Copyright
©The Author(s) 2018.
World J Clin Cases. Nov 6, 2018; 6(13): 577-588
Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.577
Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.577
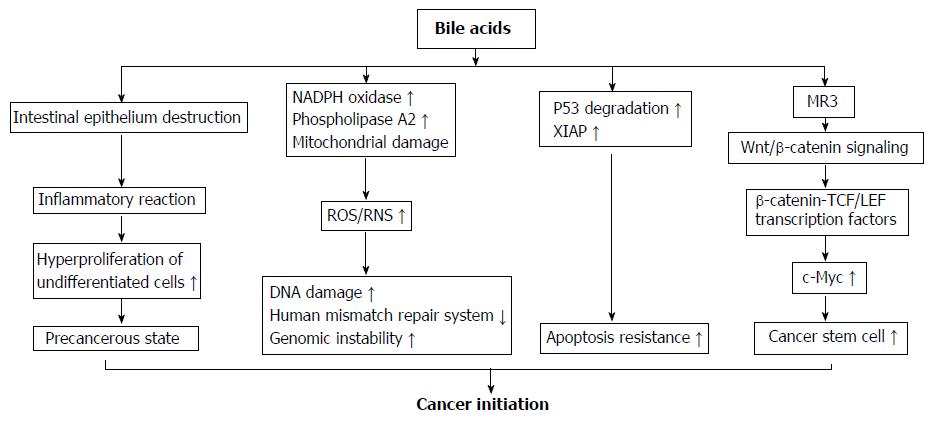
Figure 2 Mechanisms of bile acids initiating colorectal cancer development.
(1) High concentrations of bile acids (BAs) could cause a focal destruction of intestinal epithelium, subsequently stimulate repair mechanisms involving inflammatory reactions and hyper-proliferation of undifferentiated cells. These processes could cause a cell transition into a precancerous state and are considered as an early priming step in colorectal tumorigenesis; (2) BAs strongly induce reactive oxygenic species and reactive nitrogen species production via its stimulatory effect on nicotinamide adenine dinucleotide phosphate oxidase and phospholipase A2, and mitochondrial damage. These reactive species cause damage on DNA, and disrupt the base excision repair pathways; (3) After chronic exposure to BAs at physiological concentration, colon epithelial cells become resistant to apoptosis. This is because BAs induce the degradation of tumor suppressor p53, and up-regulate the expression of X-linked inhibitor of apoptosis protein protein. The cells with genomic errors coupled with apoptosis resistance ability rapidly get much further mutation and ultimately become cancer cells; (4) BAs induce colonic epithelial cells becoming CSCs through muscarinic cholinergic receptor receptor and Wnt/β-catenin signaling. This pathway leads to nuclear translocation of β-catenin to form a complex with T cell factor/lymphoid enhancer factor family transcription factors that acts as a co-activator to express c-Myc, a gene regulating cell stemness. NADPH oxidase: Nicotinamide adenine dinucleotide phosphate oxidase; MR3: Muscarinic cholinergic receptor 3; ROS: Reactive oxygenic species; RNS: Reactive nitrogen species; XIAP: X-linked inhibitor of apoptosis protein; TCF/LEF: T cell factor/lymphoid enhancer factor.
- Citation: Nguyen TT, Ung TT, Kim NH, Jung YD. Role of bile acids in colon carcinogenesis. World J Clin Cases 2018; 6(13): 577-588
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/577.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.577