Published online Feb 16, 2017. doi: 10.12998/wjcc.v5.i2.67
Peer-review started: March 22, 2016
First decision: July 20, 2016
Revised: November 14, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: February 16, 2017
Processing time: 333 Days and 3.9 Hours
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare systemic small- and medium-sized-vessel vasculitis. The literature contains only a few reports of gastrointestinal perforation with this condition. We report a patient with EPGA treated with high-dose steroid who underwent emergency surgery for intestinal perforations. We performed a simple repair of the 11 perforations. Intestinal fistulas developed 8 d postoperatively; they healed well after 60 d of continuous washing and negative pressure suction. The clinical data of 14 additional patients with EGPA or Churg-Strauss syndrome complicated with gastrointestinal perforation, which were reported from 1996 to 2014, were also collected and compared. The formation of multiple perforations and fistulas following high dosage steroid administration can have a good outcome with appropriate management. Meticulous attention to abdominal symptoms and appropriate interventions can result in timely management. Corticosteroid administration remains a very important perioperative procedure for EPGA.
Core tip: Eosinophilic granulomatosis with polyangiitis (EGPA) complicated with intestinal perforation is very rare. It needs urgent surgical intervention but hormone administration usually covers up the situation and delays the prognosis. We report a 43-year-old male diagnosed with EGPA by biopsy who experienced high-dose hormone administration, 11 intestinal perforations, postoperative intestinal fistula, and eventually recovered. This article aims to share the treatment course and experience.
- Citation: He JN, Tian Z, Yao X, Li HY, Yu Y, Liu Y, Liu JG. Multiple perforations and fistula formation following corticosteroid administration: A case report. World J Clin Cases 2017; 5(2): 67-72
- URL: https://www.wjgnet.com/2307-8960/full/v5/i2/67.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i2.67
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare type of necrotizing vasculitis affecting small to medium-sized vessels. It is typically characterized by asthma, lung infiltrates, necrotizing granulomas, and hypereosinophilia. It is an uncommon form of vasculitis with a prevalence that ranges from 10.7 to 13 cases/million[1]. Gastrointestinal involvement has been reported to occur in approximately 50% of EGPA patients. Symptoms include abdominal pain, vomiting and diarrhea[2]. Although EGPA very easily involves the digestive system, the chance of digestive tract perforation is very low and only a handful of cases have been reported. We report a case of EGPA with 11 intestinal perforations, and discuss the subsequent treatment.
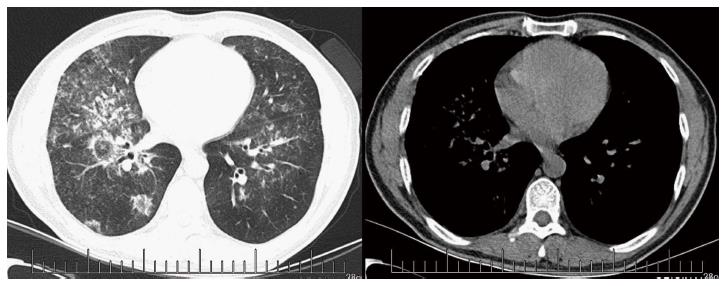
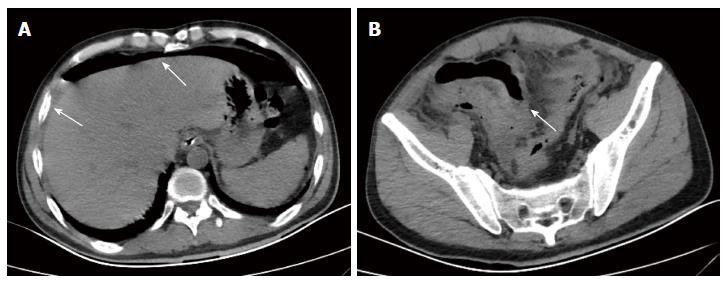
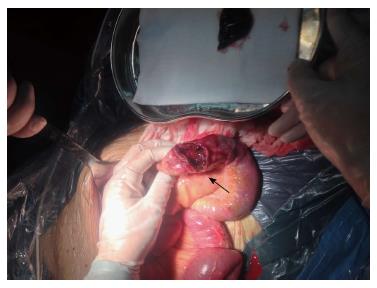
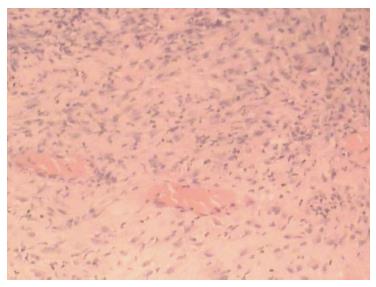
A 43-year-old male with a 3-mo history of asthma was admitted to the Rheumatoid Immune Department of Shengjing Hospital in July 2013. The patient complained of a cough of one-month duration, blood-stained sputum, 14-d peripheral purpura, 3-d diarrhea, and an oral ulcer. His height was 176 cm and weight was 72 kg when admission. His temperature was 37.2 °C, his pulse was 78 per minute, and his blood pressure was 128/78 mmHg. Multiple skin purpura was found in the physical examination. Heart sounds were clear and regular with no murmur. A few moist rales were heard with lung auscultation. Abdominal examination was unremarkable. Laboratory studies revealed leukocytosis (15600/mm3) with eosinophilia (20.3%), a marked increase in inflammatory indices (ESR: 69 mm at the first hour; CRP: 176 mg/L), rheumatoid factor (656 UI/mL), and positive pANCA antibody. A pulmonary computed tomography (CT) scan revealed multiple low-density oval shadows, with the largest one measuring about 3.2 cm × 2.6 cm (Figure 1). A diagnosis of vasculitis was made. A biopsy of the nasal mucosa revealed an eosinophilic infiltration. We diagnosed EGPA according to the revised international Chapel Hill nomenclature. The patient received methylprednisolone (120 mg intravenously daily) and ifosfamide for the treatment of the pulmonary lesions. The patient’s condition exacerbated during the first three days following admission; the methylprednisolone dose was increased to 500 mg (intravenously daily × 2) and then was gradually tapered. On the 13th day after admission (160 mg methylprednisolone intravenously daily), he experienced a sudden onset of fever and abdominal pain. The physical examination revealed generalized abdominal tenderness without apparent rebound tenderness, and muscle tension. No specific treatment was given. After 11 h, the abdominal pain exacerbated and peritonitis developed. An abdominal CT scan showed an amount of free gas in the abdominal cavity (Figure 2). A laparotomy was performed immediately, which revealed scattered eleven perforations in the intestine. The proximal one was located approximately 150 cm distal from the ligament of Treitz ligament, and the distal one located approximately 50 cm from the ileocecal valve, with the largest measuring about 3 cm × 3 cm (Figure 3). Considering the high risk of short bowel syndrome, we made a simple repair of the intestinal perforations. Histopathological examination did not reveal either eosinophilic infiltration or granuloma formation of the vessels (Figure 4).
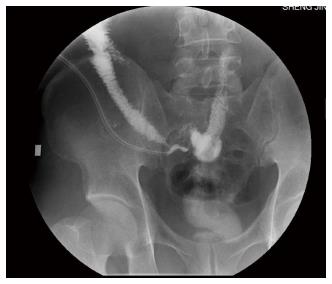
Continued intravenous therapy with intermittent ifosfamide as well as somatostatin and esomeprazole was administered postoperatively. The hormone dosage was tapered from 160 mg downward. On postoperative day 6, when the steroid was reduced to 80 mg, the patient’s vasculitis exacerbated and he developed fever and new purpura on the entire body. Eight days postoperatively, an intestinal fistula developed, which was confirmed by fistulography (Figure 5). With continuous saline washing through a double-lumen cannula connected to a negative pressure suction apparatus, the drainage was about 300 mL intestinal fluid daily; the fistula slowly healed. The patient began eating on postoperative day 45. Gastric retention occurred after eating; it was treated with decompression via a nasogastric tube, total parenteral nutrition, and a gastric motility stimulating agent. The patient resumed eating about two months postoperatively, and the drainage was minimal. When the steroid dosage was reduced to 60 mg intravenously, the fistula healed and the patient was discharged.
Unfortunately, four months later, an intestinal fistula and vasculitis recurred. Although the amount was 5 mL daily, he was readmitted and underwent continuous washing and negative pressure suction. The fistula healed slowly during one month. When last seen in the outpatient clinic in March 2014, he was in good health without any symptoms or eosinophilia. Laboratory analysis revealed 0.1% eosinophils and negative pANCA antibodies.
EGPA, formerly named churg-strauss syndrome (CSS), is characterized by the presence of severe asthma as well as blood and tissue eosinophilia. It is an uncommon form of vasculitis, with a prevalence that ranges from 10.7 to 13 cases/million. Although it is classified as vasculitis, the affected tissue usually does not show necrotizing vasculitis or granulomata, rather an apparently nondestructive infiltration of the vessel walls by eosinophils; in fact, only 40%-60% of patients with CSS have anti-neutrophil cytoplasmic antibodies (ANCAs)[3]. For this reason the diagnostic criteria remain clinical.
Gastrointestinal involvement is present in 32.6% of CSS patients. In 29.11% of the cases, the large bowel is involved, in 53.5%, the small bowel is involved, in 16.6%, the gastroduodenal region is involved, and in 6.3%, pancreatitis and cholecystitis occur. The mortality rate is 11.9%[4-6]. Although gastrointestinal symptoms are common, perforated ulcers are relatively uncommon and only a small number of cases have been documented in the literature[7]. Because multiple organs are involved and peritonitis symptoms are not typical, complicated clinical manifestations occur, which delays treatment and results in a poor prognosis.
A systematic review of the literature was performed using the keywords “Churg-Strauss syndrome/eosinophilic granulomatosis with polyangiitis” and “gastrointestinal perforation” in PubMed to search articles from 1951 to December 2014. In China, CSS/EGPA with gastrointestinal perforation is very rare, and there is no relevant report. The articles were restricted to English language only. Duplicate reports and papers with important data missing were excluded. We included the papers on digestive tract perforation with CSS/EGPA, and compared the characteristics (Table 1). The average patient age was 46.3 years (range: 16-72 years; males: 10; females: 4). A half of the patients were complicated with the symptom of asthma, and all of them had received corticosteroid treatment. Their prognosis was poor, with 4/13 (30.77%; case 13 was unknown) dying during hospitalization, although the causes of death were very different. A second perforation of the digestive system occurred in 2 (15.38%) of 13 patients (case 13 was unknown).
| Ref. | year | Age/sex | Initial symptoms | WBC (/mm3) | Eosino (%) | Hormone | Perforation | treatment | Histology | Second perforation | Prognosis |
| Ikoma et al[10] | 2014 | 19/female | Asthma | 24700 | 39 | Yes | Ileum | Anastomosis | Transmural infiltration of numerous eosinophils with thrombosis and extensive involvement of small arteries | No | Alive |
| Kaul et al[11] | 2014 | 58/male | Low-grade fever | 94000 | 42 | Yes | Ileo-caecal junction | Ileostomy | Transmural inflammation of sub-mucosal and serosal vessels with perivascular infiltrate comprising of lymphomononuclear cells and eosinophils | No | Alive |
| Assmann et al[12] | 2014 | 32/male | Dyspnea, fatigue, fever and chest pain | - | 5500/l | Yes + cyclophosphamide | Middle part of jejunum | Anastomosis | - | No | Alive |
| Assmann et al[12] | 2014 | 36/male | Dyspnea, fatigue, fever and chest pain | - | 4900/l | Yes + cyclophosphamide | Colon transversum | Anastomosis | Eosinophilic infiltration and thrombotic vessel occlusion | No | Alive |
| Çiledağ et al[8] | 2012 | 35/male | Anorexia | - | 35 | Yes | Small intestine | Repair | - | No | Died |
| Venditti et al[13] | 2011 | 69/male | Abdominal pain | - | - | Yes | Right and transverse colon | Ileostomy | Multiple ulcers, extravasal granulomas and mucosal pseudopolyps | No | Alive |
| Zanaboni et al[14] | 2008 | 43/male | Asthma | 32000 | 65 | Yes | Small intestine | Anastomosis | Necrotizing ischemic vasculitis with inflammatory granulomatous infiltrates of lymphocytes, polymorphonuclear cells and eosinophils | Yes | Alive |
| Rolla et al[15] | 2007 | 55/male | Asthma | 15500 | 21 | Yes | Ileum | Anastomosis | Granulomatous vasculitis with eosinophilic infiltration | No | Alive |
| Murakami et al[5] | 2004 | 51/female | Asthma | 27650 | 62 | Yes | Ileum | Anastomosis | Angiitis of small vessels surrounded by eosinophilic infiltration and granuloma of the vessels | No | Died |
| Nagashima et al[16] | 2002 | 67/male | Asthma | 18500 | 65 | Yes | Intestine | Anastomosis | Vasculitis in the small arteries and arterioles characterized by thrombotic occlusion with fibrinoid necrosis of the vascular wall and prominent inflammatory cell infiltration in the perivascular region | No | Alive |
| Nakamura et al[17] | 2002 | 31/male | Epigastralgia | 19700 | 40 | Yes | Jejunum and ileum | Anastomosis | Multiple ulcerative lesions with remarkable eosinophilic infiltration and thrombosis obstruction of small vessels | No | Alive |
| Alvarez et al[18] | 2002 | 64/female | Urticaria, recurrent rhinitis, and asthma | 10000 | 34 | Yes | Intestine | Anastomosis | Wall ulcerations, vascular thrombosis with fibrinoid necrosis, and eosinophilic infiltrates | Yes | Died |
| Kim et al[4] | 2000 | 72/female | Asthma | 6600 | 14 | Yes | Sigmoid colon | Anastomosis | Ulceration with heavy infiltrations of eosinophils, neutrophils and lymphoplasma cells | - | - |
| Sharma et al[19] | 1996 | 16/male | Low-grade, continuous fever and wheezing sounds in the chest | 12600 | 70 | Yes | Jejunum | Anastomosis | Necrotizing vasculitis with marked eosinophilic infiltration of medium-to-small blood vessels and extravascular granulomas | No | Died |
Ulceration, perforation, and stenosis of the gastrointestinal tract are assumed to be the results of ischemia caused by vasculitis. The small intestine is the most commonly affected site[8]. Immunosuppressive therapy, especially large-dosage corticosteroids, may play an important role in the development of an intestinal perforation. However, sometimes, it is difficult to distinguish clinically whether the intestinal perforation is due to vasculitis itself or immunosuppression[9].
Treatment of a gastrointestinal perforation in patients with active vasculitis can be challenging. A delayed diagnosis may lead to a delayed cure, as in our patient. First, we did not pay adequate attention to the digestive symptoms: Sour regurgitation, heartburn, and abdominal pain. Second, the symptoms of peritonitis are often atypical in patients receiving long-term administration of glucocorticoids. Third, when the digestive tract perforation occurred, the patient was in the rheumatic ward. As a result of consultation, diagnosis, and department transference, treatment delay can occur. Fourth, the gastrointestinal perforation may occur during a clinical remission. The perforation of this case occurred during the process of steroid tapering. Pathologic examination of the perforated bowel revealed no significant eosinophil infiltration.
In conclusion, improved awareness of gastrointestinal symptoms may allow for timely management of a perforation. Multiple perforations and fistulas caused by high dosage steroids can have a good outcome with appropriate management. Prompt surgical treatment is necessary. The choice of the appropriate surgical approach should be based on the time the perforations occurred, their size, numbers, and sites. Adequate steroid administration and intensive care play an important role during perioperative treatment.
A 43-year-old male with a 3-mo history of asthma, a cough of one-month duration, blood-stained sputum, 14 d of peripheral purpura, 3 d of diarrhea, and an oral ulcer. On the 13th day after admission, the patient experienced a sudden onset of fever and abdominal pain. And the abdominal pain exacerbated and peritonitis developed rapidly.
History of asthma, peripheral purpura, and peritonitis.
Granulomatosis with polyangiitis, microscopic polyangitis, and polyarteritis nodosa.
Laboratory studies revealed leukocytosis (15600/mm3) with eosinophilia (20.3%), a marked increase in inflammatory indices (ESR: 69 mm at the first hour; CRP: 176 mg/L), rheumatoid factor (656 UI/mL), and positive pANCA antibody.
A pulmonary computed tomography (CT) scan revealed multiple low-density oval shadows, with the largest one measuring about 3.2 cm × 2.6 cm. An abdominal CT scan showed free air in the abdomen.
A biopsy of the nasal mucosa revealed an eosinophilic infiltration.
Continued intravenous hormone therapy with intermittent ifosfamide perioperatively, and intestinal perforation repair.
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare type of necrotizing vasculitis affecting small to medium-sized vessels; it is typically characterized by asthma, lung infiltrates, necrotizing granulomas, and hypereosinophilia. Gastrointestinal involvement even perforation is more unusual and results in a poor prognosis.
EGPA with multiple intestinal perforations is very rare. Prompt surgical treatment is necessary. Adequate steroid administration and intensive care play an important role during perioperative treatment.
Reasonable amount of hormone administration is the key point during the treatment. Excess hormone may induce intestinal perforation while insufficient amount could induce disease relapse and intestinal fistula postoperatively.
This is an interesting case report and the paper is well written.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Caronna R S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Franco DL, Ruff K, Mertz L, Lam-Himlin DM, Heigh R. Eosinophilic granulomatosis with polyangiitis and diffuse gastrointestinal involvement. Case Rep Gastroenterol. 2014;8:329-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Mouthon L, Dunogue B, Guillevin L. Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (formerly named Churg-Strauss syndrome). J Autoimmun. 2014;48-49:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Sablé-Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, Blockmans D, Cordier JF, Delaval P, Puechal X. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. 2005;143:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 439] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Kim YB, Choi SW, Park IS, Han JY, Hur YS, Chu YC. Churg-Strauss syndrome with perforating ulcers of the colon. J Korean Med Sci. 2000;15:585-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Murakami S, Misumi M, Sakata H, Hirayama R, Kubojima Y, Nomura K, Ban S. Churg-Strauss syndrome manifesting as perforation of the small intestine: report of a case. Surg Today. 2004;34:788-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Mémain N, De BM, Guillevin L, Wechsler B, Meyer O. Delayed relapse of Churg-Strauss syndrome manifesting as colon ulcers with mucosal granulomas: 3 cases. J Rheumatol. 2002;29:388-391. [PubMed] |
| 7. | Riva N, Cerri F, Butera C, Amadio S, Quattrini A, Fazio R, Comola M, Comi G. Churg Strauss syndrome presenting as acute neuropathy resembling Guillain Barré syndrome: case report. J Neurol. 2008;255:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Çiledağ A, Deniz H, Eledağ S, Özkal C, Düzgün N, Erekul S, Karnak D. An aggressive and lethal course of Churg-Strauss syndrome with alveolar hemorrhage, intestinal perforation, cardiac failure and peripheral neuropathy. Rheumatol Int. 2012;32:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yildirim AC, Koçak E, Yildiz P, Yildiz M, Karakayali AŞ, Kaptanoglu B, Köklü S. Multiple intestinal perforation in a patient with Wegener’s granulomatosis: a case report and review of the literature. Gastroenterol Clin Biol. 2010;34:712-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ikoma N, Fischer UM, Covinsky M, Quesada AE, Gomez R, McCray C, Warner NB, Ko TC. Ileal perforation in a young female with Churg-Strauss syndrome. Am Surg. 2014;80:94-96. [PubMed] |
| 11. | Kaul A, Sharma RK, Jaisuresh KS, Agrawal V. Crescentic glomerulonephritis in non-asthmatic Churg-Strauss syndrome. Saudi J Kidney Dis Transpl. 2014;25:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Assmann G, Molinger M, Pfreundschuh M, Bohle R, Zimmer V. Gastrointestinal perforation due to vasculitis at primary diagnosis of eosinophilic granulomatosis with polyangiitis (EGPA) despite a high dose glucocorticosteroids treatment. Springerplus. 2014;3:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Venditti D, Valerio B, Ielpo B, Buonomo O, Petrella G. Bowel perforations in a patient affected by Churg-Strauss syndrome under high-dose steroid treatment: will alternative drugs reduce risk of surgery? Rheumatol Int. 2011;31:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Zanaboni D, Maccabruni A, Bonacci E, Caprioli M, Caporali R, Mariani B. Churg-Strauss syndrome: a diagnosis not to be forgotten. New Microbiol. 2008;31:285-290. [PubMed] |
| 15. | Rolla G, Tartaglia N, Motta M, Ferrero N, Bergia R, Guida G, Heffler E. Warning nonrespiratory symptoms in asthma: catastrophic abdominal involvement in a case of Churg-Strauss syndrome. Ann Allergy Asthma Immunol. 2007;98:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Nagashima T, Cao B, Takeuchi N, Chuma T, Mano Y, Fujimoto M, Nunomura M, Oshikiri T, Miyazaki K, Dohke M. Clinicopathological studies of peripheral neuropathy in Churg-Strauss syndrome. Neuropathology. 2002;22:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Nakamura Y, Sakurai Y, Matsubara T, Nagai T, Fukaya S, Imazu H, Hasegawa S, Ochiai M, Funabiki T, Mizoguchi Y. Multiple perforated ulcers of the small intestine associated with allergic granulomatous angiitis: report of a case. Surg Today. 2002;32:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Alvarez C, Asensi V, Rodriguez-Guardado A, Casado L, Ablanedo P, Alvarez-Navascués C. Unusual complications in the Churg-Strauss syndrome. Ann Rheum Dis. 2002;61:94-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Sharma MC, Safaya R, Sidhu BS. Perforation of small intestine caused by Churg-Strauss syndrome. J Clin Gastroenterol. 1996;23:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |