Published online Jun 16, 2018. doi: 10.12998/wjcc.v6.i6.99
Peer-review started: April 8, 2018
First decision: April 23, 2018
Revised: April 27, 2018
Accepted: May 30, 2018
Article in press: May 31, 2018
Published online: June 16, 2018
Processing time: 76 Days and 9.3 Hours
To compare the clinical outcomes of patients with portal hypertension (PH) who underwent treatment with splenectomy plus simplified pericardial devascularisation (SSPD) or splenectomy plus traditional pericardial devascularisation (STPD).
We conducted a single-centre retrospective study of 1045 PH patients treated with either SSPD (S Group, 357 patients) or STPD (T Group, 688 patients) between January 2002 and February 2017. In all, 37 clinical indicators were compared to evaluate the efficacy of SSPD.
Perioperative indicators in the S Group were significantly better than those in the T Group (P < 0.05). In both groups, the postoperative long-term portal vein diameter and Model for End-Stage Liver Disease score were significantly lower than those in the preoperative and postoperative short-term groups (P < 0.05). The incidence of complications in the S Group was significantly lower than that in the T Group (P < 0.05). Compared to the T Group, postoperative short-term WBC (white blood cell) and platelet counts were significantly lower and the short-term Hb (haemoglobin) level was significantly higher in the S Group (P < 0.05). In the S Group, postoperative long-term total bilirubin, direct bilirubin, alanine transaminase, and aspartate transaminase and postoperative serum creatinine and cystatin C levels were significantly lower than those in the T Group (P < 0.05), and postoperative albumin was significantly higher than that in the T Group (P < 0.05).
Compared to STPD, SSPD is a simple and easy procedure resulting in less tissue damage. Patients recovered smoothly and steadily with fewer complications. Short-term liver and kidney function damage was less severe, and long-term liver function recovery was better. Therefore, SSPD is worthy of clinical promotion and application for the treatment of PH.
Core tip: We performed the use of splenectomy plus simplified pericardial devascularisation (SSPD) in 2002. In this study, we compared the clinical data of patients treated with SSPD or splenectomy plus traditional pericardial devascularisation to evaluate the efficacy of SSPD. A total of 1045 portal hypertension patients were included, and the results suggest that SSPD is simple and easy to perform, resulting in less tissue damage and a reduced inflammatory reaction. Patients recovered smoothly and steadily after SSPD, with lower rates of thrombosis and other complications. Liver and kidney function damage are less severe and long-term liver function recovery is better.
- Citation: Zhang YF, Ji H, Lu HW, Lu L, Wang L, Wang JL, Li YM. Comparison of simplified and traditional pericardial devascularisation combined with splenectomy for the treatment of portal hypertension. World J Clin Cases 2018; 6(6): 99-109
- URL: https://www.wjgnet.com/2307-8960/full/v6/i6/99.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i6.99
Due to the high incidence of hepatitis B and hepatitis C in Asia, especially in China, an increasing number of patients suffer from portal hypertension (PH) secondary to cirrhosis. Oesophagogastric varices and hypersplenism are the main clinical manifestations of PH. The incidence and mortality of severe oesophagogastric variceal haemorrhage are extremely high and seriously threaten patients’ lives and health[1,2]. Currently, the main purpose of surgical treatment for PH is to address the bleeding caused by oesophagogastric variceal rupture, followed by the resolution of splenomegaly and hypersplenism[3,4].
The surgical treatment of PH mainly consists of shunt and devascularisation. Although a shunt may reduce portal vein pressure, liver function may be compromised due to decreased hepatic blood flow from the portal vein, potentially resulting in hepatic encephalopathy, and patients may ultimately die from liver failure. Compared with a shunt, the greatest advantage of devascularisation is that it does not reduce portal vein blood flow to the liver, does not affect liver nutrition, does not compromise liver function, and is associated with a lower likelihood of hepatic encephalopathy. Therefore, patient survival after the operation is better[5]. Due to the difference of etiology, the treatment of PH in western countries is mainly shunt, while in Asian countries, especially in China, are mainly devascularization. Splenectomy plus pericardial devascularisation (SPD) can simultaneously address the problems of bleeding, thrombocytopenia and leukocyte reduction and has therefore become the most effective surgical treatment for PH[5]. This method has been continuously improved and has achieved excellent clinical effects[6-8]. However, splenectomy plus traditional pericardial devascularisation (STPD) needs to cut the serous layer and damage the seromuscular layer of the stomach and the esophagus. The high postoperative rebleeding rate, the complexity of the procedure and severe resultant tissue damage can cause extensive liver and renal function injury, which affect many patients and seriously threaten people’s lives.
Based on the experiences of domestic and foreign experts, we simplified the traditional pericardial devascularisation method and developed a splenectomy plus simplified pericardial devascularisation (SSPD) technique, which achieved good initial treatment effects[9]. In this study, we retrospectively analysed the short-term and long-term clinical efficacy of SSPD vs STPD from 2002 to the present.
PH patients with oesophagogastric varices and hypersplenism who were seen in our department from January 2002 to February 2017 were screened for this single-centre retrospective cohort study. According to the patients’ and their relatives’ choices of surgical method, the patients were divided into an S Group and a T Group. The patients in the S Group were treated with SSPD, and the patients in the T Group were treated with STPD. The two surgical methods were performed by the same group of doctors. In this study, clinical indicators of the two groups were compared and analysed to evaluate the efficacy of SSPD. This research was approved by the Ethical Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. All procedures were conducted in accordance with the Helsinki Declaration of the World Medical Association and with the ethical standards of the committee responsible for human experimentation (institutional and national). The requirement for written informed patient consent was waived due to the retrospective and anonymous nature of this study; all data were used only for statistical analysis.
The inclusion criteria were as follows: (1) PH patients diagnosed with oesophagogastric varices and hypersplenism based on clinical symptoms combined with laboratory, digestive endoscopy or image examinations; (2) PH patients classified as grade A or B according to the Child-Pugh grading criteria or Child-Pugh grade C at admission assigned a reduced classification to preoperative Child-Pugh grade A or B after liver preservation therapy to attain appropriate surgical indications; and (3) patients who could tolerate general anaesthesia and had no surgical contraindications. The exclusion criteria were as follows: (1) patients with acute heart failure, shock, or other vital organ diseases; (2) patients in an acute haemorrhagic state with unstable vital signs; and (3) patients with poor function of the heart, lung, liver or kidney.
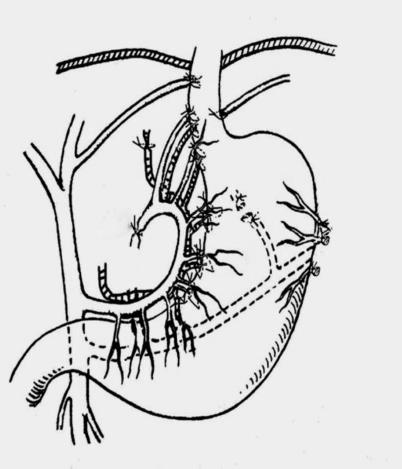
An arc-shaped incision was made in the left ribs with the patient under general anaesthesia. The abdominal cavity was explored, and the spleen was removed. The short gastric and posterior gastric vessels were cut, and the left diaphragmatic blood vessel was sutured and ligated. The venae coronaria ventriculi and arteriae gastrica sinistra were sutured and ligated from the roots. The vessels from the arteriovenous gastrica sinistra to the tributaries of the stomach were sutured and ligated one by one. The rami oesophagei and high rami oesophagei were sutured and ligated. The peritoneal cavity was routinely closed (Figure 1). The patients underwent traditional STPD surgery, as described previously[9].
The perioperative indicators were postoperative hospital stay, operation fee, total hospitalisation cost, operative time, intraoperative blood loss, intraoperative transfusion, time to first flatus, mortality and reoperation for rebleeding. The short-term and long-term recovery indicators and complications after the operation were portal vein diameter, Model for End-Stage Liver Disease (MELD) score and complications. The short-term and long-term postoperative routine blood tests were white blood cell (WBC), haemoglobin (Hb), and platelets. The short-term and long-term postoperative liver function tests were total bilirubin (TBIL), DBIL (direct bilirubin), alanine transaminase (ALT), aspartate transaminase (AST), albumin (ALB), and globulin (GLB). The short-term and long-term postoperative renal function tests were serum creatinine (Scr) and cystatin C (Cys C). Short-term refers to within one month after the operation, and long-term refers to more than one month after the operation.
Continuous variables are presented as the mean ± SD and were evaluated using an independent samples t test or the Wilcoxon rank test. Categorical variables are expressed as the frequencies and percentages and were compared via the χ2 test or Fisher’s exact test (in cases of small numbers, indicated by n ≤ 5). A P-value < 0.05 was considered statistically significant. The statistical analysis was performed with SPSS software (version 22.0, SPSS Inc., Chicago, IL, United States).
A total of 1045 PH patients were included, with 357 in the S Group and 688 in the T Group. No significant differences were observed in the clinical parameters, including gender, age, aetiology of disease, history of abdominal surgery, history of variceal bleeding, ascites, oesophageal varices grade, MELD score at admission, and preoperative portal vein diameter, between the two groups (P > 0.05). However, significant differences were identified between the two groups in Child-Pugh grade and the incidence of portal hypertensive gastropathy (PHG) at admission (P < 0.05). In the S Group, the proportions of patients with Child grades A, B and C disease were 42.86%, 52.66% and 4.48%, respectively. In the T Group, the proportions were 52.18%, 43.60% and 4.22%, respectively. The incidence of PHG was 33.05% in the S Group and 25.0% in the T Group (Table 1).
| Parameters | S Group (n = 357) | T Group (n = 688) | Statistic | P-value |
| Gender, male | 196 (54.90) | 368/320 (53.49) | χ2 = 0.19 | 0.66 |
| Age (yr) | 47.68 ± 11.71 | 47.13 ± 11.98 | t = 0.71 | 0.48 |
| Aetiology | χ2 = 7.52 | 0.11 | ||
| Hepatitis B | 261 (73.11) | 454 (65.99) | ||
| Hepatitis C | 39 (10.92) | 82 (11.92) | ||
| Autoimmune hepatitis | 9 (2.52) | 33 (4.80) | ||
| Nonspecific hepatitis | 40 (11.20) | 94 (13.66) | ||
| Others | 8 (2.24) | 25 (3.63) | ||
| History of abdominal surgery | 81 (22.69) | 183 (26.60) | χ2 = 1.90 | 0.17 |
| History of variceal bleeding | 181 (50.70) | 388 (56.40) | χ2 = 3.07 | 0.08 |
| Ascites | 268 (75.07) | 490 (71.22) | χ2 = 1.75 | 0.19 |
| Child-Pugh grade at admission | χ2 = 8.60 | 0.01 | ||
| A | 153 (42.86) | 359 (52.18) | ||
| B | 188 (52.66) | 300 (43.60) | ||
| C | 16 (4.48) | 29 (4.22) | ||
| Oesophageal varices grade | χ2 = 0.30 | 0.86 | ||
| Mild | 44 (12.32) | 93 (13.52) | ||
| Moderate | 114 (31.93) | 219 (31.83) | ||
| Severe | 199 (55.74) | 376 (54.65) | ||
| PHG at admission | 118 (33.05) | 172 (25.00) | χ2 = 7.60 | < 0.05 |
| Preoperative MELD score | 5.97 ± 0.40 | 5.95 ± 0.40 | t = 0.61 | 0.54 |
| Preoperative portal vein diameter (cm) | 1.53 ± 1.52 | 1.39 ± 0.43 | t = 0.86 | 0.4 |
Statistically significant differences were observed in postoperative hospital stay, operation fee, total hospitalisation cost, operative time, intraoperative blood loss, intraoperative transfusion, and time to first flatus between the two groups (P < 0.05); the S Group exhibited significantly better results compared to the T Group. In the T Group, 4 patients died due to rebleeding or liver and renal failure during the perioperative period (3 in Child B, 1 in Child C), and 1 patient underwent reoperation for rebleeding. No perioperative death or rebleeding occurred in the S Group (Table 2).
| Parameters | S Group (n = 357) | T Group (n = 688) | Statistic | P-value |
| Postoperative hospital stay (d) | 10.70 ± 5.35 | 14.96 ± 5.66 | t = -3.64 | < 0.05 |
| Operation fee (Yuan) | 3638.85 ± 1144.17 | 4168.06 ± 960.53 | t = 3.60 | < 0.05 |
| Total hospitalisation cost (Yuan) | 29654.60 ± 17475.10 | 35331.13 ± 18165.41 | t = 2.67 | 0.01 |
| Operative time (min) | 125.60 ± 46.08 | 144.83 ± 54.06 | t = 3.96 | < 0.05 |
| Intraoperative blood loss (mL) | 291.46 ± 208.86 | 573.42 ± 409.38 | t = 4.76 | < 0.05 |
| Intraoperative transfusion (mL) | 700.82 ± 541.92 | 986.60 ± 627.75 | t = 5.30 | < 0.05 |
| Time to first flatus (d) | 3.74 ± 1.21 | 4.61 ± 1.60 | t = -7.83 | < 0.05 |
| Mortality | 0 | 4 | ||
| Reoperation | 0 | 1 |
No significant differences in the short-term and long-term portal vein diameters or MELD scores were observed between the two groups after surgery (P > 0.05), but the postoperative long-term indicators were significantly lower than the preoperative and postoperative short-term indicators (P < 0.05). The incidence rates of short-term ascites, portal vein thrombosis and pleural effusion in the S Group were significantly lower than those in the T Group (P < 0.05). Moreover, the incidence rates of long-term ascites, portal vein thrombosis and rebleeding in the S Group were significantly lower than those in the T Group (P < 0.05) (Table 3). The number of rebleeding patients was 21 (5.88%) for S group (rebleeding site: 13 in esophageal and gastric vein, 8 in gastric mucosa) and 89 (12.94%) for T group (rebleeding site: 52 in esophageal and gastric vein, 37 in gastric mucosa). For rebleeding patients, we performed drug hemostasis (vasopressin, somatostatin, etc.) or endoscopic ligation. While for severe bleeding patients, surgical treatment was choosed.
| Parameters | S Group (n = 357) | T Group (n = 688) | Statistic | P-value |
| Short-term portal vein diameter (cm) | 1.27 ± 0.18 | 1.28 ± 0.20 | t = -0.07 | 0.94 |
| Long-term portal vein diameter (cm) | 1.13 ± 0.141 | 1.16 ± 0.171 | t = -1.16 | 0.25 |
| Short-term MELD score | 5.88 ± 0.54 | 5.97 ± 0.48 | t = -1.39 | 0.16 |
| Long-term MELD score | 5.79 ± 0.501 | 5.78 ± 0.421 | t = 0.27 | 0.79 |
| Short-term complications | ||||
| Ascites | 105 (29.41) | 298 (43.31) | χ2 = 19.17 | < 0.05 |
| Portal vein thrombosis | 49 (13.73) | 153 (22.24) | χ2 = 10.92 | < 0.05 |
| Pleural effusion | 66 (18.49) | 271 (39.39) | χ2 = 47.00 | < 0.05 |
| Wound infections | 10 (2.80) | 37 (5.38) | χ2 = 3.63 | 0.06 |
| Hepatic encephalopathy | 3 (0.84) | 5 (0.73) | χ2 = 0.04 | 1 |
| Rebleeding | 0 (0.00) | 3 (0.44) | χ2 = 1.56 | 0.56 |
| Long-term complications | ||||
| Ascites | 36 (10.08) | 122 (17.73) | χ2 = 10.71 | < 0.05 |
| Portal vein thrombosis | 25 (7.00) | 78 (11.34) | χ2 = 4.97 | 0.03 |
| Pleural effusion | 57 (15.97) | 95 (13.81) | χ2 = 0.88 | 0.35 |
| Hepatic encephalopathy | 3 (0.84) | 7 (1.02) | χ2 = 0.08 | 1 |
| Rebleeding | 21 (5.88) | 89 (12.94) | χ2 = 12.42 | < 0.05 |
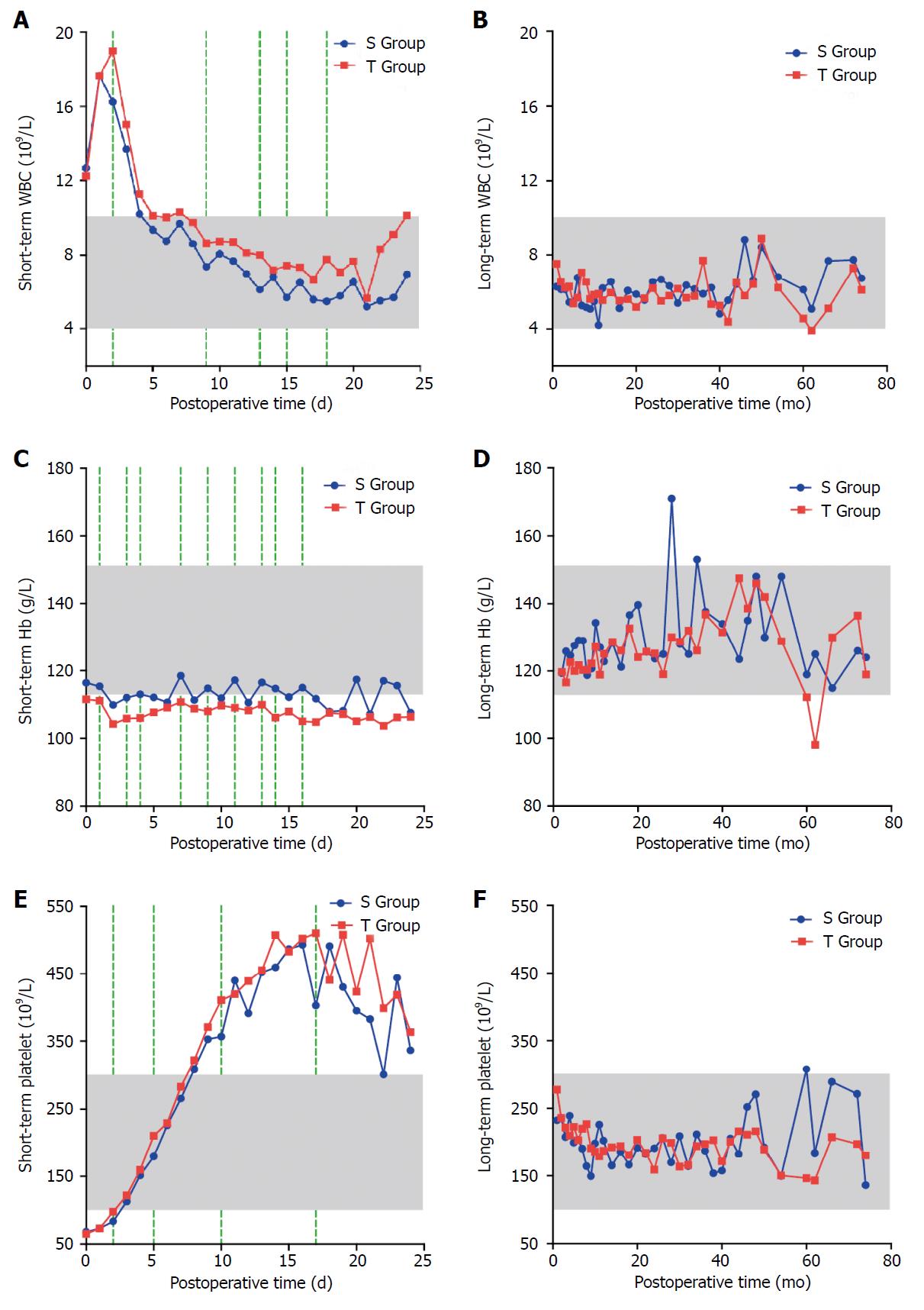
Within 2 d after the operation, the number of WBCs peaked rapidly in the two groups and then gradually decreased. The short-term WBC count in the S Group was lower than that in the T Group, and the difference was statistically significant on the second, ninth, thirteenth, fifteenth, and eighteenth days (P < 0.05). However, no significant difference was found between the two groups in the long-term WBC count (P > 0.05), which was within the normal reference range in both groups. The short-term Hb level was decreased in both groups, but the level in the T Group was lower than the normal reference range. The short-term Hb level in the S Group was higher than that in the T Group, and the difference was statistically significant on the first, third, fourth, seventh, ninth, eleventh, thirteenth, fourteenth, and sixteenth days (P < 0.05). No significant difference between the two groups was observed in the long-term Hb level (P > 0.05), which was within the normal reference range. The platelet number in the two groups gradually increased and peaked on approximately the fifteenth day after the operation, and then gradually decreased to a normal value. The short-term platelet number in the S Group was lower than that in the T Group, and the difference was statistically significant on the second, fifth, tenth, and seventeenth days (P < 0.05). However, no significant difference was found between the two groups in the long-term number of platelets (P > 0.05) (Figure 2).
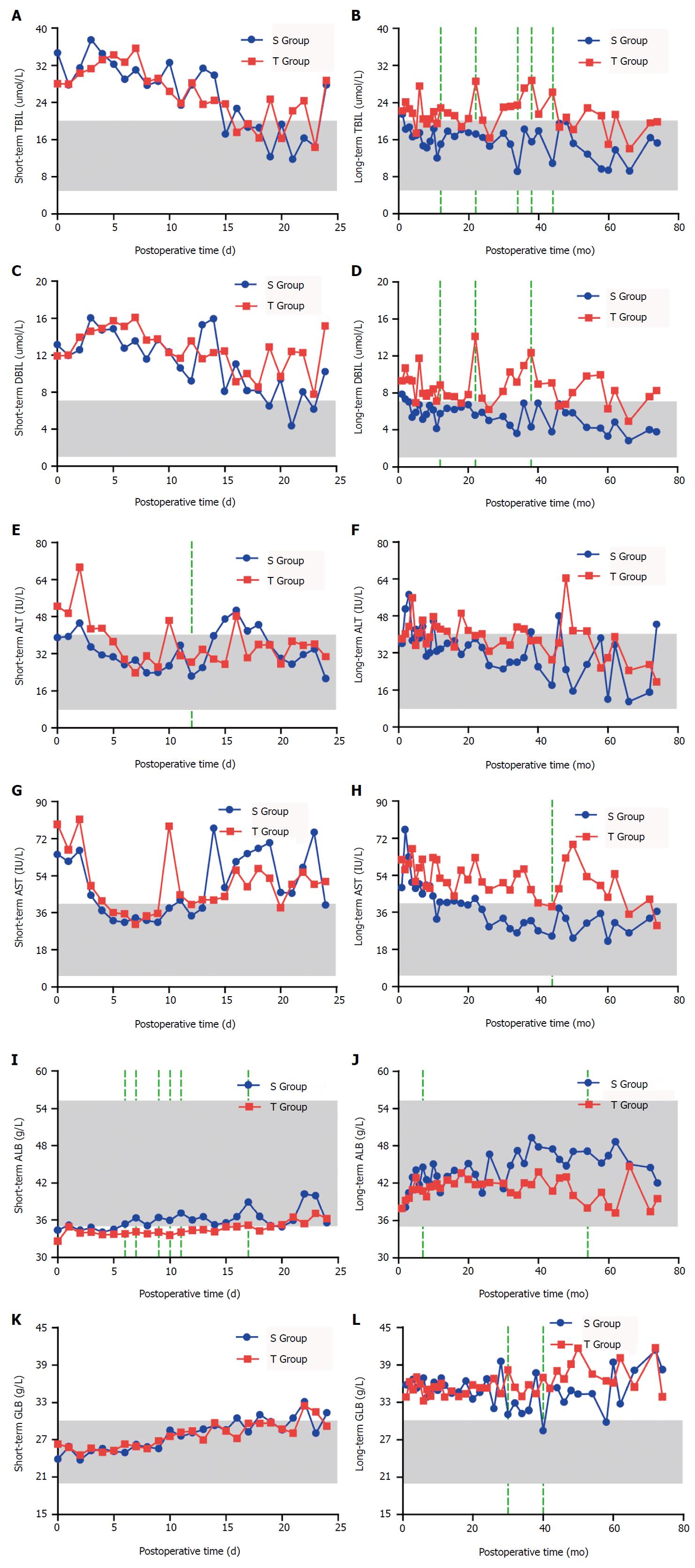
No significant differences in the short-term TBIL and DBIL levels were observed between the two groups (P > 0.05). The long-term TBIL and DBIL levels in the S Group were lower than those in the T Group, and the differences were statistically significant at the twelfth, twenty-second, thirty-fourth, thirty-eighth, and forty-fourth months for TBIL (P < 0.05) and at the twelfth, twenty-second, thirty-eighth, and forty-fourth months for DBIL (P < 0.05). ALT and AST levels in the S Group were lower than those in the T Group one week after the operation, but the difference was not statistically significant (P > 0.05). The short-term ALT and AST levels in the S Group were significantly lower than those in the T Group only on the twelfth day after the operation (P < 0.05). The long-term ALT and AST levels in the S Group were lower than those in the T Group, with a significant difference at the forty-fourth month (P < 0.05). The short-term and long-term levels of ALB in the S Group were higher than those in the T Group, and the difference was statistically significant on the sixth, seventh, ninth, tenth, eleventh, and seventeenth days and at the seventh and fifty-fourth months after the operation (P < 0.05). The short-term and long-term levels of GLB in the S Group were significantly lower than those in the T Group only at the thirtieth and fortieth months after the operation (P < 0.05) (Figure 3).
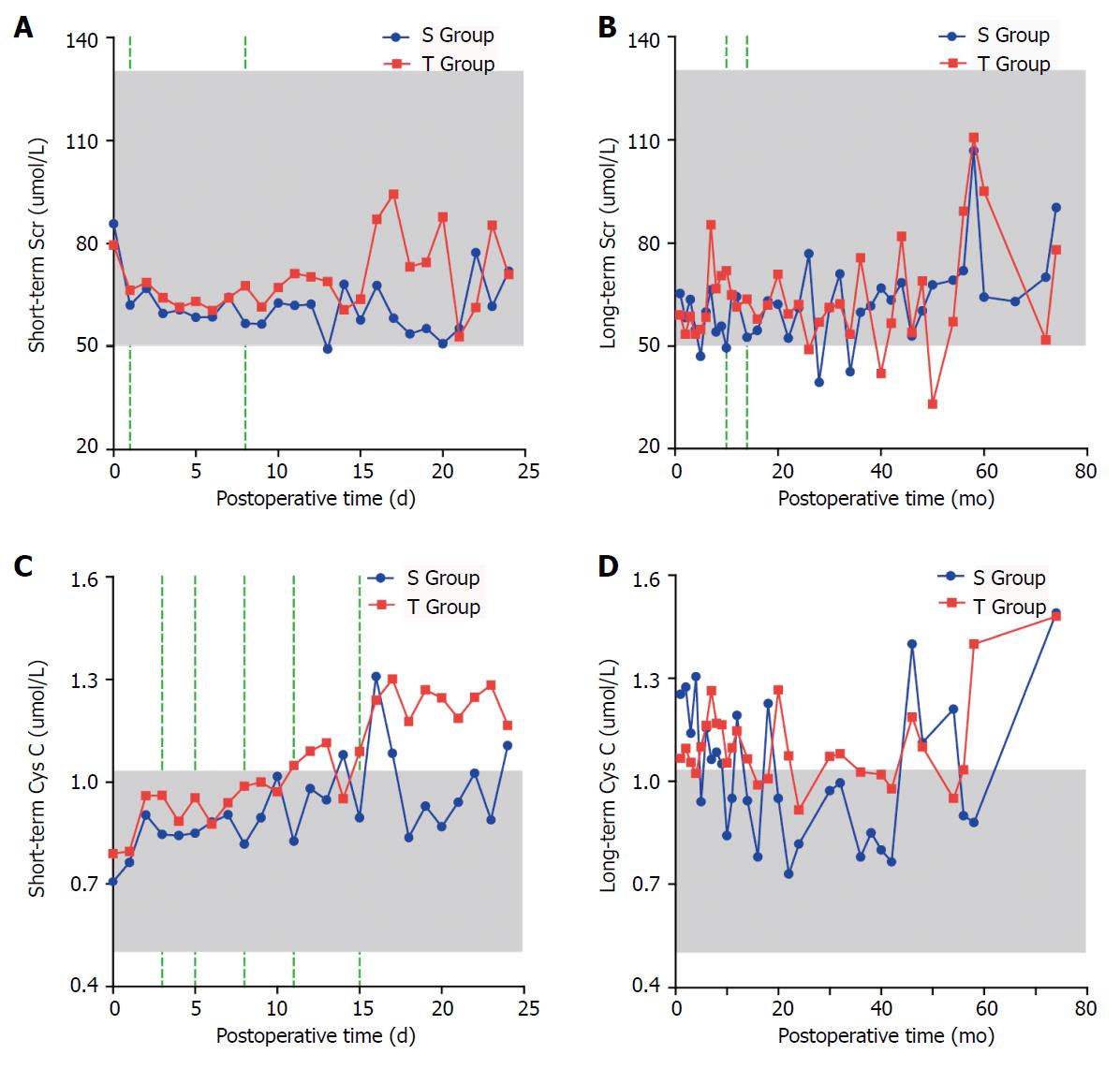
The short-term Scr and Cys C levels in the S Group were lower than those in the T Group; the difference was statistically significant for Scr on the first and eighth days, and Cys C was significantly different on the third, fifth, eighth, eleventh and fifteenth days (P < 0.05). The long-term Scr level in the S Group was lower than that in the T Group, and the difference was statistically significant at the tenth and fourteenth months (P < 0.05). However, no significant difference was found between the two groups in the long-term Cys C level (P > 0.05) (Figure 4).
The pathogenesis and mechanism of PH are varied and complicated and are associated with haemodynamic and mechanical dynamic changes in the liver and circulatory system. PH develops due to increased portal resistance and prograde portal flow. Blood flows through the collateral circulation from the high-pressure portal vein system to the relatively low-pressure systemic venous system, resulting in corresponding complications[10,11]. After the formation of PH, the rami anastomoticus opens, the collateral circulation expands, and haemorrhage occurs; the most important clinical complication is the formation of oesophagogastric varices[12,13].
Surgery is the main treatment for PH in patients with cirrhosis[14]. Liver transplantation appears to be the most effective treatment for PH. However, the considerable lack of liver donors and high medical costs limit its broad clinical application. Therefore, SPD has become the most commonly used method to treat PH[15,16]. STPD can achieve a remarkable curative effect by maintaining prograde portal flow and protecting liver function and haemostasis[17]. However, STPD is complex and can lead to severe tissue damage and many complications. For PH patients with poor liver function, STPD increases the burden of the liver and increases the risk of complications such as hepatic coma and hepatorenal syndrome[3]. Therefore, we simplified STPD and introduced SSPD, which includes cutting and ligating the pericardial vessels to the posterior gastric vessels and suturing the left gastric vessels along the lesser curvature of the stomach to the lower oesophageal vessels. Preserving the paraoesophageal vein and severing only the perforating vein can block reflux of the abdominal oesophagus, lower the portal vein pressure and ensure thorough haemostasis, thus reducing congestion of the gastric mucosa and reducing the occurrence of PHG and then reducing the postoperative rebleeding. Using a suture and not cutting the muscle layer minimise injury to the wound and reduce blood oozing during the operation. Additionally, the operative time is shortened significantly by simplifying the operation, thereby reducing the liver burden caused by the procedure. In the dynamic balance of two systems of coagulation and anticoagulation, liver plays an important regulatory role[18]. Therefore, better liver function can reduce portal vein thrombosis. The patients recovered well, with a low incidence of complications such as bleeding, liver and kidney dysfunction and jaundice. In this study, the clinical efficacy and safety of SSPD were retrospectively compared and evaluated.
The results of this study showed that the S Group exhibited significantly better outcomes compared to the T Group in terms of postoperative hospital stay, operation fee, total hospitalisation cost, operative time, intraoperative blood loss, intraoperative transfusion, and time to first flatus. When cutting and ligating blood vessels, STPD includes cutting the serosa, which will cause additional wounds, increase intraoperative bleeding and prolong the operation[8,19]. This study also found no significant differences in short-term and long-term portal vein diameters or MELD scores between the two groups after the operation, while the long-term postoperative indicators of the two groups were significantly lower than the preoperative and postoperative short-term indicators, demonstrating that both surgical methods have good long-term effects and that SSPD is superior to STPD in reducing portal vein pressure and improving the long-term prognosis. The incidence rates of short-term and long-term ascites, portal vein thrombosis and pleural effusion and long-term rebleeding in the S Group were significantly lower, indicating that SSPD is significantly better than STPD in reducing long-term and short-term postoperative complications. Ascites, portal vein thrombosis and rebleeding are the most serious and most common complications after pericardial devascularisation, seriously affecting patients’ prognoses and threatening PH patients’ lives[8]. SSPD has a better clinical effect compared to STPD.
WBC elevation after the operation is related to severe tissue injury and an intense inflammatory reaction[20]. Our results showed that fewer WBCs were detected in the short term in the S Group compared to the T Group, illustrating that SSPD is simpler and easier than STPD, causes less tissue damage, and minimises inflammatory reactions. The short-term Hb levels in both groups were decreased, and the levels in the T Group were lower than the normal reference range. The short-term Hb level in the S Group was higher. This was related to less tissue injury, a reduced stress response and less bleeding with SSPD vs STPD. The platelet count in the S Group was lower in the short term. Greater thrombocythemia corresponds to a higher risk of thrombosis[21]. Thrombosis is a common, serious complication after pericardial devascularisation that substantially affects the prognosis of the patients. This study shows that SSPD has a certain advantage in reducing the number of platelets and decreasing the risk of thrombosis postoperatively in the short term.
In the T Group, 4 participants died due to rebleeding or liver and renal failure during the perioperative period, and 1 patient underwent reoperation for rebleeding; no death or rebleeding occurred in the S Group. STPD requires carving of the serosa, which causes significant damage to the tissue and increases intraoperative bleeding, prolongs the operation, and increases the burden on the liver, thereby increasing the risk of complications such as hepatic coma and hepatorenal syndrome. The long-term TBIL and DBIL levels in the S Group were lower than those in the T Group. SSPD is a simplified version of STPD that can relieve the liver burden due to the operation. The patients recovered smoothly after SSPD, especially in terms of long-term liver function recovery. The short-term and long-term ALT and AST levels in the S Group were significantly lower than those in the T Group. ALT is a sensitive marker of acute hepatocyte damage[22]. The results showed that short-term liver injury was mild and that long-term liver function was significantly better in the S Group compared to that in the T Group. The short-term and long-term ALB levels were higher in the S Group. The short-term and long-term GLB levels were significantly lower in the S Group, indicating that SSPD has significant advantages for protecting liver function. The short-term and long-term Scr and Cys C levels were lower in the S Group than those the T Group, suggesting that the S Group experienced a significant advantage of reduced renal function injury, especially in the short term. Notably, significant differences in Child-Pugh grade and the incidence of PHG at admission were observed between the two groups. Patients’ conditions in the S Group were more serious compared to those in the T Group, and the indicators in the S Group were better than those in the T Group, reflecting the advantages of SSPD.
Our study has some limitations. First, it was a retrospective cohort study. Because it was not a randomised research study, we cannot exclude a residual selection bias, which may have affected our results[23]. However, due to pragmatic and ethical reasons, a randomised clinical trial would be difficult to perform. Second, because we do not have complete follow-up data, such as survival, haemodynamics, and others (this is the focus of our next study), we were only able to evaluate in-hospital indicators. Third, the study was limited to Chinese patients, and due to differences in aetiology and other factors, our results may not be directly applicable to other ethnic groups. In Western countries, for example, PH is mainly caused by alcoholic cirrhosis, and shunting surgery may therefore have a better effect. In addition, this study is a single-centre study, and due to differences in treatments and national conditions, some of the results may not be generalisable to other institutions. For example, some hospitals tend to prolong the postoperative hospital stay to reduce complications, while hospitals in some other countries try to shorten the hospitalisation time to lower hospital expenses. A randomised, controlled multi-centre study with larger samples and long-term follow-up data are still needed to evaluate SSPD. Despite the above limitations, we believe that our study contributes evidence in support of SSPD for the treatment of PH.
In summary, SSPD is simple and easy to perform, resulting in less tissue damage and minimising inflammatory reactions. Operative time, intraoperative blood loss, postoperative hospital stay and expenses are all lower for SSPD compared with those for STPD. After SSPD, patients recovered smoothly and steadily, which effectively reduced the risk of thrombosis and the incidence of complications in the short term and long term. Short-term liver and kidney function damage was mild, and long-term liver function recovery was better. Therefore, SSPD is an excellent surgical method for the treatment of PH patients and is worthy of clinical promotion and application, especially in primary hospitals. Additional long-term follow-up data are still needed.
Splenectomy plus pericardial devascularisation is the main surgical treatment for portal hypertension (PH) caused by virus hepatitis. However, the procedure of splenectomy plus traditional pericardial devascularisation (STPD) is complex with high rates of postoperative rebleeding and complications. A simplified pericardial devascularisation was extremely needed. We developed a splenectomy plus simplified pericardial devascularisation (SSPD), whose better short-term and long-term prognosis compared to STPD were reported in the present study.
The procedure of SSPD is simple and easy to perform resulting in less tissue damage and less liver and kidney function injury, which is worthy of clinical promotion and application.
The main objectives of this retrospective study were to evaluate the short-term and long-term clinical efficacy of SSPD vs STPD.
We retrospectively analyzed the perioperative indicators, short-term and long-term prognosis indicators and complications and short-term and long-term blood biochemical indexes of 1045 PH patients who underwent SSPD or STPD. The patients were divided into an S Group (underwent SSPD) and a T Group (underwent STPD). We analyzed the short-term and long-term clinical efficacy of SSPD vs STPD.
Perioperative indicators in the S Group (underwent SSPD) were significantly better than those in the T Group (underwent STPD). In both groups, the postoperative long-term portal vein diameter and MELD score were significantly lower than those in the preoperative and postoperative short-term groups. The incidence of complications in the S Group was significantly lower than that in the T Group. Compared to the T Group, postoperative short-term white blood cell and platelet counts were significantly lower and the short-term haemoglobin level was significantly higher in the S Group. In the S Group, postoperative long-term total bilirubin, direct bilirubin, alanine transaminase, and aspartate transaminase and postoperative serum creatinine and cystatin C levels were significantly lower than those in the T Group, and postoperative albumin was significantly higher than that in the T Group.
SSPD is a simple and easy procedure resulting in less tissue damage and less liver and kidney function injury, which is worthy of clinical promotion and application, especially in primary hospitals.
Long-term survival and hemodynamic indexes should be further studied between the two operation types in the future research.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Garbuzenko DV, Hashimoto N, Manenti A, Mercado MA S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. Portal hypertension and gastrointestinal bleeding. Semin Liver Dis. 2008;28:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5205] [Article Influence: 520.5] [Reference Citation Analysis (0)] |
| 3. | Mercado MA. Surgical treatment for portal hypertension. Br J Surg. 2015;102:717-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kimer N, Wiese S, Mo S, Møller S, Bendtsen F. Advances in the treatment of portal hypertension in cirrhosis. Expert Rev Gastroenterol Hepatol. 2016;10:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Sugiura M, Futagawa S. Esophageal transection with paraesophagogastric devascularizations (the Sugiura procedure) in the treatment of esophageal varices. World J Surg. 1984;8:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Lu CL, Cao YJ, Cheng H, Pan YM, Bao SH, Xie M. Clinical factors that influence the outcome of selective devascularization in the treatment of portal hypertension. Oncotarget. 2016;7:50635-50642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Hong D, Cheng J, Wang Z, Shen G, Xie Z, Wu W, Zhang Y, Zhang Y, Liu X. Comparison of two laparoscopic splenectomy plus pericardial devascularization techniques for management of portal hypertension and hypersplenism. Surg Endosc. 2015;29:3819-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Lu H, Liu S, Zhang Y, Shang H, Ji H, Li Y. Therapeutic effects and complications of simplified pericardial devascularization for patients with portal hypertension. Int J Clin Exp Med. 2015;8:14036-14041. [PubMed] |
| 10. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 650] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 12. | Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, Abu-Elmagd K, Connor J; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol. 2003;38 Suppl 1:S54-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Choy TY, Simoens C, Thill V, Mboti F, Vandaele S, Mendes da Costa P. Results of surgical treatment of uncontrollable upper gastrointestinal hemorrhage using endoscopy. Hepatogastroenterology. 2011;58:89-95. [PubMed] |
| 15. | Bao H, He Q, Dai N, Ye R, Zhang Q. Retrospective Study to Compare Selective Decongestive Devascularization and Gastrosplenic Shunt versus Splenectomy with Pericardial Devascularization for the Treatment of Patients with Esophagogastric Varices Due to Cirrhotic Portal Hypertension. Med Sci Monit. 2017;23:2788-2795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Wu J, Li Z, Wang Z, Han X, Ji F, Zhang WW. Surgical and endovascular treatment of severe complications secondary to noncirrhotic portal hypertension: experience of 56 cases. Ann Vasc Surg. 2013;27:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Yang L, Yuan LJ, Dong R, Yin JK, Wang Q, Li T, Li JB, Du XL, Lu JG. Two surgical procedures for esophagogastric variceal bleeding in patients with portal hypertension. World J Gastroenterol. 2013;19:9418-9424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Bedreli S, Sowa JP, Gerken G, Saner FH, Canbay A. Management of acute-on-chronic liver failure: rotational thromboelastometry may reduce substitution of coagulation factors in liver cirrhosis. Gut. 2016;65:357-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Zhang YB, Lu Y, Wu WD, Zhang CW, Shen GL, Hong dF. Indocyanine green retention is a potential prognostic indicator after splenectomy and pericardial devascularization for cirrhotic patients. Hepatobiliary Pancreat Dis Int. 2016;15:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Yu S, Arima H, Heeley E, Delcourt C, Krause M, Peng B, Yang J, Wu G, Chen X, Chalmers J. White blood cell count and clinical outcomes after intracerebral hemorrhage: The INTERACT2 trial. J Neurol Sci. 2016;361:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Colli A, Gana JC, Yap J, Adams-Webber T, Rashkovan N, Ling SC, Casazza G. Platelet count, spleen length, and platelet count-to-spleen length ratio for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev. 2017;4:CD008759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Mangus RS, Fridell JA, Kubal CA, Davis JP, Tector AJ. Elevated alanine aminotransferase (ALT) in the deceased donor: impact on early post-transplant liver allograft function. Liver Int. 2015;35:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Kammerman LA, Grosser S. Statistical considerations in the design, analysis and interpretation of clinical studies that use patient-reported outcomes. Stat Methods Med Res. 2014;23:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |