Published online May 16, 2018. doi: 10.12998/wjcc.v6.i5.88
Peer-review started: January 15, 2018
First decision: February 28, 2018
Revised: March 4, 2018
Accepted: April 1, 2018
Article in press: April 1, 2018
Published online: May 16, 2018
Plexiform schwannoma is an extremely rare variant of schwannoma, accounting for approximately 5% of cases. Due to the rarity and lack of typical symptoms, signs and radiological images, a definite diagnosis of plexiform schwannoma may not be made by clinicians prior to biopsy. In the present study, we report the first case (to our knowledge) of perianal plexiform schwannoma arising from the overlapped skin of the ischioanal fossa, and we propose an intratumorally nonenhanced circumferential capsule dividing the tumour into multiple homogeneously enhanced nodules as a magnetic resonance imaging feature to aid in the differential diagnosis of plexiform schwannoma from ancient schwannoma, cavernous haemangioma, liposarcoma and plexiform neurofibroma.
Core tip: Plexiform schwannoma is an extremely rare variant of schwannoma, without typical symptoms, signs and radiological images. Hence, a definite diagnosis of plexiform schwannoma may not be made by clinicians prior to biopsy. In this work, to our knowledge, we report the first case of perianal plexiform schwannoma arising from the overlapped skin of ischioanal fossa, and we propose an intratumorally nonenhanced circumferential capsule dividing the tumour into multiple homogeneously enhanced nodules as an magnetic resonance imaging feature to aid in the differential diagnosis of plexiform schwannoma from common nodular lesions, such as ancient schwannoma, cavernous haemangioma, liposarcoma and plexiform neurofibroma.
- Citation: Sun XL, Wen K, Xu ZZ, Wang XP. Magnetic resonance imaging findings for differential diagnosis of perianal plexiform schwannoma: Case report and review of the literature. World J Clin Cases 2018; 6(5): 88-93
- URL: https://www.wjgnet.com/2307-8960/full/v6/i5/88.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i5.88
Plexiform schwannoma (PS) is a rare subtype of schwannoma, accounting for approximately 5% of cases[1]. To date, the literature on PS is all case reports. The differential diagnosis of PS predominantly depends on histopathological examination, which weakens the role of diagnosis according to symptoms, signs and radiological images, and makes a dilemma for planning treatment protocol[1-3]. Hence, it is difficult for clinicians to make a definite diagnosis of PS. The head, neck, trunk and extremities are common lesion sites, while colon, rectum, penis and clitoris involvements have been documented[2-5]. To our knowledge, we report the first case of PS located in the perianal region and demonstrate a magnetic resonance imaging (MRI) feature for differential diagnosis in the present paper.
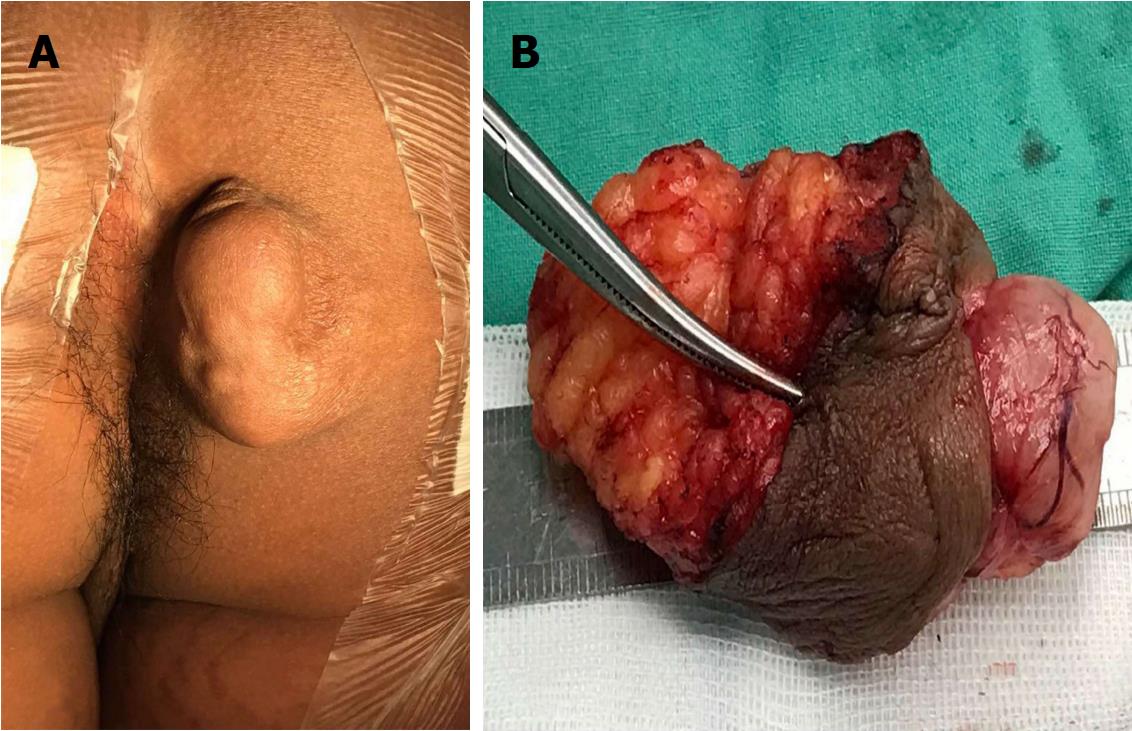
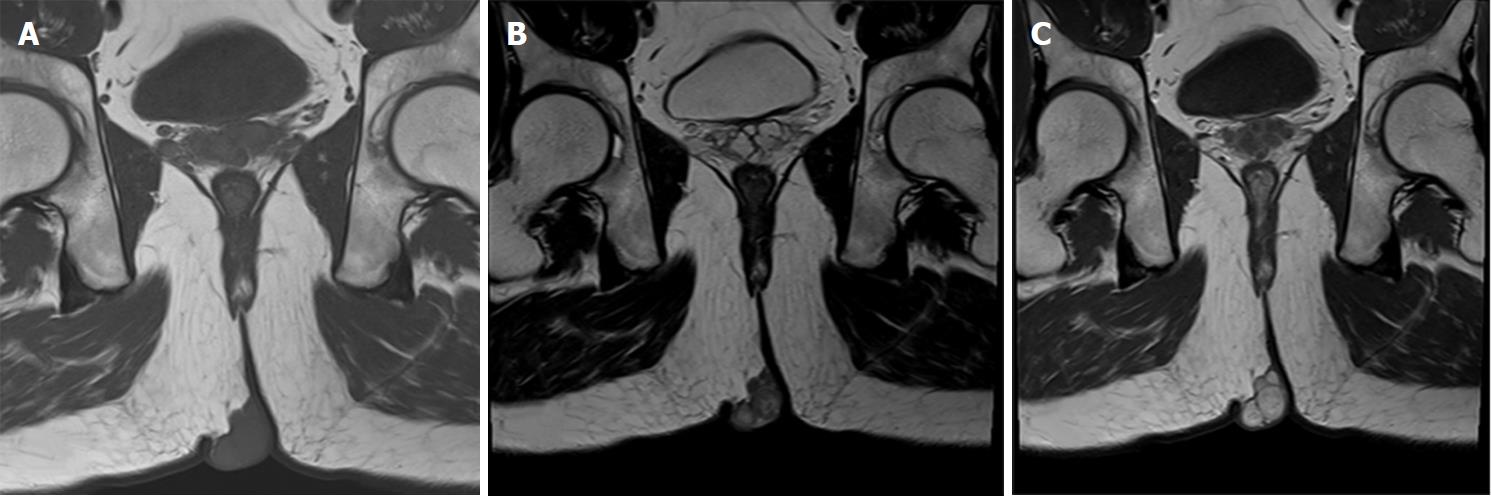
A nineteen-year-old male presented with a five-year history of a slowly growing, painless mass on the right ischioanal fossa. Haematochezia and change in bowel habit were not observed. Before referral to our institution, perianal percutaneous colour Doppler ultrasound revealed a solid lesion with abundant blood flow, and then, a diagnosis of haemangioma was made. After admission, physical examination revealed a multinodular cystic-solid mass of 5 cm × 3 cm on the perianal region, which was ductile, elastic, non-tender, movable and smooth (Figure 1A). No fistula tract could be palpated around the mass. Rectal digital examination could not detect any abnormity. No cafe-au-lait spots, axillary freckling or enlarged inguinal lymph nodes were observed. The results of routine laboratory tests and computed tomography of the chest and abdomen were normal. Pelvic MRI showed a well-circumscribed lobulated mass by a thin complete capsule, which demonstrated a T1-isointense signal and heterogeneously T2-hyperintense signal compared to striated muscle, and homogeneously intense enhancement on contrast-enhanced images (Figure 2A-C). A neurogenic tumour was considered by the radiologist.
Local excision without preoperative biopsy was the treatment protocol because the anal sphincters and gluteus maximus were uninvaded. The operative procedure referenced the Bascom cleft lift technique[6]. After intraspinal anaesthesia, the patient was placed in the prone jack-knife position. A longitudinal spindle incision was made with a 1 cm surgical margin. After the lesion completely resected, a skin flap 1 cm thick was freed from the right buttock until the wound could be sutured without tension. The defect was restored by suturing the fat cushion with vicryl suture. A negative-pressure drainage tube was put upon the fat cushion. The wound was closed by interrupted suture and then dressed with pressure by using an elastic abdominal bandage.
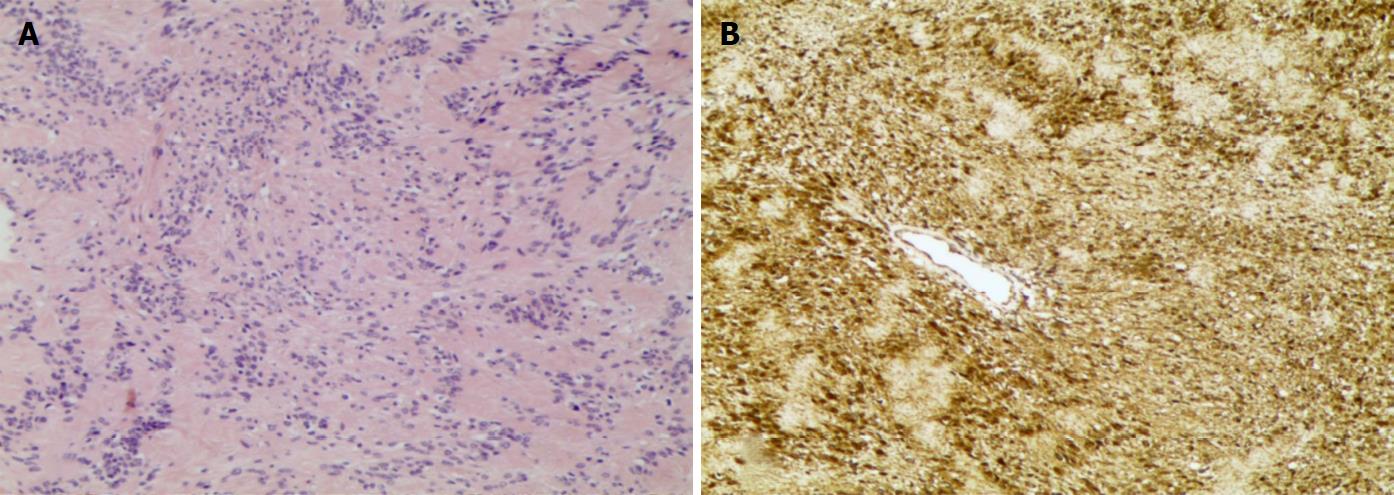
Histologically, the mass was composed of 10 variably sized nodules that appeared white-grey and were individually encapsulated. Observation in high-power fields confirmed a predominant Antoni type A structure with hypercellularity, Verocay bodies and a rare Antoni type B structure with hypocellularity. Spindle cells of the tumour were shown with elongated nuclei and palisading parallel rows (Figure 3A). Increased vascularity and sporadic haemorrhage were evident. Nuclear atypia and mitotic figures were not evident. Immunostains showed that the tumour cells were diffusely positive for vimentin, S100 protein and CD56 and negative for desmin, smooth muscle actin, CD34 and CD68 (Figure 3B). The Ki-67 labelling index was 3%. A definitive diagnosis of PS was made.
Although pelvic MRI has been widely applied for staging and differential diagnosis of perianal neoplasms and thus assists in making treatment protocols, literature regarding the MRI findings of PS is scarce. Hence, a radiological misdiagnosis might be easily made and lead to a misguided treatment strategy. The knowledge of MRI features of PS is required for differential diagnosis.
Compared to striated muscle, PS in our case presented as a T1-isointense signal, which was evidently distinguished from the surrounding tissue by a circumferential hypointense capsule. An intratumoral T2-hypointense capsule divided the tumour into multiple nodules that exhibited a predominant isointense signal area mixed with a minor hyperintense signal area. Contrast-enhanced images showed that the nodules were homogeneously enhanced and encapsulated by a nonenhanced capsule. A target sign characterized by the rim of a T2-hyperintense signal surrounding a central hypointense signal was absent on MR images of PS, which is one of the characteristics of peripheral nerve sheath tumours. This was the reason that a histopathologically hypercellular area accompanied by fibrous tissue is predominant while a hypocellular matrix area is rare or even absent, and haemorrhagic foci can be found in some lesions.
To our knowledge, only one previous case report of solitary PS depicted the MRI feature that aids in the radiologic diagnosis. This feature is that both a T1- and a T2-hypointense thin capsule separated the multinodular tumour from the surrounding soft tissue[7]. However, this feature might not make it easy to differentially diagnose PS from those multinodular lesions with pseudocapsule. In the present paper, we noted a more specific radiological feature: intratumoral smooth capsules that displayed both T1- hypointense and T2-hypointense signals and were not enhanced on contrast-enhanced scan divided the tumour into multiple nodules with heterogeneously T2-hyperintense signal and homogeneous enhancement, which may aid in the radiological diagnosis of this extremely rare perianal tumour that seems no specific nerve of origin.
Perineal ancient schwannoma (AS) has been reported in few literatures, which is characterized by degenerative changes including cyst formation, haemorrhage and calcification[8,9]. Both PS and AS as rare variants of schwannoma are encapsulated with a true capsule consisting of the epineurium, that is demonstrated as a T1-hypointense and T2-hypointense rim. However, a slight to intense enhancement of the fibrous capsule may be expected in a few cases of AS, which is differential from PS[10,11]. AS is divided into a multilocular cystic-solid mass by intratumoural fibrous septa and shows a T1-hypointense and heterogeneously T2-hyperintense signal. An enhanced marginal crescent solid component of AS combining with unenhanced cystic area is the MRI feature for differentiating from homogeneously enhanced multinodular PS[10].
Morphologically, PS mimicking haemangioma has been depicted in two reports[12,13]. The dilatancy growth of PS increased vascularity in the overlapped skin and made a superficial vessel implant into the tumour (Figure 1B). Hence, sonographically abundant blood flow led to a misdiagnosis of haemangioma in our clinical practice. Among the three subtypes of haemangioma, capillary haemangioma, cavernous haemangioma and sclerosing haemangioma, giant cavernous haemangioma measuring over 4 cm in diameter presents as a well-defined nodular mass resembling PS. On MRI, giant cavernous haemangioma demonstrates a T1-hypointense and heterogeneously T2-hyperintense signal with liquefaction, thrombus, blood calculus, vascular smooth muscle and discontinuously fibrous septa components[14]. The vascular flowing-void effect may be seen in some lesions. A heterogeneously nodular peripheral enhancement is the characteristic MRI finding of cavernous haemangioma[14].
PS on the perianal fat space should be differentiated from lobulated liposarcoma (LPS), which comprises 20% of soft tissue sarcomas and has a low degree of malignancy and potential risk of metastasis[15]. The World Health Organization divides LPS into 3 broad categories: well-differentiated (containing atypical lipomatous tumours) and dedifferentiated LPS; myxoid LPS (containing round-cell LPS); and pleomorphic LPS[16]. The presence of visible fat is their common MRI feature. Well-differentiated LPS with a predominant adipose tissue component demonstrates homogeneously T1-hyperintense and T2-hyperintense signals and a hypointense signal on fat-suppressed images, which makes it easy to differentiate PS from LPS[17]. Dedifferentiated LPS appears as a lipomatous tumour with a prominent soft tissue component, intense heterogeneous enhancement, but no encapsulation[17].
As PS tends to occur in adolescents and young adults, similar to our case, myxoid LPS also often occurs in young adults and is the mainstay of differential diagnosis[18,19]. However, clinicians may face a radiologic diagnostic dilemma, as visible fat accounts for < 10% of the tumour volume in < 40% of myxoid LPS cases. Myxoid LPS with high water content demonstrates T1-isointense and homogeneously T2-hyperintense signals with encapsulated margins[17]. In addition, intratumorally discontinuous fatty septa instead of intact fibrous capsules divide the tumour into a multinodular pattern of heterogeneously intense enhancement, which is the differentiating feature from PS. The absence of encapsulated margins, peritumoral oedema and nodular enhancement correlate with high-grade histology.
Pleomorphic LPS with significant metastatic potential mostly occurs in the deep soft tissue of the extremities in elderly patients[19]. T1-isotense, heterogeneously T2-hyperintense signals and heterogeneously intense enhancement with intratumoral haemorrhage and necrosis are the MRI manifestations of pleomorphic LPS[17]. The lack of encapsulated margins and enhanced intratumoral fatty septa are differentiating features from PS.
PS and plexiform neurofibroma (PN) are both neurogenic tumours with multinodular growth patterns. Differential diagnosis is essential due to the potential malignant risk of PN, whose clinical diagnosis may be made according to cafe-au-lait spots or axillary freckling[20]. Radiologically, PN demonstrates T1-hypointense and heterogeneously T2-hyperintense signals or typical target sign with a central fibrocollagenous and peripheral myxoid stroma component[21]. Usually, encapsulated margins are not shown. Mild enhancement is displayed on contrast-enhanced images, corresponding to the absence of flow on colour Doppler ultrasound[21]. The MRI feature of the tumour orientated longitudinally in the nerve distribution and pierced by the peripheral nerve is more significant for diagnosis, as the target sign may not always be present. The above features make it easy to differentiate PS from PN. Heterogeneous enhancement, peritumoral oedema, intratumoral cystic lesions and maximum diameter of the mass > 5 cm indicate the potential of malignant transformation of PN[22].
Absence of Verocay bodies and fibrous capsules and a 30%-40% immunopositive rate for S100 are immunohistopathological features of PN. In contrast, PS displays Verocay bodies, fibrous capsules, and diffuse positivity for S100 and vimentin[2]. Mitotic figures and a Ki-67 labelling index > 5% may be observed once PN transforms into malignancy[23].
Local excision is the treatment strategy for benign PS, but incomplete resection may cause recurrence[13]. Preoperative biopsy was not conducted in our practice because local excision is utilized as a total biopsy to thoroughly assess any perianal neoplasm without involvement of the anal sphincter. Postoperatively, a large defect might not be primarily sutured without tension, which could prolong the healing time, increase the infection risk of the perianal wound and form extensive scarring that hinders sitting. The Bascom advancement flap was applied to primarily suture the defect in our patient, which was utilized as an off-midline tension-free repair technique for the sacrococcygeal pilonidal sinus. The Bascom advancement flap provides a shorter learning curve, lower pain scores, lower flap necrosis risk and better quality of life[24,25].
In conclusion, we have described the first case of perianal PS characterized by an intratumoral nonenhanced capsule dividing the tumour into multiple homogeneously enhanced nodules, which we propose as the radiologically key point to differentially diagnose PS from AS, cavernous haemangioma, LPS and PN (Table 1), all with a capsule or a pseudocapsule. The Bascom advancement flap provides a convenient and safe modality to restore perianal defects.
| Item | T1 signal | T2 signal | T2 homogeneity | Enhancement | Enhancement homogeneity | Intratumoral capsule |
| Plexiform schwannoma | Isointense | Hyperintense | Heterogeneous | Intense | Homogeneous | circumferential fibrous capsule |
| Ancient schwannoma | Hypointense | Hyperintense | Heterogeneous | Intense | Heterogeneous | Fibrous septa |
| Liposarcoma | ||||||
| Well-differentiated | Hyperintense | Hyperintense | Homogeneous | Mild | Homogeneous | Fibrous septa |
| Myxoid | Isointense | Hyperintense | Homogeneous | Intense | Heterogeneous | Fatty septa |
| Pleomorphic | Isointense | Hyperintense | Heterogeneous | Intense | Heterogeneous | Fatty septa |
| Cavernous haemangioma | Hypointense | Hyperintense | Heterogeneous | Intense | Heterogeneous | Fibrous septa |
| Plexiform neurofibroma | Hypointense | Hyperintense | Heterogeneous | Mild | Heterogeneous | Fibrocollagenous septa |
A nineteen-year-old male presented with a slowly growing, painless, multinodular mass on the right ischioanal fossa.
Clinical diagnosis was a dilemma due to no characteristic clinical feature observed.
Magnetic resonance images and histopathologic examination were focused on to differentially diagnose plexiform schwannoma from multilocular or multinodular ancient schwannoma, liposarcoma, cavernous haemangioma and plexiform neurofibroma.
There was no specific laboratory testing contributing to the diagnosis of plexiform schwannoma.
Magnetic resonance images showed an intratumoral nonenhanced capsule dividing the tumour into multiple homogeneously enhanced nodules.
Neoplasm biopsy revealed Verocay bodies, fibrous capsules, and diffuse positivity for S100 and vimentin.
Complete resection combined with the Bascom advancement flap to suture a large defect was performed to prevent a recurrence.
Plexiform schwannoma commonly located on head, neck, trunk and extremities was definitely diagnosed based on postoperative histopathologic examination, whereas the foci located in the perianal region was rarer and was more difficult for preoperative diagnosis without a specific nerve of origin.
Perianal plexiform schwannoma is an extremely rare variant of schwannoma and is difficultly diagnosed in clinic owing to the absence of a specific nerve of origin and a characteristic clinical manifestation.
Due to a multinodular growth pattern, a suspectable plexiform schwannoma can be preoperatively confirmed according to the magnetic resonance imaging feature of an intratumoral nonenhanced capsule dividing the tumour into multiple homogeneously enhanced nodules, which contributes to plan treatment protocol.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Majbar AM S- Editor: Wang XJ L- Editor: A E- Editor: Tan WW
| 1. | Kudose S, Kyriakos M, Awad MM. Gastric plexiform schwannoma in association with neurofibromatosis type 2. Clin J Gastroenterol. 2016;9:352-357. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Jacobson BC, Hirsch MS, Lee JH, Van Dam J, Shoji B, Farraye FA. Multiple asymptomatic plexiform schwannomas of the sigmoid colon: a case report and review. Gastrointest Endosc. 2001;53:801-804. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Kawaguchi S, Yamamoto R, Yamamura M, Oyamada J, Sato H, Fuke H, Yabana T. Plexiform schwannoma of the rectum. Dig Endosc. 2014;26:113-116. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Pan F, Li B, Kunwar KJ, Zhang Q, Xiao Y, Zeng F. Neuroimage: giant plexiform schwannoma of the penis. Eur Neurol. 2013;69:118. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Sammarco AG, Abualnadi NM, Andraska EA, Tracy PV, Berger MB, Haefner HK. Plexiform schwannoma: an unusual clitoral mass. Am J Obstet Gynecol. 2017;216:319.e1-319.e2. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Bascom J, Bascom T. Failed pilonidal surgery: new paradigm and new operation leading to cures. Arch Surg. 2002;137:1146-50; discussion 1151. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Yamada K, Harada M, Kunitoku N, Goto S, Kochi M, Ushio Y. MR imaging features of a scalp plexiform schwannoma. AJNR Am J Neuroradiol. 2004;25:291-294. [PubMed] [Cited in This Article: ] |
| 8. | Pantè S, Terranova ML, Leonello G, Fedele F, Ascenti G, Famulari C. Perineal schwannoma. Can J Surg. 2009;52:E8-E9. [PubMed] [Cited in This Article: ] |
| 9. | Majbar A, Hrora A, Jahid A, Ahallat M, Raiss M. Perineal schwannoma. BMC Res Notes. 2016;9:304. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Takeuchi M, Matsuzaki K, Nishitani H, Uehara H. Ancient schwannoma of the female pelvis. Abdom Imaging. 2008;33:247-252. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Vlychou M, Dailiana ZH. Ancient schwannoma of the hand. J Hand Surg Am. 2011;36:2030-2033. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Lo S, How P, Moss AL. Plexiform schwannoma mimicking haemangioma: pitfalls in clinical diagnosis and histological interpretation. Br J Dermatol. 2007;157:838-839. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Nambirajan A, Mridha AR, Kumar P, Ray R. Congenital cellular plexiform schwannoma mimicking a vascular lesion: Potential pitfalls in clinical and histopathological assessment. Indian J Dermatol Venereol Leprol. 2016;82:79-81. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Klotz T, Montoriol PF, Da Ines D, Petitcolin V, Joubert-Zakeyh J, Garcier JM. Hepatic haemangioma: common and uncommon imaging features. Diagn Interv Imaging. 2013;94:849-859. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416-21; discussion 421-2. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Wortman JR, Tirumani SH, Jagannathan JP, Tirumani H, Shinagare AB, Hornick JL, Ramaiya NH. Primary Extremity Liposarcoma: MRI Features, Histopathology, and Clinical Outcomes. J Comput Assist Tomogr. 2016;40:791-798. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Punia RS, Dhingra N, Mohan H. Cutaneous plexiform schwannoma of the finger not associated with neurofibromatosis. Am J Clin Dermatol. 2008;9:129-131. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | O’Regan KN, Jagannathan J, Krajewski K, Zukotynski K, Souza F, Wagner AJ, Ramaiya N. Imaging of liposarcoma: classification, patterns of tumor recurrence, and response to treatment. AJR Am J Roentgenol. 2011;197:W37-W43. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Tchernev G, Chokoeva AA, Patterson JW, Bakardzhiev I, Wollina U, Tana C. Plexiform Neurofibroma: A Case Report. Medicine (Baltimore). 2016;95:e2663. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Gosein M, Ameeral A, Banfield R, Mosodeen M. Plexiform neurofibroma of the wrist: imaging features and when to suspect malignancy. Case Rep Radiol. 2013;2013:493752. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Wasa J, Nishida Y, Tsukushi S, Shido Y, Sugiura H, Nakashima H, Ishiguro N. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194:1568-1574. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Vera-Sempere F, Vera-Sirera B. Intraosseus plexiform schwannoma of the mandible: immunohistochemical differential diagnosis. J Craniofac Surg. 2010;21:1820-1824. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Guner A, Boz A, Ozkan OF, Ileli O, Kece C, Reis E. Limberg flap versus Bascom cleft lift techniques for sacrococcygeal pilonidal sinus: prospective, randomized trial. World J Surg. 2013;37:2074-2080. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Guner A, Ozkan OF, Kece C, Kesici S, Kucuktulu U. Modification of the Bascom cleft lift procedure for chronic pilonidal sinus: results in 141 patients. Colorectal Dis. 2013;15:e402-e406. [PubMed] [DOI] [Cited in This Article: ] |