Published online Feb 16, 2017. doi: 10.12998/wjcc.v5.i2.27
Peer-review started: August 20, 2016
First decision: October 21, 2016
Revised: October 29, 2016
Accepted: November 21, 2016
Article in press: November 22, 2016
Published online: February 16, 2017
Conductor externalization and insulation failure are frequent complications with the recalled St. Jude Medical Riata implantable cardioverter-defibrillator (ICD) leads. Conductor externalization is a “unique” failure mechanism: Cables externalize through the insulation (“inside-out” abrasion) and appear outside the lead body. Recently, single reports described a similar failure also for Biotronik leads. Moreover, some studies reported a high rate of electrical dysfunction (not only insulation failure) with Biotronik Linox leads and a reduced survival rate in comparison with the competitors. In this paper we describe the case of a patient with a Biotronik Kentrox ICD lead presenting with signs of insulation failure and conductor externalization at fluoroscopy. Due to the high risk of extraction we decided to implant a new lead, abandoning the damaged one; lead reimplant was uneventful. Subsequently, we review currently available literature about Biotronik Kentrox and Linox ICD lead failure and in particular externalized conductors. Some single-center studies and a non-prospective registry reported a survival rate between 88% and 91% at 5 years for Linox leads, significantly worse than that of other manufacturers. However, the preliminary results of two ongoing multicenter, prospective registries (GALAXY and CELESTIAL) showed 96% survival rate at 5 years after implant, well within industry standards. Ongoing data collection is needed to confirm longer-term performance of this family of ICD leads.
Core tip: Conductor externalization and insulation failure are frequent complications with the recalled St. Jude Medical Riata implantable cardioverter-defibrillator leads. Cables can externalize through the insulation (“inside-out” abrasion) and appear outside the lead body. Recently similar failure mechanisms have also been described for Biotronik leads. Some studies reported a high rate of electrical dysfunction (including insulation failure) with Biotronik Linox leads and a survival rate between 88% and 91% at 5 years, significantly worse than that of other manufacturers. However, the preliminary results of two ongoing multicenter, prospective registries showed 96% survival rate at 5 years, well within industry standards.
- Citation: De Maria E, Borghi A, Bonetti L, Fontana PL, Cappelli S. Externalized conductors and insulation failure in Biotronik defibrillator leads: History repeating or a false alarm? World J Clin Cases 2017; 5(2): 27-34
- URL: https://www.wjgnet.com/2307-8960/full/v5/i2/27.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i2.27
Implantable cardioverter-defibrillator (ICD) is a well established life-saving therapy for patients at risk of sudden cardiac death from ventricular arrhythmias. “Achille’s heel” of the ICD system is the lead, because of its susceptibility to mechanical and electrical defects[1]. Incidence of lead failure can be as high as 0.58%/year, increasing with time (up to 10%-15% at 10 years of follow up)[2,3]. Lead failure has a broad range of clinical presentations and outcomes, the most dreaded being potentially lethal proarrhythmia and inability to interrupt spontaneous ventricular arrhythmias.
Conductors fracture and insulation failure are the main mechanisms responsible for lead failure[1-3]: Two classical examples are the recalled Medtronic Sprint Fidelis leads and the St. Jude Riata leads family.
Sprint Fidelis leads (Medtronic Inc., St. Paul, Minnesota, United States) were recalled from the market, in October 2007, because of a high failure rate due to conductor fracture. On the other side, Riata family of ICD silicone leads (St. Jude Medical, Sylmar, California, United States) underwent class I recall by the Food and Drug Administration, in December 2011, because of insulation failure. In particular, Riata leads are susceptible to a unique failure mechanism: The conductor cables can externalize through the silicone insulation (“inside-out” abrasion) and appear outside the lead body[3].
Recently, single case reports described a “Riata like” insulation failure mechanism also for Biotronik Kentrox and Linox ICD leads (Biotronik, Berlin, Germany)[4-9]. Moreover, some single-center studies and a non-prospective registry reported a high rate of electrical dysfunction (including but not limited to insulation failure) with Biotronik Linox leads and a reduced survival rate in comparison with the competitors[10-12]. Nevertheless, the preliminary results of two ongoing multicenter, prospective registries (GALAXY and CELESTIAL) showed 96% survival rate at 5 years after implant, well within industry standards and not different from that of other manufacturers[13].
In this paper, we describe (beyond the already published case reports) a patient, managed at our institution, with a Biotronik Kentrox ICD lead presenting with signs of insulation failure and conductor externalization at fluoroscopy. Subsequently, we review currently available literature about Biotronik ICD lead failure and in particular insulation failure with externalized conductors.
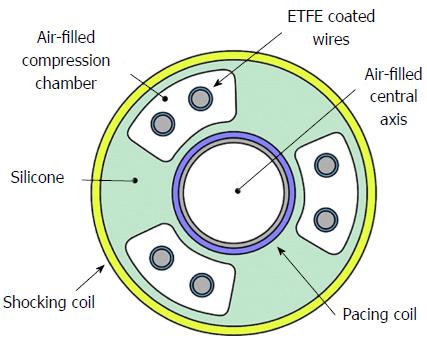
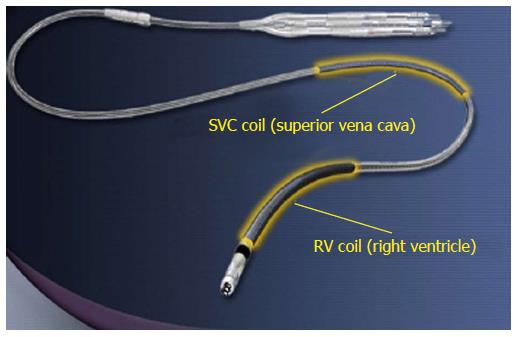
Each ICD lead has several components[3]: Conductor, insulation material, defibrillation coil, electrode, fixation mechanism to myocardium, division point of single conductors and connector. Most manufacturers use similar materials, even if assembled in different ways. All modern ICD leads have a multi-lumen design. High-voltage shock conductors include a low-resistance core of silver-platinum and are coated with polytetrafluoroethylene and ethylenetetrafluoroethylene (ETFE); they lie in a silicone cylinder with 3-6 lumens. Low-voltage conductors are made of alloy of cobalt, nickel, chromium silver and molybdenum. A central coil conductor used for the pacing-sensing cathode (tip) allows for stylet insertion and extension/retraction of the fixation helix. Conductors for the pacing-sensing anode (ring) and high voltage coils are built in parallel cables around the central coil (Figure 1). Lead design may vary among manufacturers: Coils can be placed in symmetric or asymmetric manner; compression lumens can be present or not, etc. All leads, anyway, will have minimum one distal right ventricular (RV) shock coil, necessary for the delivery of high-voltage shock therapy. Dual-coil leads have another shock coil, usually located in the superior vena cava (SVC). Dual-coil leads may ensure greater defibrillation efficacy, expecially in right-sided implants, but they involve greater procedural difficulties and risks, when extraction is required, due to fibrotic tissue around the proximal coil (Figure 2).
Last generation ICD leads use a DF-4 connection, that has replaced the old, multicomponent yokes (DF-1/IS-1). DF-4 connection has the pace-sense conductors and the defibrillation conductor(s) connected to a single pin. The new connection has the advantage of a reduced pocket bulk and prevents the accidental reversal of high-voltage connections during implantation or replacement procedures.
ICD leads always have bipolar sensing and the tip electrode is always used as a cathode. However two types of sensing design exist. The dedicated bipolar lead has a ring electrode as sensing dedicated anode. On the other side, the integrated bipolar lead has the RV defibrillation coil, integrated within the shock circuit, as the anode. Therefore, a dedicated bipolar lead is more complex because it requires two conductors, versus one in an integrated bipolar lead. Dedicated and integrated leads show no difference regarding sensing of ventricular fibrillation (VF). However, an integrated bipolar lead has a larger “antenna”, more prone to oversensing of diaphragmatic myopotentials, electromagnetic interference (EMI) and atrial “far field” signals. Dedicated bipolar leads are more prone to oversense the T wave. Current ICD leads diameters vary from 6.3 to 8.6 French.
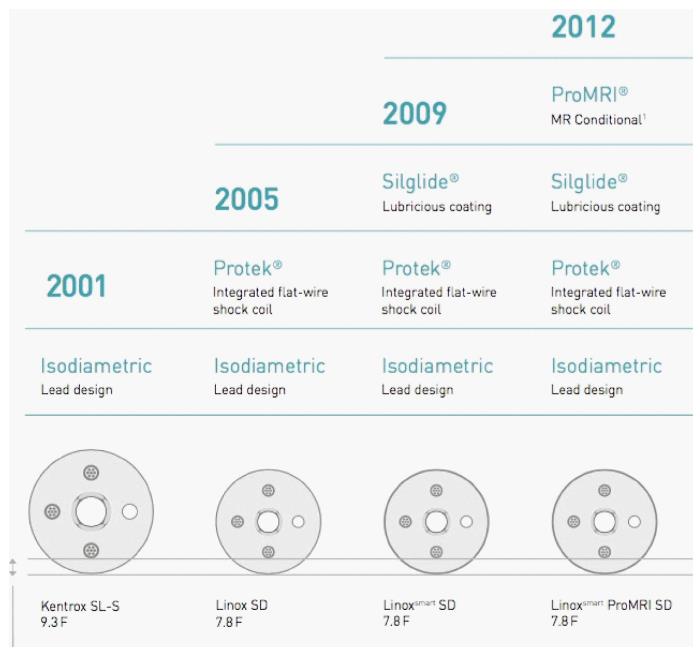
Kentrox SL (marketed from 2001 to 2005) is a 9.3 French, passive fixation ICD lead with an isodiametric design, coated with fractal iridium. The sensing is dedicated bipolar. The lead is insulated with silicone rubber similar to first generation Riata leads insulation. In contrast to Riata lead, Kentrox does not present a “redundant” design that can facilitate the movement of the cables within the lumen (and was supposed to be implied in “inside-out” abrasion)[6]. Nevertheless, the mechanism of Kentrox externalization described in literature (see next paragraphs) seems to be very similar to that of Riata leads: “Inside-out” abrasion (movement of the conductors within the insulation, leading to cable externalization through the outer layer) rather than “outside-in” (contact with another lead or anatomic structures, e.g., tricuspid valve).
Linox family leads (marketed in 2005) are 7.8 French with an isodiametric design and dedicated bipolar sensing. The cross-section of Linox lead is comparable to that of Kentrox and, although having a smaller diameter, the thickness of the silicone layer is equal. Moreover, Linox is equipped with integrated flat-wire shock coil (Protek®) which reduces fibrotic tissue ingrowth.
LinoxSmart lead (marketed in 2009, from 2012 proMRI model) is additionally treated with Silglide®, a surface treatment which ensures lubricious coating, improved gliding, low friction and reduces the risk of abrasion (Figure 3). In a similar manner, Riata ST and Durata St. Jude Medical models were provided with an additional abrasion-resistant silicone-polyurethane co-polymer (OptimTM). Silicone rubber is inert and more biostable compared to polyurethane, but has a higher coefficient of friction and is more vulnerable to abrasion and breaches. On the other side, polyurethane is too stiff to be used as the only insulation material in ICD leads. That is why each manufacturer attempted to “reinforce” silicone with different proprietary solutions.
Finally, Protego family leads (marketed in 2013) are almost identical to Linox, their new feature is the introduction of a DF-4 connection.
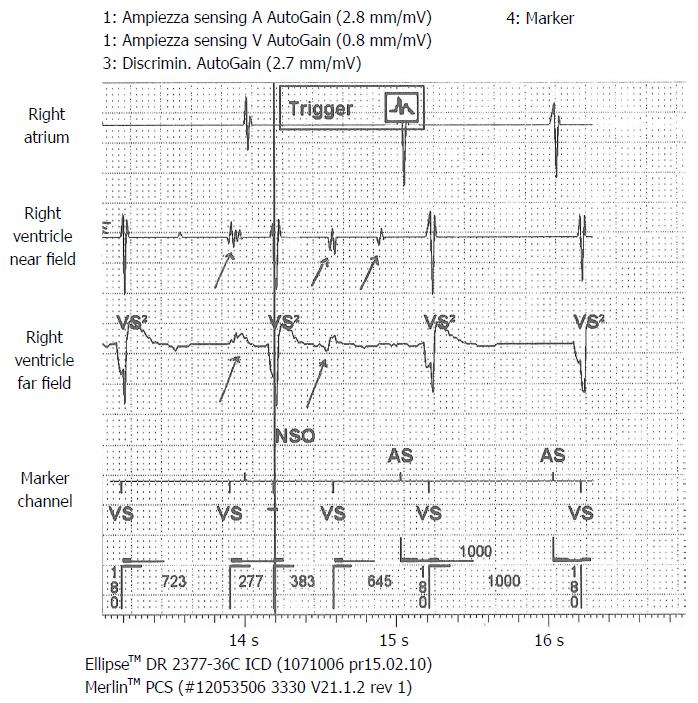
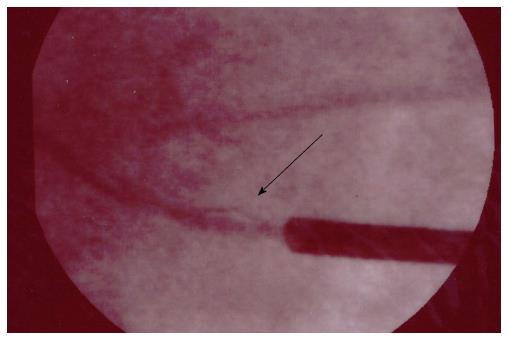
A 71-year-old man, with ischemic dilated cardiomyopathy, was evaluated in our center for a suspect ICD malfunction. He had been implanted, 10 years before, with a Biotronik defibrillator and a Kentrox dual-coil lead as primary prevention. In 2013 the device was replaced (normal battery depletion) and an Ellipse St. Jude defibrillator was implanted and connected to the old Kentrox lead. Early in 2016 at device interrogation we found abnormally low pacing impedance values (< 200 Ohm) and repetitive nonphysiological high-rate sensed events on sensing channels (both near and far field), suggesting an insulation defect. These episodes were of brief duration, therefore they did not trigger inappropriate shocks (Figure 4). At fluoroscopy conductor externalization was evident just proximal to the ventricular coil (Figure 5). Due to a deemed high risk of extraction we decided to implant a new lead, abandoning the damaged one; reimplant was uneventful. Subsequent defibrillation testing on induced VF was performed successfully.
In our center we have followed a total of 35 patients with Biotronik ICD leads: 5 Kentrox, 27 Linox and 3 Protego models, implanted between 2005 and 2016. The above-mentioned case is the only failure we had to face so far.
Shoemaker et al[4] was the first to report the phenomenon of conductor externalization in a Linox lead. The lead was dual-coil, implanted 4 years before, in a patient with a persistent left and absent right SVC. The externalized conductors, proximal to the caval coil, were incidentally discovered during a coronary angiogram. There was no change in baseline electrical performance of the lead which was, however, extracted. It is notewhorty that a lead with externalized conductors may still function normally because high-voltage and pace-sense ring cables are covered with ETFE, which serves as a second insulation. However, if ETFE abrades, electrical short circuits can occur during shock delivery with inability to defibrillate and catastrophic consequences.
In a successive paper, Manfredi et al[5] described a dual-coil Linox lead, implanted 8 years before, in a 53-year-old man with ischemic dilated cardiomyopathy for primary prevention. During an electrophysiological study, the lead had conductor wires protruding outside the body lead, between caval and ventricular coil. Also in this case, device interrogation revealed normal baseline electrical values. The patient was not pacing dependent and had never received appropriate or inappropriate ICD therapy; therefore, it was decided to follow the lead closely without extracting or replacing it.
Abi-Saleh et al[6] were the first to describe externalized conductor in a Kentrox lead. The dal-coil lead had been implanted, 7 years before, in a 38-year-old man with Brugada syndrome after cardiac arrest from VF. The patient presented with multiple inappropriate shocks due to noise which was evident on intracardiac electrogram (suggesting insulation failure); moreover, device check also revealed high pacing and shock impedance (suggesting concomitant conductors fracture). Fluoroscopy showed externalized conductors at the level of tricuspid valve. The patient underwent Kentrox extraction and reimplantation of a new ICD lead.
Another case of Kentrox failure was described by Bogossian et al[7]. The paper reports the first evidence of conductor externalization in a single-coil Biotronik lead, which had been implanted, 11 years before, in a 14-year-old boy after cardiac arrest from idiopathic VF. The lead presented externalized conductors at the region of tricuspid valve. Electrical measurements of the lead showed a significant decrease of sensing values, as well as a high-voltage shock impedance > 300 Ohm (suggesting both insulation failure and conductor fracture). The malfunctioning lead was explanted and a subcutaneous ICD was implanted.
In the case report by Reichlin et al[8], a Linox dual-coil lead presented with multiple inappropriate shocks due to noise on the sensing channel, suggesting insulation failure. Baseline electrical parameters (sensing values, pacing threshold and impedances) were normal. The lead had been implanted, 8 years before, in a 54-year-old man with non-ischemic dilated cardiomyopathy. At fluoroscopy externalized conductor cables were seen just proximal to the ventricular coil. The lead was extracted and visual inspection confirmed the externalization of pace-sense cables, putative source of noise.
Finally, Wutzler et al[9] described the case of a 31-year-old man with VF which was not interrupted by his ICD. The device was explanted: An area of burn marks on the surface with a small hole in the titanium can was found. Further analysis by the manufacturer showed a defect of the defibrillator output stage, indicating shock delivery via a low impedance shock path and premature battery discharge. These findings suggest an isolation defect of the ICD lead (Biotronik LinoxSmart).
Given this background and the published case reports, some centers started to systematically review all Biotronik leads implanted in their institutions.
Howe et al[10] reviewed all Biotronik ICD leads implanted in Royal Victoria Hospital, Belfast, United Kingdom, between 2006 and 2014. They included Vigila and Volta ICD leads marketed by Sorin (Sorin Group, Milan, Italy) but produced by Biotronik and identical to Linox. A total of 98 leads were included in their retrospective analysis (86 Linox and 12 Vigila/Volta). The authors identified a total of 4 lead failures, corresponding to 4% of all Biotronik leads. The failed leads presented with signs of insulation failure: 3 cases of nonphysiological high rate noise sensing leading to VF detection; 1 case with a significant decrease in pacing lead impedance. Only 1 case of externalized conductors was evident at fluoroscopy. All malfunctional leads were subsequently replaced.
Noti et al[11] reported their experience with Biotronik ICD leads, implanted in their center (University Hospital of Bern, Switzerland) between 2006 and 2014. They retrospectively compared performance of all Linox/Vigila leads (n = 93) with that of all Boston Scientific Endotak Reliance integrated bipolar leads (n = 190) and Medtronic Sprint Quattro dedicated bipolar lead (n = 202), implanted during the same period. Moreover, all Linox/Vigila leads were screened with fluoroscopy for conductor externalization. Lead failure was defined as follows: Recurrent nonphysiological high-rate sensing unrelated to EMI or T-wave oversensing; a sudden rise in pacing or shock impedance unrelated to perforation or dislodgment; sudden increase in pacing threshold and/or sudden decrease in R-wave sensing; visual evidence of fracture or insulation failure or externalization. The authors identified 9 cases of lead failure in Biotronik leads (9.7%): 2 cases of externalization, 6 cases of nonphysiological high rate noise sensing (5 cases with inappropriate shocks), 1 case of high-voltage conductor fracture. All failures concerned Linox leads (not Vigila). Lead failure was about 1% for Boston and Medtronic leads. Notably, lead survival at 5 years was 88% for Biotronik, 97.5% for Boston, 100% for Medtronic leads. Moreover, the median time from implant to failure was shorter with Biotronik leads (46 mo) compared with Boston and Medtronic (60 mo). A total of 10 patients died during the study period but circumstances of death were not systematically evaluated. The authors concluded that survival of Biotronik ICD leads was significantly worse than that of other leads; insulation failure was the most common presentation even if conductor externalization was seen only in a minority of failed leads. Younger age was found to be and independent predictor of failure.
In 2015, Padfield et al[12] published the results of a multicenter retrospective ICD registry, performed in British Coumbia (BC) region of Canada. Following the introduction of Linox leads the authors began to observe cases of early failure, some of which associated with conductors externalization. Therefore, they systematically evaluated the long term performance of all Linox leads implanted in BC, using St. Jude Medical Durata ICD leads (implanted during the same period) as comparator. This retrospective analysis included a total of 477 Linox and 838 Durata leads, implanted between 2008 and 2014. Definition of lead failure was almost identical to the above-mentioned paper of Noti et al[11]. Over a median of 39 (27-50) mo Linox leads had a higher failure rate than the Durata: 16/477 cases of Linox failure vs 4/838 for Durata (3.4% vs 0.4%). Linox failure type in detail was as follows: 11 cases of recurrent nonphysiological high-rate sensing; 7 cases of sudden impedance rise consistent with lead fracture; insulation failure was confirmed in 6 cases (exposed conductors in the pocket, insulation abrasion, “outside-in” abrasion, etc). Notably, no clear case of “Riata like” externalized conductor was evident at fluoroscopy, but systematic radiographic analysis was not performed. Survival rate at 5 years was 91.6% for Linox vs 99.4% for Durata (P < 0.0001). Failure occurred earlier with Biotronik leads compared to Durata. Female sex was the only independent risk factor for Linox failure in this study (P = 0.004). Patient survival analysis was not an end-point of the study and not reported.
Taken togheter these 3 studies[10-12] (with the limitations that we will discuss thereafter) analyzed 668 Biotronik ICD leads and showed a worrisome incidence of Linox leads failure, ranging from 3.4% to 9.7% (vs 1% of Endotak Reliance and Sprint Quattro leads and 0.4% of Durata). Survival rate at 5 years (88%-91%) was significantly lower in comparison with the competitors. Failure occurred earlier with Biotronik leads compared to the others. Insulation defect was the main mechanism of failure, while conductor fracture was less frequent but not negligible. Conductor externalization was present only in a minority of cases but fluoroscopic screening was not performed systematically in all studies.
In contrast to the above-mentioned studies, Linox survival rate was 96%-97% at 5 years of follow-up in a product performance report published by Biotronik in July 2015 (http://www.biotronik.com/files/38E6CFB4E275DE2CC1257EC800531F89/$FILE/Product_Performance_Report_July_2015.pdf), well within industry standards. Anyway, it is well known that reported failure rates from manufacturers are frequently based on voluntary product return and not on systematic data collection, so they are prone to under-reporting bias.
Conflicting results of spontaneous studies vs Biotronik report prompted to evaluate post-market, long-term performance of Linox leads in 2 ongoing large, multicenter, prospective, non-randomized, independent registries GALAXY (NCT00836589) and CELESTIAL (NCT00810264)[13].
GALAXY registry was designed to obtain long-term safety and reliability data on Linox family leads implanted in 98 United States sites. Enrollment started in 2009 end was completed in 2011, a total of 1997 patients being included. CELESTIAL post-approval registry was originally designed to evaluate long-term performance of Biotronik Corox family of bipolar left ventricular leads. However many Linox were implanted and included in the study of this ICD lead. The enrollment (2499 patients in 97 United States sites) started in 2008 and was completed in 2013.
A total of 3.933 Linox leads were implanted for both registries and included in the analysis. All patients were implanted with a Biotronik ICD or biventricular defibrillator. The GALAXY and CELESTIAL registry protocols collected adverse events (AEs) related to the implanted system or procedure. A “system-related” AE was defined as follows: (1) an event related to the implanted system occurred; and (2) an action was taken to address the event, or lead use was continued despite a known performance issue, which would have otherwise implicated an action to be taken (e.g., patient too ill for extraction).
The median follow-up was 3.6 years for Linox models and 2.3 years for LinoxSmart. The analysis of Linox leads showed an excellent performance over time: The estimated cumulative survival rate probability was 96.3% at 5 years after implant for Linox models and 96.6% at 4 years for LinoxSmart leads. A relatively low rate of chronic AEs was observed (2.31%). The most common AEs were: Oversensing (23, 0.58%); conductor fracture (14, 0.36%); failure to capture (13, 0.33%); insulation breach (10, 0.25%); high pacing impedance (8, 0.20%).
The authors concluded that Linox leads are safe, reliable and rarely associated with lead-related adverse events, with a clinically acceptable estimated survival probability that is well within industry standards. Data collection is still ongoing an will be updated in the near future.
Transvenous ICD leads are prone to failure over time, representing the weakest link of a defibrillation system. Lead models from various manufacturers have different performance records. Endotak Reliance (Boston), Sprint Quattro (Medtronic) and Durata (St. Jude Medical) have a very low incidence of failure (between 0.4% and 1%); this is expecially true for the leads marketed from a longer time and with longer follow-up duration (Endotak and Sprint Quattro). On the opposite site, other leads have been withdrawn from the market because of a very high rate of failure, and this is the case of Medtronic Sprint Fidelis (over 268000 leads implanted worldwide) and St. Jude Riata (over 227000 implants worldwide).
Recent case reports[4-9] and some studies[10-12]. have raised doubts about the performance of Biotronik ICD leads too. Over 140.000 Biotronik ICD leads have been marketed worldwide (including Kentrox, Linox and Vigila/Volta) so it is of utmost importance to have a high level of awareness and attention when following patients with these leads. For this reason expert consensus exists that systematic post-market surveillance of (all) ICD leads is essential to evaluate their long-term performance.
Linox and Riata leads share some structural similarities: Silicone insulation without outer coating, a coaxial lead design, a rather small diameter. The unique insulation defect described for Riata leads (“inside-out” abrasion) has been consistently reported also for Kentrox and Linox leads. The exact failure mechanism of Biotronik lead is not fully clear, but it is plausible that it is very similar to Riata. While case reports focused the attention on conductors externalization, other studies have shown that this phenomenon was present only in a minority of cases, even if fluoroscopic screening was not always performed systematically. More importantly, insulation defect was the main mechanism of failure for Biotronik leads (independently from conductor externalization). Conductor fracture was less frequent but (differently from Riata) not negligible.
A very important point is to explain the discrepancies existing between single-center studies plus the Canadian retrospective registry[10-12] on one side, and the results of the United States prospective registries (CELESTIAL and GALAXY)[13] on the other side. The former showed a worrisome incidence of Linox leads failure (from 3.4% to 9.7% vs 0.4%-1% of competitors) with a significantly lower lead survival rate at 5 years (88%-91%). The latter (CELESTIAL and GALAXY registries) substantially confirm the results of product performance report published by Biotronik, with a 96% survival rate at 5 years and a relatively low rate of chronic adverse events (2.3%). First of all, single-center and retrospective studies have a relatively small sample size (a total of 668 leads) compared to the 2 United States prospective registries (3.933 leads), so it is harder to draw conclusions with smaller numbers. Secondly, the 2 United States registries[13] have a prospective design and a more complete protocol which are best suited to address the question of lead performance. Finally, in the BC Canadian registry Linox leads were predominately connected to a Medtronic device, while most Durata leads were connected to a St. Jude Medical ICD. This is important because each manufacturer has its own proprietary sensing filters and algorithms. Medtronic devices have a proprietary Lead Integrity Alert (LIATM) which is sensitive to nonphysiological short V-V sensing intervals: this algorithm can be useful to assess lead performance, including Linox[14], but when used with non-Medtronic leads it can potentially overestimate the incidence of failure.
In conclusion CELESTIAL and GALAXY registries are quite reassuring, even if Linox performance seems to be slightly inferior to Endotak Reliance, Durata and Sprint Quattro. Data collection from the registries is still ongoing an will be updated in the near future to confirm longer-term performance of this family of ICD leads. Meantime, Biotronik leads can be managed according to usual clinical practice. Literature data do not support the need for a routine fluoroscopic screening, but (in our opinion) it is reasonable to have the lead connected to a Biotronik device whenever possible. Finally, remote monitoring should be activated for early detection of potential nonphysiological high-rate sensing before the occurrence of inappropriate shocks.
This statement is to certify that all authors have seen and approved the manuscript being submitted, have contributed significantly to the work, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to the Journal. We attest that the article is the Authors’ original work, has not received prior publication and is not under consideration for publication elsewhere. On behalf of all Co-Authors, the corresponding Author shall bear full responsibility for the submission. Any changes to the list of authors, including changes in order, additions or removals will require the submission of a new author agreement form approved and signed by all the original and added submitting authors. Patient’s consent was obtained. The authors report no relationships that could be construed as a conflict of interest.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chawla M, Nam GB S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, Weisse U, Seidl K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of & gt; 10 years. Circulation. 2007;115:2474-2480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 2. | Kalahasty G, Ellenbogen KA. Management of the patient with implantable cardioverter-defibrillator lead failure. Circulation. 2011;123:1352-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Swerdlow CD, Kalahasty G, Ellenbogen KA. Implantable Cardiac Defibrillator Lead Failure and Management. J Am Coll Cardiol. 2016;67:1358-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Shoemaker MB, Rottman JN. Conductor extrusion in a persistent left superior vena cava. Europace. 2012;14:307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Manfredi JA, Smithgall SM, Kircher CM, Lollis MA. Insulation failure with externalized conductor of a Linox SD lead: a case report. J Cardiovasc Electrophysiol. 2014;25:440-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Abi-Saleh B, Refaat MM, Khoury M, Wilkoff B. Conductor externalization of the Biotronik Kentrox internal cardioverter-defibrillator lead: the tip of another iceberg? Heart Rhythm. 2014;11:1648-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Bogossian H, Mijic D, Frommeyer G, Winter J. Insulation failure and externalized conductor of a single-coil Kentrox lead: an ongoing story? J Cardiovasc Electrophysiol. 2015;26:226-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Reichlin T, Kühne M, Sticherling C. Repetitive inappropriate implantable cardioverter-defibrillator shocks due to insulation failure with externalized conductor cables of a Biotronik Linox SD ICD lead. Europace. 2016;18:686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Wutzler A, Attanasio P, Haverkamp W, Blaschke F. Near-Fatal ICD Lead Dysfunction with Implications for ICD Testing. Pacing Clin Electrophysiol. 2016;39:105-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Howe AJ, McKeag NA, Wilson CM, Ashfield KP, Roberts MJ. Insulation Failure of the Linox Defibrillator Lead: A Case Report and Retrospective Review of a Single Center Experience. J Cardiovasc Electrophysiol. 2015;26:686-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Noti F, Lam A, Klossner N, Seiler J, Servatius H, Medeiros-Domingo A, Nam Tran V, Haeberlin A, Fuhrer J, Tanner H. Failure rate and conductor externalization in the Biotronik Linox/Sorin Vigila implantable cardioverter-defibrillator lead. Heart Rhythm. 2016;13:1075-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Padfield GJ, Steinberg C, Karim SS, Tung S, Bennett MT, Le Maitre JP, Bashir J. Early failure of the Biotronik Linox implantable cardioverter defibrillator lead. J Cardiovasc Electrophysiol. 2015;26:274-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Good ED, Cakulev I, Orlov MV, Hirsh D, Simeles J, Mohr K, Moll P, Bloom H. Long-Term Evaluation of Biotronik Linox and Linox(smart) Implantable Cardioverter Defibrillator Leads. J Cardiovasc Electrophysiol. 2016;27:735-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Steinberg C, Padfield GJ, Hahn E, Flavelle S, McILROY C, VAN Bremen O, Yeung-Lai-Wah JA, Kerr CR, Deyell MW, Tung SK. Lead Integrity Alert Is Useful for Assessment of Performance of Biotronik Linox Leads. J Cardiovasc Electrophysiol. 2015;26:1340-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |