Published online Apr 16, 2016. doi: 10.12998/wjcc.v4.i4.112
Peer-review started: November 11, 2015
First decision: December 22, 2015
Revised: January 12, 2016
Accepted: February 23, 2016
Article in press: February 24, 2016
Published online: April 16, 2016
Processing time: 155 Days and 1.3 Hours
One of the most common symptoms presenting in patients with chronic pancreatitis is pancreatic-type pain. Obstruction of the main pancreatic duct in chronic pancreatitis can be treated by a multitude of therapeutic approaches, ranging from pharmacologic, endoscopic and radiologic treatments to surgical interventions. When the conservative treatment approaches fail to resolve symptomatic cases, however, endoscopic retrograde pancreatography with pancreatic duct drainage is the preferred second approach, despite its well-recognized drawbacks. When the conventional transpapillary approach fails to achieve the necessary drainage, the patients may benefit from application of the less invasive endoscopic ultrasound (EUS)-guided pancreatic duct interventions. Here, we describe the case of a 42-year-old man who presented with severe abdominal pain that had lasted for 3 mo. Computed tomography scanning showed evidence of chronic obstructive pancreatitis with pancreatic duct stricture at genu. After conventional endoscopic retrograde pancreaticography failed to eliminate the symptoms, EUS-guided pancreaticogastrostomy (PGS) was applied using a fully covered, self-expandable, 10-mm diameter metallic stent. The treatment resolved the case and the patient experienced no adverse events. EUS-guided PGS with a regular biliary fully covered, self-expandable metallic stent effectively and safely treated pancreatic-type pain in chronic pancreatitis.
Core tip: Endoscopic treatment for chronic pancreatitis is one of the most challenging advanced therapeutic interventions applied in clinical practice. While endoscopic retrograde pancreaticography remains the preferred treatment option, alternative therapeutic approaches are available for application upon failure of the first-line approach, including endoscopic ultrasound (EUS)-guided pancreatic interventions, radiological interventions, or surgery. Here, we describe a case of chronic pancreatitis that failed first-line therapy and was effectively and safely resolved by EUS-guided pancreaticogastrostomy using a biliary-type, fully covered, self-expandable metallic stent.
- Citation: Chang A, Aswakul P, Prachayakul V. Chronic pancreatic pain successfully treated by endoscopic ultrasound-guided pancreaticogastrostomy using fully covered self-expandable metallic stent. World J Clin Cases 2016; 4(4): 112-117
- URL: https://www.wjgnet.com/2307-8960/full/v4/i4/112.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i4.112
Chronic pancreatitis is characterized by chronic progressive inflammation, destruction and scarring of the pancreatic tissue, all of which can lead to permanent loss of pancreatic function in both the exocrine and endocrine glands of the organ. As a condition with appreciable prevalence and incidence worldwide, accumulated research efforts have provided substantive insights into its etiology, pathophysiological course, and clinical manifestations[1]. The resultant advancements in radiologic and endoscopic techniques for examining this condition have increased the rate of diagnosis and helped to identify cases eligible for treatment in a more timely manner.
The most common clinical signs of chronic pancreatitis are deficiencies in pancreatic exocrine and endocrine enzymes (known as pancreatic insufficiency) and chronic abdominal pain. The pancreatic-type pain manifests from major pancreatic duct obstruction consequent to ductal stone blockade, peri-pancreatic ductal fibrosis, or parenchymal calcification. A multitude of therapeutic approaches are available for treatment of main pancreatic duct (MPD) obstruction in chronic pancreatitis, and range from pharmacologic, endoscopic and radiologic approaches to surgical intervention[2-4]. These approaches are also applied to resolve obstructions of the MPD associated with post-operative anastomotic stricture resulting from the Whipple procedure or surgeries to address disconnected pancreatic tail syndrome or necrotizing pancreatitis with complete duct rupture[5]. Until resolution, the obstruction of drainage of pancreatic juices causes ductal hypertension which manifests as pancreatic-type pain[6,7].
The pharmacologic treatments, such as pancreatic enzyme supplementation and analgesics, can relieve the pain symptom but do not resolve the obstruction-induced ductal hypertension[4,8]. Resolutive treatments, on the other hand, include physical interventions, using surgical or the less-invasive endoscopic approaches. Endoscopic retrograde pancreatography (ERP) with pancreatic duct drainage is the current preferred first line treatment[5-9], and reportedly leads to complete or partial relief of the pain symptom in up to 80% of cases[9,10]; however, the ERP intervention reportedly fails to achieve pancreatic duct drainage in 5%-10% of cases, even when performed by experienced endoscopists[11,12]. The reported reasons for failure of pancreatic drainage are various and include chronic inflammation-related changes in the tissues of the periampullary region, complete MPD obstruction, post-surgical anastomotic changes, tortuous configuration of MPD, and obstructions of the gastric outlet and duodenum[11,13-15]. Surgery remains the most effective intervention for chronic pancreatitis since it can resolve ductal hypertension while facilitating resection of pathologic foci; in addition, the surgical approach allows for localized disease treatment in the absence of ductal dilatation[11]. When MPD dilatation is present, however, the surgical drainage is most frequently achieved by longitudinal pancreaticojejunostomy; although, this procedure carries high rates of complications (reportedly up to 30%) and mortality (reportedly up to 2%)[4,16-20].
For patients with contraindications for surgery or who decline the surgical treatment, endoscopic intervention is considered. ERP with pancreatic duct drainage is the least invasive of the available endoscopic treatments and is often offered as the first line treatment for this alterative therapeutic approach[21-23]. However, if pancreatic duct cannulation by the standard ERP approach fails, alternative endoscopic techniques are available; these techniques include the endoscopic ultrasound (EUS)-guided rendezvous technique using a transpapillary approach or antegrade stenting and the EUS-guided transmural drainage technique[24].
Here, we report the successful application of EUS-guided pancreaticogastrostomy (EUS-PGS) using a fully covered, self-expandable metallic stent (SEMS) to resolve a case a chronic pancreatitis.
A 42-year-old man presented to our clinic with complaint of post-prandial chronic epigastric pain that had lasted for 3 mo. At intake, the patient reported no pre-existing illness but revealed a 14-year history of heavy alcohol intake. The patient described the abdominal pain as very severe and radiating into his back. Other reported symptoms included anorexia, nausea and vomiting, with unintentional weight loss of a few kilogram. Physical examination revealed mild epigastric tenderness without rebound tenderness or abdominal mass. Blood workup showed complete blood count in the normal range, high fasting glucose (115 mg/dL; reference range: 74-99 mg/dL), normal total/direct bilirubin (0.6/0.2 mg/dL; reference range: 0.0-1.2 mg/dL), normal serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase (SGOT/SGPT) (21/15 U/L; reference range: 0-41 U/L), normal alkaline phosphatase (75 U/L; reference range: 40-130 U/L), low amylase (25 U/L; reference range: 28-100 U/L), and low lipase (12 U/L; reference range: 13-60 U/L). Coagulation tests were normal.
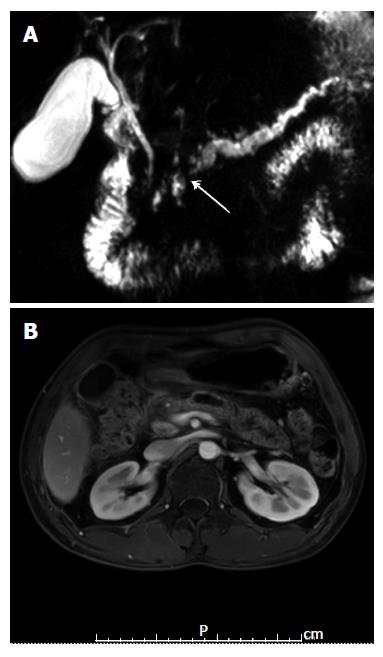
The patient underwent esophagogastroduodenostomy, which showed unremarkable findings. Radiologic examinations of the upper abdomen by computed tomography and magnetic resonance imaging demonstrated atrophic pancreatic parenchyma with heavy calcification; in addition, the pancreatic duct showed a beaded appearance and dilation, with duct size measuring 7 mm at the maximal diameter. Accumulation of pancreatic juices was detected in the MPD adjacent to the pancreatic head, and multifocal narrowing and focal wall thickening was detected in the cystic duct and extrahepatic common bile duct. The collective radiologic findings strongly suggested a diagnosis of chronic pancreatitis (Figure 1).
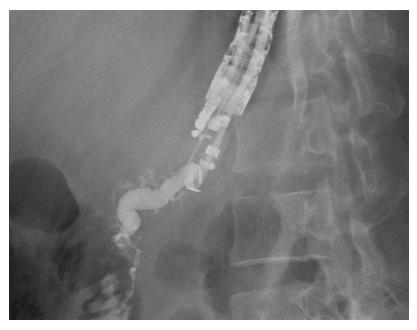
Pharmacological treatment was initiated and consisted of pancreatic enzyme supplements, tricyclic anti-depressant, and analgesic agents. When the nearly 1-year treatment produced no improvement in symptoms, including of the abdominal pain, the patient was referred for endoscopic pancreatic duct decompression. Initially, ERP with transpapillary drainage was attempted but the procedure could not be completed due to tight stricture of the pancreatic duct at the pancreatic genu that hindered passage of the guidewire.
The patient developed post-ERP pancreatitis and at day 3 post-ERP was offered the alternative treatment options of EUS-guided pancreatic duct drainage (rendezvous or PGS) as well as surgery. The patient and his family were informed of all procedure-related risks, benefits and long-term outcomes. At this time, the patient was discharged for decision-making and he returned 14 d later to undergo the selected EUS-guided intervention. Upon admission for the procedure, the patient provided informed consent for the procedure and publication of any and all data related to his case. For the treatment, the patient was positioned on the left lateral decubitus in the supine position and was put under intravenous sedation using propofol.
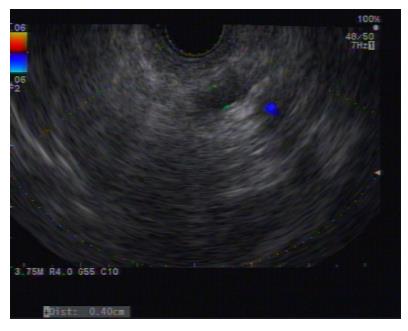
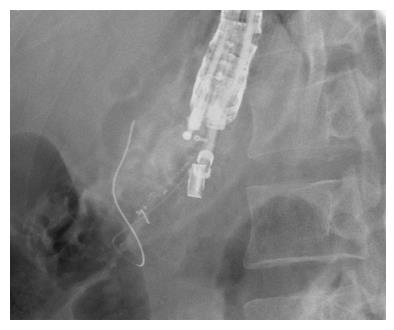
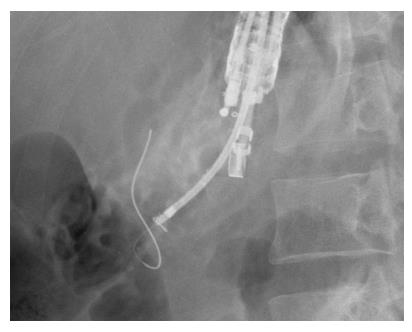
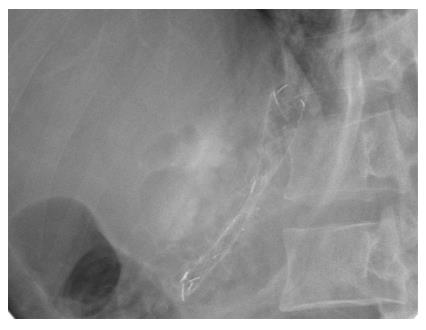
A curvilinear echoscope (GF-UCT 140-AL5; Olympus, Tokyo, Japan) was used for scanning. The endoview was unremarkable, and the echoview demonstrated non-homogeneous pancreatic parenchyma with hyperechoic foci and strands, lobulation, and parenchymal calcifications. The MPD, from the body to tail, was irregular, tortuous, and dilated up to 7 mm (Figure 2). An optimal puncture site was located to access the full MPD, from the body to tail, and a 19-gauge needle (Expect™ Slimline; Boston Scientific Corp., Spencer, IN, United States) was inserted. The aspirated liquid was clear. Contrast media was injected for the pancreatography, and the MPD was observed to be tortuous and dilated to between 6 mm and 8 mm in diameter (Figure 3). A 0.025 stiff guidewire (VisiGlide™; Terumo Medical Corp., Somerset, NJ, United States) was negotiated proximally, but passage down through the papilla was not possible (Figure 4). Therefore, the endoscopist decided to perform PGS. The neo-tract was created using a needle knife (MicroKnife™; Boston Scientific Corp., El Coyol, Alajuela, Costa Rica), followed by dilation using a 5 Fr Soehendra biliary dilation tapered tip catheter (Wilson-Cook Medical, Inc., Winston-Salem, NC, United States), a 7 Fr Soehendra dilation catheter, a Soehendra stent retriever size 8.5 Fr, and an 8.5 Fr Soehendra dilator (Figure 5). Then, a 10 mm × 60 mm fully covered metallic stent (Niti-S Biliary Covered Stent™; TaeWoong Medical Co, Ltd., Gimpo-si, Gyeonggi-do, Korea) was inserted and deployed between the MPD and gastric cavity; good position and satisfactory drainage was achieved (Figure 6). After 5 d of uneventful in-hospital recovery, the patient was discharged.
At the 6-mo follow-up appointment, the patient reported no recurrence of abdominal pain and weight gain of 3 kg. The blood workup showed results within normal range for complete blood count and for all liver function markers. The patient continued taking the pancreatic enzyme supplement, but analgesics and anti-depressants were deemed unnecessary and discontinued. At the last follow-up (attended at 6 mo), the patient was healthy and had gained an additional 3 kg. The blood workup again showed results within the normal ranges, including those for lipase, amylase, and CA19-9. Pancreatic stent removal was planned for 6 mo to 9 mo following this appointment.
When conventional endoscopic techniques for pancreatic drainage fail, two alternative endoscopic approaches may be considered. The first technique is the rendezvous technique followed by transpapillary approach, and is recommended by some expert endoscopists as the first line option due to its general safety and efficacy[24-27]. For the case described herein, we attempted to perform this technique initially but the attempt failed when the guidewire could not be negotiated down through the stricture point, possibly due to the patient’s complete MPD obstruction at the pancreatic genu. The second technique is the EUS-PGS. Since its first description in the literature in 1995 by Harada et al[28] several case series successfully treated by this technique have been reported, but with the majority having used plastic stents[24,26,29-34]. However, the safety profile of plastic stents is negatively impacted by their potential to migrate from the intrapancreatic location[35], the not infrequent failure rate of achieving stent placement, and the association with pancreatic fluid leakage following placement[36,37].
Recent case reports have demonstrated the advantages of using fully covered SEMS, which include longer stent patency, applicability to larger sized pancreaticogastric fistula, reduced rates of re-intervention, and increased ease of re-intervention to resolve cases of stent obstruction[38,39]. Moon et al[40] reported a case series of 25 patients with painful obstructive pancreatitis, including 10 cases of chronic pancreatitis, all of who failed the initial ERP treatment and then underwent EUS-guided pancreatic drainage using fully covered SEMS. The study showed a 100% technical success rate and 100% clinical success rate, without any occurrences of stent-related adverse events (mean follow-up: 221.1 d).
The stents used in the study by Moon et al[40] were either 6 mm or 8 mm in diameter, which are not readily available in the global medical device market. For example, our hospital did not have ready access to stents of those sizes and only a regular-sized biliary stent, of 10-mm, was available. Therefore, we performed the EUS-PGS using the 10-mm biliary stent and drainage was achieved without occurrence of any stent-related adverse event during the 6-mo follow-up period.
In conclusion, we have shown that a regular biliary fully covered SEMS can be used effectively and safely for EUS-PGS.
A 42-year-old man presented with severe abdominal pain that had lasted for 3 mo. Computed tomography scanning showed evidence of chronic obstructive pancreatitis with pancreatic duct stricture at genu. After conventional endoscopic retrograde pancreaticography failed to eliminate the symptoms, endoscopic ultrasound (EUS)-guided pancreaticogastrostomy (PGS) was applied using a fully covered, self-expandable, 10-mm diameter metallic stent.
Chronic pancreatitis with pancreatic duct stricture.
Pancreatic duct stone.
Increased blood glucose (115 mg/dL; reference range: 74-99 mg/dL); Liver chemistry results: normal total/direct bilirubin (0.6/0.2 mg/dL; reference range: 0.0-1.2 mg/dL), normal serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase (21/15 U/L; reference range: 0-41 U/L), normal alkaline phosphatase (75 U/L; reference range: 40-130 U/L), low amylase (25 U/L; reference range: 28-100 U/L), low lipase (12 U/L; reference range: 13-60 U/L).
Magnetic resonance imaging showed trophic pancreatic parenchyma with heavy calcification and the pancreatic duct with a beaded appearance and dilatation of 7 mm at the maximal diameter; in addition, multifocal narrowing and focal wall thickening was observed in the cystic duct and extrahepatic cystic bile duct.
EUS-guided PGS using a 10-mm, fully covered self-expandable metallic stent (FCSEMS).
EUS is a novel endoscopic intervention applied through the gastrointestinal tract for diagnostic evaluation and therapeutic interventions. EUS-guided PGS is a therapeutic intervention used to create a new connection between the main pancreatic duct and stomach to achieve pancreatic drainage.
A 10-mm FCSEMS represents a feasible and safe tool for achieving drainage of the pancreatic duct via the EUS-guided PGS procedure.
This is an interesting case report aimed at describing and explaining the treatment of pancreatic duct obstruction by EUS-guided PGS using a fully covered metallic stent that is 10 mm in diameter.
P- Reviewer: Fu D, Gupta RA, Stasi C S- Editor: Qi Y L- Editor: A E- Editor: Jiao XK
| 1. | Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ, Levy MJ, Kwon R, Lieb JG, Stevens T. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 276] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Cahen DL, Gouma DJ, Nio Y, Rauws EA, Boermeester MA, Busch OR, Stoker J, Laméris JS, Dijkgraaf MG, Huibregtse K. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 497] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 3. | Schnelldorfer T, Adams DB. Outcome after lateral pancreaticojejunostomy in patients with chronic pancreatitis associated with pancreas divisum. Am Surg. 2003;69:1041-1044; discussion 1045-1046. [PubMed] |
| 4. | Byrne RL, Gompertz RH, Venables CW. Surgery for chronic pancreatitis: a review of 12 years experience. Ann R Coll Surg Engl. 1997;79:405-409. [PubMed] |
| 5. | Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, Binmoeller KF. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Gabbrielli A, Pandolfi M, Mutignani M, Spada C, Perri V, Petruzziello L, Costamagna G. Efficacy of main pancreatic duct endoscopic drainage in patients with chronic pancreatitis, continuous pain and dilated duct. Gastrointest Endosc. 2005;61:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Ebbehøj N, Borly L, Bülow J, Rasmussen SG, Madsen P. Evaluation of pancreatic tissue fluid pressure and pain in chronic pancreatitis: A longitudinal study. Scand J Gastroenterol. 1990;25:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Attasaranya S, Abdel Aziz AM, Lehman GA. Endoscopic management of acute and chronic pancreatitis. Surg Clin North Am. 2007;87; 1379-1402, vii. [PubMed] |
| 9. | Wilder-Smith CH, Hill L, Osler W, O’Keefe S. Effect of tramadol and morphine on pain and gastrointestinal motor function in patients with chronic pancreatitis. Dig Dis Sci. 1999;44:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Rösch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Delhaye M, Arvanitakis M, Verset G, Cremer M, Devière J. Long-term clinical outcome after endoscopic pancreatic ductal drainage for patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol. 2004;2:1096-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Widmer J, Sharaiha RZ, Kahaleh M. Endoscopic ultrasonography-guided drainage of the pancreatic duct. Gastrointest Endosc Clin N Am. 2013;23:847-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Giovannini M. Endoscopic ultrasonography-guided pancreatic drainage. Gastrointest Endosc Clin N Am. 2012;22:221-230, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Will U, Fueldner F, Thieme AK, Goldmann B, Gerlach R, Wanzar I, Meyer F. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Ginès A, Varadarajulu S, Napoleon B. EUS 2008 Working Group document: evaluation of EUS-guided pancreatic-duct drainage (with video). Gastrointest Endosc. 2009;69:S43-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Widmer JL, Michel K. Endoscopic Ultrasound-Guided Treatment beyond Drainage: Hemostasis, Anastomosis, and Others. Clin Endosc. 2014;47:432-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Reid-Lombardo KM, Ramos-De la Medina A, Thomsen K, Harmsen WS, Farnell MB. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007;11:1704-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Adams DB, Ford MC, Anderson MC. Outcome after lateral pancreaticojejunostomy for chronic pancreatitis. Ann Surg. 1994;219:481-487; discussion 487-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 97] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Prinz RA, Greenlee HB. Pancreatic duct drainage in 100 patients with chronic pancreatitis. Ann Surg. 1981;194:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Sharma V, Rana SS, Bhasin DK. Endoscopic ultrasound guided interventional procedures. World J Gastrointest Endosc. 2015;7:628-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Cremer M, Devière J, Delhaye M, Baize M, Vandermeeren A. Stenting in severe chronic pancreatitis: results of medium-term follow-up in seventy-six patients. Endoscopy. 1991;23:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 238] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, Petruzziello L, Familiari P, Mutignani M. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Dumonceau JM, Devière J, Le Moine O, Delhaye M, Vandermeeren A, Baize M, Van Gansbeke D, Cremer M. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 151] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Cremer M, Deviere J, Delhaye M, Vandermeeren A, Baize M. Non-surgical management of severe chronic pancreatitis. Scand J Gastroenterol Suppl. 1990;175:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Jalleh RP, Aslam M, Williamson RC. Pancreatic tissue and ductal pressures in chronic pancreatitis. Br J Surg. 1991;78:1235-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Fink AS, Perez de Ayala V, Chapman M, Cotton PB. Radiologic pitfalls in endoscopic retrograde pancreatography. Pancreas. 1986;1:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Harada N, Kouzu T, Arima M, Asano T, Kikuchi T, Isono K. Endoscopic ultrasound-guided pancreatography: a case report. Endoscopy. 1995;27:612-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg. 1994;129:374-379; discussion 379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Gress F, Ikenberry S, Sherman S, Lehman G. Endoscopic ultrasound-directed pancreatography. Gastrointest Endosc. 1996;44:736-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | François E, Kahaleh M, Giovannini M, Matos C, Devière J. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002;56:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Brauer BC, Chen YK, Fukami N, Shah RJ. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video). Gastrointest Endosc. 2009;70:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Itoi T, Yasuda I, Kurihara T, Itokawa F, Kasuya K. Technique of endoscopic ultrasonography-guided pancreatic duct intervention (with videos). J Hepatobiliary Pancreat Sci. 2014;21:E4-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Ergun M, Aouattah T, Gillain C, Gigot JF, Hubert C, Deprez PH. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Chantarojanasiri T, Aswakul P, Prachayakul V. Uncommon complications of therapeutic endoscopic ultrasonography: What, why, and how to prevent. World J Gastrointest Endosc. 2015;7:960-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Fujii LL, Topazian MD, Abu Dayyeh BK, Baron TH, Chari ST, Farnell MB, Gleeson FC, Gostout CJ, Kendrick ML, Pearson RK. EUS-guided pancreatic duct intervention: outcomes of a single tertiary-care referral center experience. Gastrointest Endosc. 2013;78:854-864.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Katanuma A, Maguchi H, Fukazawa M, Kurita A, Ichiya T, Kin T, Osanai M, Takahashi K. Endoscopic ultrasonography-guided pancreaticogastrostomy for a case of occlusion of gastro-pancreatic anastomosis after pancreaticoduodenectomy. Dig Endosc. 2009;21 Suppl 1:S87-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Will U. Endoscopic Ultrasound-Guided Pancreaticogastrostomy. Video J Encyclopedia GI Endosc. 2013;1:563-566. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Moon SH, Kim MH, Park do H, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010;72:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |