Published online Jul 16, 2016. doi: 10.12998/wjcc.v4.i7.172
Peer-review started: March 2, 2016
First decision: March 22, 2016
Revised: April 14, 2016
Accepted: May 7, 2016
Article in press: May 9, 2016
Published online: July 16, 2016
Neuromuscular electrical stimulation (NMES) and testosterone replacement therapy (TRT) are effective rehabilitation strategies to attenuate muscle atrophy and evoke hypertrophy in persons with spinal cord injury (SCI). However both interventions might increase heterotopic ossification (HO) size in SCI patients. We present the results of two men with chronic traumatic motor complete SCI who also had pre-existing HO and participated in a study investigating the effects of TRT or TRT plus NMES resistance training (RT) on body composition. The 49-year-old male, Subject A, has unilateral HO in his right thigh. The 31-year-old male, Subject B, has bilateral HO in both thighs. Both participants wore transdermal testosterone patches (4-6 mg/d) daily for 16 wk. Subject A also underwent progressive NMES-RT twice weekly for 16 wk. Magnetic resonance imaging scans were acquired prior to and post intervention. Cross-sectional areas (CSA) of the whole thigh and knee extensor skeletal muscles, femoral bone, and HO were measured. In Subject A (NMES-RT + TRT), the whole thigh skeletal muscle CSA increased by 10%, the knee extensor CSA increased by 17%, and the HO + femoral bone CSA did not change. In Subject B (TRT), the whole thigh skeletal muscle CSA increased by 13% in the right thigh and 6% in the left thigh. The knee extensor CSA increased by 7% in the right thigh and did not change in the left thigh. The femoral bone and HO CSAs in both thighs did not change. Both the TRT and NMES-RT + TRT protocols evoked muscle hypertrophy without stimulating the growth of pre-existing HO.
Core tip: Neuromuscular electrical stimulation (NMES) and testosterone replacement therapy (TRT) are effective rehabilitation strategies in restoring muscle size and lean mass in persons with spinal cord injury (SCI). However, the effects on ectopic bony growth similar to heterotopic ossification (HO) have yet to be determined. The current two case reports demonstrated that TRT with or without NMES applications for 16 wk are considered safe rehabilitation strategies in persons with SCI who have HO formation. Both the TRT and NMES + TRT protocols evoked muscle hypertrophy without stimulating the growth of pre-existing HO.
- Citation: Moore PD, Gorgey AS, Wade RC, Khalil RE, Lavis TD, Khan R, Adler RA. Neuromuscular electrical stimulation and testosterone did not influence heterotopic ossification size after spinal cord injury: A case series. World J Clin Cases 2016; 4(7): 172-176
- URL: https://www.wjgnet.com/2307-8960/full/v4/i7/172.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i7.172
Great attention is currently directed towards developing rehabilitation strategies to offset the negative consequences of spinal cord injury (SCI) on the musculoskeletal, metabolic and cardiovascular systems[1]. Both electro-therapeutic and pharmaceutical interventions have been successful in reversing skeletal muscle atrophy following SCI[2-6]. Moreover, persons with SCI suffer decreased levels of anabolic hormones such as testosterone (T)[7,8]. Sixty percent of men with SCI have low T levels beginning within 6 mo after SCI[8]. Testosterone replacement therapy (TRT) has been shown to promote lean mass, reduce fat mass and increase basal metabolic rate[2].
Surface neuromuscular electrical stimulation resistance training (NMES-RT) has induced muscle hypertrophy up to 30%-40%[3-5]. Previously, significant muscle hypertrophy of the thigh muscles was accompanied by decreased intramuscular fat, increased leg lean mass and fatigue resistance, and improved metabolic profile[4,5]. Combining TRT with NMES-RT may prove beneficial in promoting positive changes in body composition in persons with SCI.
A common problem impacting the quality of life following SCI is the development of heterotopic ossification (HO). HO is estimated to affect up to 53% of the SCI population[9]. HO grows in non-bony areas near large joints below the level of injury and contributes to reduction in range of motion and pain resulting from compression of vascular and peripheral nerve structures. The purpose of this report was to determine if the anabolic effects of TRT with or without NMES-RT would trigger the growth of pre-existing HO in two persons with motor complete SCI.
Two men with chronic traumatic motor complete SCI participated in a clinical trial (NCT01652040) addressing the effects of NMES-RT + TRT vs TRT on parameters of body composition and metabolic profile. The physical characteristics of both participants are listed in Table 1. Both participants reported a past history of HO. X-ray records confirmed the presence of HO in subjects A and B. Both participants had functional range of motion of their hip, knee, and ankle joints during their initial physical exam before starting the study. The study was approved by the local Institutional Review Boards, and both participants enrolled in the study after reading and signing consent forms.
| Subject A | Subject B | |
| Age (yr) | 49 | 31 |
| Weight (kg) | 106.5 | 93 |
| Height (cm) | 188.5 | 181.5 |
| BMI (kg/m2) | 30 | 28.4 |
| Ethnicity | AA | W |
| LOI | T4 | T8 |
| TSI (yr) | 24 | 5 |
| AIS | A | A |
| Cause | GSW | Fall |
| HO | Right leg | Both legs |
A Theratouch NMES unit (NMES unit: Rich-Mar Corp, 4120 S Creek Rd, Chattanooga, TN 37406) was used along with two conductive adhesive gel electrodes (8-cm × 10-cm). A single electrode was placed 2-3 cm above the superior aspect of the patella over the vastus medialis muscle; the other was placed lateral to and 30 cm above the patella over the vastus lateralis muscle. Stimulation parameters were set at a frequency of 30 Hz, a pulse duration of 450-μs and an amplitude of current (mA) reasonable to evoke full knee extension from a sitting position in the participant’s wheelchair[3-5].
The training protocol was conducted twice weekly for 16 wk using surface NMES and ankle weights while sitting in the wheelchair. The first 2 wk, exercise was performed without ankle weights to condition the paralyzed knee extensors; weights were then progressively increased by 2 lbs. weekly after 40 repetitions (4 sets × 10) of full knee extension were achieved in one session. A rest period of 2-min was allowed between exercising sets. Increasing the current amplitude caused the knee extensors to contract and dynamically move the leg and weights against gravity. After full knee extension was maintained for 3-5 s, the current was gradually decreased to allow the exercising leg to slowly drop to the initial position. These methods have been previously published in detail elsewhere[3-5,10,11].
TRT was administered via transdermal shoulder patches (TRT: Watson Pharma, Inc. A subsidiary of Watson Pharmaceuticals, Inc. Corona, CA 92880, United States) that delivered 4-6 mg/d. Patches were worn daily, including weekends, for 16 wk on dry skin, alternating daily between shoulders. Before post-intervention testing (week 17), both participants were asked to remove the patches on the last day of week 16 to avoid acute effects from the TRT patches.
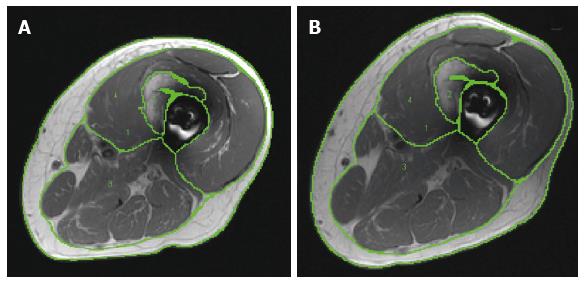
Whole thigh magnetic resonance imaging (MRI) scans were conducted at baseline and one week post-intervention to measure skeletal muscle cross-sectional areas (CSA; Figure 1)[2,4]. Transaxial images, 8 mm thick and 4 mm apart, were obtained from the hip to the knee joints using a regional body coil with a 1.5-T magnet (repetition time 550 ms, echo time 14 ms, field of view 20 cm, matrix size 256 × 256)[5]. The anatomical boundaries of the thigh muscle groups and HO were traced and analyzed using the computer program Winvessel (Dr. Ronald Meyer, Michigan State University)[2,4].
Both participants completed the assigned protocols without any study-related serious adverse events.
Eight weeks after beginning the trial, patch placement resulted in skin irritation that was completely resolved using topical hydrocortisone cream. Subject A showed an adherence of 97% (31 visits of the assigned 32 visits) and 100% compliance with his TRT protocol. The whole thigh skeletal muscle and knee extensor CSAs increased by 10% and 17%, respectively from baseline to post-intervention (Table 2). Because it was difficult to separate the HO region from the subperiosteal femoral bone, analysis was done to present the results of HO + femoral bone CSA which did not change in size after 16 wk (Table 2 and Figure 2).
| Subject A | Subject B | |||
| Baseline | Post-intervention | Baseline | Post-intervention | |
| Whole thigh muscle CSA (cm2)-right | 155.3 ± 26 | 170 ± 29 | 138 ± 18 | 156 ± 18 |
| Knee extensor CSA (cm2)-right | 60 ± 10 | 71 ± 12 | 62 ± 8 | 66 ± 6 |
| Subperiosteal femoral bone CSA (cm2)-right | 9.2 ± 1.3 | 9.4 ± 1 | ||
| HO CSA (cm2)-right | 24 ± 11 | 23 ± 11 | 3.6 ± 2 | 3.7 ± 1.9 |
| Whole thigh muscle CSA (cm2)-left | 124 ± 18 | 132 ± 19 | ||
| Knee extensor CSA (cm2)-left | 62 ± 8 | 66 ± 6 | ||
| Subperiosteal femoral bone CSA (cm2)-left | 13 ± 4 | 13 ± 4 | ||
| HO CSA (cm2)-left | 3.6 ± 2 | 3.7 ± 1.9 | ||
Subject B showed 100% compliance with his TRT program. During the last 2 wk, he developed cellulitis in his right lower extremity and received antibiotics (Bactrim). For the right thigh, the whole thigh skeletal muscle and knee extensor CSAs increased by 13% and 7%, respectively from baseline to post-intervention. The subperiosteal femoral bone and HO CSAs remained unchanged. For the left thigh, the whole thigh skeletal muscle CSA increased by 6% from baseline to post-intervention, with no changes in the knee extensor CSA. The subperiosteal femoral bone and HO CSAs did not change after 16 wk (Table 2 and Figure 2).
The primary purpose of this report was to determine whether NMES-RT + TRT or TRT would increase the size of pre-existing HO in persons with SCI. The results clearly demonstrate that TRT with or without NMES-RT did not impact the size of HO, suggesting that both interventions are safe in persons with motor complete SCI.
Both TRT and RT stimulate muscle growth which can induce bone growth through the hypertrophied muscles placing additional strain on the bone[12-14]. TRT can also affect bone development directly through the androgen receptor. TRT protects against bone loss in the male skeleton and has been associated with increased bone thickness and volume[12-14]. NMES-RT may cause micro-trauma in the paralyzed limbs of SCI patients which could aggravate existing HO[9,15]. After immobilization, passive range of motion exercises can cause soft muscle tissue to tear causing micro-trauma[9,15]. This micro-trauma triggers a local inflammatory response releasing osteogenic cytokines into the surrounding tissues which can induce mesenchymal stem cell differentiation stimulating the osteogenesis needed for HO growth[9,15,16].
Fortunately, despite the T and possible inflammation from micro-trauma, the HO size in these two patients did not increase. The T dosage (4-6 mg/d) is consistent with other studies using transdermal patch delivery, but T dose-dependently affects bone with only higher doses of T producing significant skeletal benefits in men[14]. The trial duration (16 wk) also limits the likelihood of detecting changes in bone structure. Furthermore, in male mice, T attenuated the benefits to bone structure usually induced by mechanical loading[12]. In this study, muscle hypertrophy was detected without evidence of HO growth. In future studies, it would be advisable to identify patients with existing HO prior to entrance in clinical trials and monitor the size of their HO. For longitudinal studies, periodic X-rays throughout the trial may be needed.
Replacement dose TRT with and without NMES-RT did not aggravate HO in two men with chronic SCI. After 16 wk, notable increases were measured in the skeletal muscle CSAs of the whole thigh and the knee extensors without changes in the subperiosteal femoral bone or HO CSAs.
We would like to thank Hunter Holmes McGuire Research Institute and Spinal Cord Injury Services and Disorders for providing the environment to conduct clinical human research trials. The project described was supported by VHA RR&D# B7867-W.
Both subjects had pre-existing heterotopic ossification (HO).
Pre-existing HO.
Magnetic resonance imaging.
Spinal cord injury with HO.
AIS: American spinal cord injury impairment scale classification; BMI: Body mass index; CSA: Cross-sectional area; GSW: Gunshot wound; HO: Heterotopic ossification; LOI: Level of injury; NMES: Neuromuscular electrical stimulation; RT: Resistance training; SCI: Spinal cord injury; T: Testosterone; TRT: Testosterone replacement therapy; TSI: Time since injury.
Testosterone replacement therapy with or without exercise interventions may be safe, because they did not stimulate HO growth in persons with spinal cord injury.
This is an overall well-designed case report with a new message to provide.
Manuscript source: Invited manuscript
P- Reviewer: Angoules A, Taheri S, Tokuhashi Y, Zhao JB S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37:693-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 2. | Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, Spungen AM. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res. 2011;43:574-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44:165-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 133] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Gorgey AS, Caudill C, Khalil RE. Effects of once weekly NMES training on knee extensors fatigue and body composition in a person with spinal cord injury. J Spinal Cord Med. 2016;39:99-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med. 2010;33:90-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, Ivy JL. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol. 2009;19:614-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med. 2014;37:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Durga A, Sepahpanah F, Regozzi M, Hastings J, Crane DA. Prevalence of testosterone deficiency after spinal cord injury. PM R. 2011;3:929-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002;40:313-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Dolbow DR, Gorgey AS, Moore JR, Gater DR. Report of practicability of a 6-month home-based functional electrical stimulation cycling program in an individual with tetraplegia. J Spinal Cord Med. 2012;35:182-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Dolbow DR, Gorgey AS, Dolbow JD, Gater DR. Seat pressure changes after eight weeks of functional electrical stimulation cycling: a pilot study. Top Spinal Cord Inj Rehabil. 2013;19:222-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Sinnesael M, Laurent MR, Jardi F, Dubois V, Deboel L, Delisser P, Behets GJ, D’Haese PC, Carmeliet G, Claessens F. Androgens inhibit the osteogenic response to mechanical loading in adult male mice. Endocrinology. 2015;156:1343-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, Kaufman JM, T’Sjoen G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab. 2012;97:2503-2511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Yarrow JF, Conover CF, Beggs LA, Beck DT, Otzel DM, Balaez A, Combs SM, Miller JR, Ye F, Aguirre JI. Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J Neurotrauma. 2014;31:834-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Zotz TG, Paula JB. Influence of transcutaneous electrical stimulation on heterotopic ossification: an experimental study in Wistar rats. Braz J Med Biol Res. 2015;48:1055-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |