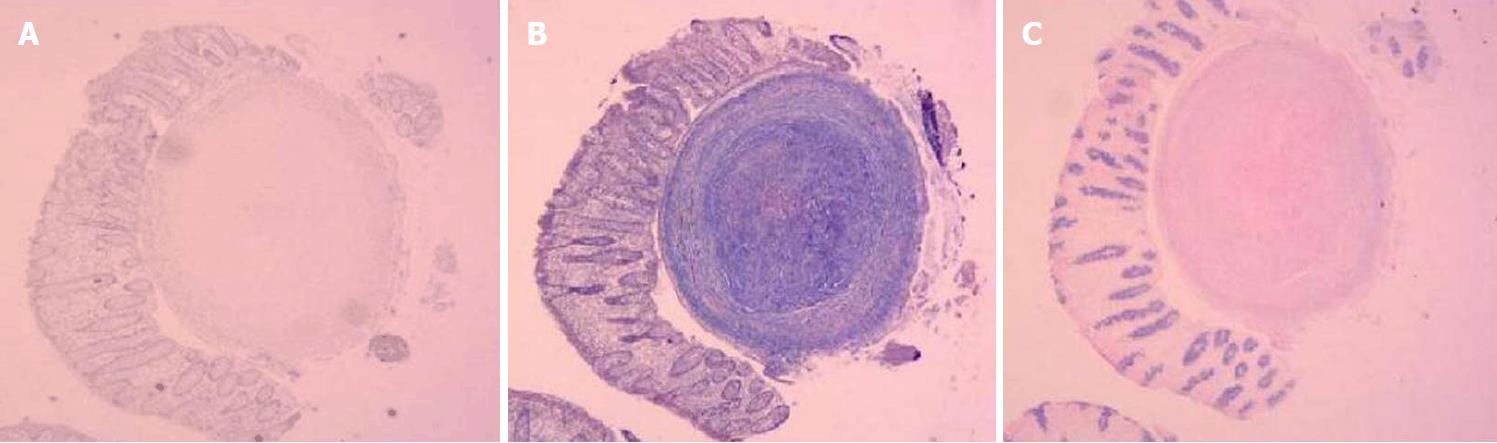
Pathological examination was performed with hematoxylin and eosin staining on samples collected from the digestive tract. In the esophagus, the squamous epithelium in the tissue was mildly proliferative and there were multiple small nodules in the submucosa. Figure 3A shows that the central portion of an esophagus biopsy sample contained a homogeneous powder and dyed structure, which is surrounded by inflammatory cells and fibrous tissues. In the ileum and descending colon, chronic inflammation was observed in the mucous tissue. Figure 3B shows that the biopsy sample of the ileum contained multiple homogeneous powder dyeing without structural small nodules in the submucosal layer. Moreover, staining of samples from the esophagus with Congo red, Masson Blue, and Periodic Acid-Schiff was positive (Figure 4).
According to the above findings, the patient was diagnosed with primary amylolysis in the digestive tract[10,11]. An oral iron supplement (Ferrous fumarate, 0.2 g one time, 3-4 times/d) was prescribed for one year for anemia between December 2014 and December 2015, along with medications such as esomeprazole magnesium (Nexium®), Talcid®, Domperidone for the treatments of abdominal discomfort. The anemia gradually improved and was eventually cured.
The patient was regularly followed up for three years. Blood tests performed in October 2017 showed that the hemoglobin and serum ferritin were restored to 124 g/L (normal range 110-150 g/L) and 39.8 ng/mL (normal range 10-291 ng/mL), respectively, without abnormalities for other routine parameters. During this period, the patient only occasionally reported mild abdominal discomfort. Gastroscopy and colonoscopy performed on October 2017 showed that the numbers and sizes of nodular protuberances in the mucosa of the esophagus, ileum and colon had no obvious change compared with those observed three years ago (Figure 5).
Amyloidosis is categorized into primary, secondary and inheritable based on the types of deposited proteins, and primary amyloidosis is the most common type[11-13]. Clinically, primary amyloidosis is mainly due to the deposition of immunoglobulin light chain, which commonly occurs in the digestive system and affects its functions[14,15]. Gastrointestinal involvement by amyloidosis has been reported as common. The most common gastrointestinal manifestations were weight loss, abdominal pain/dyspepsia, and nausea/vomiting[16]. The frequency of amyloid deposition was highest in the duodenum, and the duodenal biopsy is sensitive for diagnosing amyloidosis[17]. Amyloidosis in the small intestine may be characterized by diarrhea, fat diarrhea, bleeding, obstruction, constipation, and similar symptoms[18,19]. Colonic amyloidosis manifests similarly to inflammatory bowel disease and ischemic bowel disease, and is characterized by mucous congestion, erosion, ulcer, nodular protuberance or polypoid changes[20-22]. Our patient had multiple nodular protuberances in the esophagus, ileum, colon and rectum, with a history of moderately daily abdominal discomfort for two years, which was improved by symptomatic treatment. Thus, the case was characterized by multiple nodular protuberances covering the upper, middle and lower digestive tract, as well as relatively mild, non-specific and stable clinical symptoms.
Due to the lack of specificity in the clinical manifestation of primary amyloidosis in the digestive tract, laboratory and imaging examinations are prone to misdiagnosis and missed diagnosis. At present, auxiliary examinations for gastrointestinal amyloidosis mainly rely on gastroscopy and colonoscopy. In our case, transoral and transanal enteroscopy was applied. Theoretically, enteroscopy specifically covers the entire small intestine and extends the examination to the whole digestive tract, and thus, would improve the diagnosis of amyloidosis in the whole digestive tract. Moreover, endoscopic ultrasonography performed on the case showed that the lesions involved the submucosa, and a partial invasion of the intrinsic myometrium was hypoechoic, which is consistent with the pathological examinations. Endoscopic ultrasonography has unique characteristics that enable a diagnosis of amyloidosis of the digestive tract and reduces the rate of misdiagnosis. We propose that accurate and multi-site biopsy of the digestive tract under the guidance of endoscopic ultrasonography enhances the pathological diagnosis. If eosinophil infiltration and wax-like substance deposition are found in the pathological routine, hematoxylin and eosin staining, as well as specific Congo red staining should be performed to assist the diagnosis. In our opinion, enteroscopy and endoscopic ultrasonography play important roles in the diagnosis of the amyloidosis disease, and pathology is the gold standard for diagnosis. These techniques are highly recommended techniques for the diagnosis of digestive tract amyloidosis. Specifically, for a case with a moderate abdominal discomfort and anemia for a couple of years, the physician should take digestive tract amyloidosis into consideration and apply transoral and transanal enteroscopy, along with endoscopic ultrasonography and pathological examinations for the diagnosis. Several factors, such as the clinical and endoscopic features, the qualification and experience of the evaluating endoscopist, the choice of reasonable endoscopic and pathological examinations, may influence the diagnosis of digestive tract amyloidosis. For example, we usually apply gastroscopy and colonoscopy to diagnose gastrointestinal amyloidosis; however, the lesions are prominent in the small intestine in some patients. Therefore, transoral and transanal enteroscopy is required in these cases in order to avoid misdiagnosis and missed diagnosis. It should be mentioned that the procedure of transoral and transanal enteroscopy needs special equipment and an experienced endoscopist, and thus may not be applied in the other settings. However, it is important for a physician, especially a gastroenterologist, to be aware of the existence of digestive tract amyloidosis and refer the suspected patients to enteroscopy, along with endoscopic ultrasonography in clinical practice to diagnose or rule out digestive tract amyloidosis.
Chemotherapy and stem cell transplantation are currently the standard of treatment for primary amyloidosis[23]. For patients with primary amyloidosis in the digestive tract, studies have shown that a risk stratification score using cardiac troponin T, N-terminal pro-brain natriuretic peptide, and uric acid is helpful to identify patients at risk of early mortality, and prospective identification of these patients allows the development of risk-adapted strategies and help develop risk-adapted therapies[24,25]. In our case, the patient had no bad diet habits without a family history of a similar disease. The two major symptoms were abdominal discomfort and anemia. The former was treated symptomatically and relieved to a great extent, and the latter was treated and cured with an iron supplement. Close follow-up was maintained for three years after admission. The condition of the patient remained stable, clinical symptoms were not progressively aggravated, and endoscopic observation showed no obvious changes in the digestive tract, indicating a benign disease course. Ideally, the subtype of the amyloidosis should be further investigated to rule out multiple myeloma. We indeed tried to persuade the patient to take bone marrow examination during the course, but the patient refused. Nevertheless, there was no manifestation of multiple myeloma during the 3-year follow-up, and thus multiple myeloma could be ruled out.
The prognosis of primary gastrointestinal amyloidosis is usually poor but largely depends on the underlying etiology for amyloid deposition and the degree of organ involvement. A prospective study of a cohort of 155 patients with systemic amyloidosis found that the median survival time was 16 mo for 131 cases without gastrointestinal involvement and 8 mo for 24 cases with gastrointestinal involvement[19]. The patient presented here was a rare case of gastrointestinal amyloidosis with a benign clinical course, and the prognosis was deemed as good. However, the mechanism linking the pathogenesis and prognosis is unknown, and needs to be further investigated. We will continue to closely follow up the case and conduct protein mass spectrometry analysis to identify the deposited proteins and genetic analysis to explore the genes involved in the development of amyloidosis.
We acknowledge that the three-year follow-up is still short to determine the future outcome of the patient, and it remains too early to make a final conclusion on whether multiple organ dysfunctions, such as heart, liver, lung and kidney, which were normal in the previous admission examination, will occur in the future. Therefore, more tests and careful analysis of clinical reports are needed in the follow-up visits.
In conclusion, the present patient represents a case of primary gastrointestinal amyloidosis with multiple nodular protuberances in the digestive tract with controllable moderate abdominal discomfort and anemia and a benign course. Enteroscopy and endoscopic ultrasonography, which are used for the diagnosis of this case, may play an important role in the diagnosis of primary gastrointestinal amyloidosis, although pathology remains the gold standard for diagnosis.
A case with multiple nodular protuberances in the digestive tract with controllable moderate abdominal discomfort and anemia and a benign course.
After admission, a comprehensive physical examination demonstrated upper abdominal tenderness and moderate anemia.
Theoretically, enteroscopy specifically covers the entire small intestine and extends the examination to the whole digestive tract and thus would improve the diagnosis of amyloidosis in the whole digestive tract, and endoscopic ultrasonography has unique characteristics that enable a diagnosis of amyloidosis of the digestive tract and reduces the rate of misdiagnosis.
The laboratory tests showed low plasma levels of hemoglobin (84 g/L, normal range 110-150 g/L) and ferritin (1.2 ng/mL, normal range 10-291 ng/mL) and normal values for urine and stool routines at administration, and normal plasma levels of hemoglobin 124 g/L and 39.8 ng/mL without abnormalities for other routine parameters at three-year follow-up.
Transoral and transanal enteroscopy showed multiple nodular protuberances in the esophagus, ileum, colon and rectum, and endoscopic ultrasonography showed the nodular protuberances stemmed from the submucosa and partially invaded the intrinsic myometrium.
Pathological examinations found multiple small nodules in the submucosa and dyed structures.
An oral iron supplement (Ferrous fumarate, 0.2 g/time, 3-4 times/d) were prescribed for one year for the anemia, along with medications such as esomeprazole magnesium (Nexium®), Talcid®, Domperidone for the treatment of abdominal discomfort.
Amyloidosis is categorized into primary, secondary and inheritable, and primary amyloidosis is the most common type.
Primary gastrointestinal amyloidosis can be presented with controllable moderate abdominal discomfort and anemia and a benign course. Enteroscopy and endoscopic ultrasonography may play an important role in the diagnosis.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Amornyotin S, Nakajima N, Osawa S S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Dogan A. Amyloidosis: Insights from Proteomics. Annu Rev Pathol. 2017;12:277-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Picken MM. Modern approaches to the treatment of amyloidosis: the critical importance of early detection in surgical pathology. Adv Anat Pathol. 2013;20:424-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, Pepys MB, Hawkins PN. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786-1791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Lavatelli F, Vrana JA. Proteomic typing of amyloid deposits in systemic amyloidoses. Amyloid. 2011;18:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Aguirre MA, Boietti BR, Nucifora E, Sorroche PB, González Bernaldo de Quirós F, Giunta DH, Posadas-Martínez ML. Incidence rate of amyloidosis in patients from a medical care program in Buenos Aires, Argentina: a prospective cohort. Amyloid. 2016;23:184-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Hemminki K, Li X, Försti A, Sundquist J, Sundquist K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health. 2012;12:974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Pinney JH, Smith CJ, Taube JB, Lachmann HJ, Venner CP, Gibbs SD, Dungu J, Banypersad SM, Wechalekar AD, Whelan CJ. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161:525-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Levy DJ, Franklin GO, Rosenthal WS. Gastrointestinal bleeding and amyloidosis. Am J Gastroenterol. 1982;77:422-426. [PubMed] [Cited in This Article: ] |
| 9. | Gilowski W, Stryjewski P, Gilowska M, Liszniański P, Nowak J. [The rapid progress of heart failure due to systemic amyloidosis with cardiac involvement--case report]. Przegl Lek. 2015;72:697-700. [PubMed] [Cited in This Article: ] |
| 10. | Djunić I, Tomin D, Perunicić M, Vidović A, Cemerikić V, Djurasinović V, Jaković L, Janković G. [Diagnosis and the treatment of primary amyloidosis]. Vojnosanit Pregl. 2010;67:781-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Pinney JH, Hawkins PN. Amyloidosis. Ann Clin Biochem. 2012;49:229-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Hemminki K, Li X, Försti A, Sundquist J, Sundquist K. Incidence of hereditary amyloidosis and autoinflammatory diseases in Sweden: endemic and imported diseases. BMC Med Genet. 2013;14:88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Czeyda-Pommersheim F, Hwang M, Chen SS, Strollo D, Fuhrman C, Bhalla S. Amyloidosis: Modern Cross-sectional Imaging. Radiographics. 2015;35:1381-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Freudenthaler S, Hegenbart U, Schönland S, Behrens HM, Krüger S, Röcken C. Amyloid in biopsies of the gastrointestinal tract-a retrospective observational study on 542 patients. Virchows Arch. 2016;468:569-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641-2654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 559] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 16. | Said SM, Grogg KL, Smyrk TC. Gastric amyloidosis: clinicopathological correlations in 79 cases from a single institution. Hum Pathol. 2015;46:491-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Yilmaz M, Unsal A, Sokmen M, Harmankaya O, Alkim C, Kabukcuoglu F, Ozagari A. Duodenal biopsy for diagnosis of renal involvement in amyloidosis. Clin Nephrol. 2012;77:114-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Gould M, Zarrin-Khameh N, Sellin J. Small bowel amyloidosis. Curr Gastroenterol Rep. 2013;15:350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Lim AY, Lee JH, Jung KS, Gwag HB, Kim DH, Kim SJ, Lee GY, Kim JS, Kim HJ, Lee SY. Clinical features and outcomes of systemic amyloidosis with gastrointestinal involvement: a single-center experience. Korean J Intern Med. 2015;30:496-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Antonini F, Goteri G, Macarri G. Bleeding localized amyloidosis of the colon. Dig Liver Dis. 2014;46:e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Li J, Lv YM. Endoscopic and pathological diagnostic significance of gastroenteric amyloidosis. Chin J Digest Endo. 2002;19:6-8. [DOI] [Cited in This Article: ] |
| 22. | Liu JX, Wu HL, Wang XD. Analysis of difficult case review: tongue hypertrophy, after chest tightness hematochezia. Chin Med J. 2012;92:2295-2297. [DOI] [Cited in This Article: ] |
| 23. | Cohen AD, Comenzo RL. Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program. 2010;2010:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 614] [Cited by in F6Publishing: 701] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 25. | Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman SR, Buadi FK, Short-Detweiler K, Zeldenrust SR, Leung N, Greipp PR. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 2011;86:12-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |