Published online Dec 16, 2016. doi: 10.12998/wjcc.v4.i12.413
Peer-review started: April 12, 2016
First decision: May 19, 2016
Revised: September 7, 2016
Accepted: September 21, 2016
Article in press: September 22, 2016
Published online: December 16, 2016
Processing time: 240 Days and 13.2 Hours
Lymphocytic esophagitis (LyE) is a rare condition characterised histologically by high numbers of esophageal intraepithelial lymphocytes without significant granulocytes infiltration, in addition to intercellular edema (“spongiosis”). The clinical significance and natural history of LyE is poorly defined although dysphagia is reportedly the most common symptom. Endoscopic features range from normal appearing esophageal mucosa to features similar to those seen in eosinophilic esophagitis, including esophageal rings, linear furrows, whitish exudates, and esophageal strictures/stenosis. Symptomatic gastroesophageal reflux disease is an inconsistent association. LyE has been associated in paediatric Crohn’s disease, and recently in primary esophageal dysmotility disorder in adults. There are no studies assessing effective treatment strategies for LyE; empirical therapies have included use of proton pump inhibitor and corticosteroids. Esophageal dilatation have been used to manage esophageal strictures. LyE has been reported to run a benign course; however there has been a case of esophageal perforation associated with LyE. Here, we describe the clinical, endoscopic and histopathological features of three patients with lymphocytic esophagitis along with a review of the current literature.
Core tip: Lymphocytic esophagitis (LyE) is a histological subset of esophagitis that is rare with poorly defined clinical significance and associations. We present the clinical, endoscopic and histopathological features of three patients with LyE followed by the most up-to-date literature on the condition, including description of the newly postulated association of primary esophageal dysmotility.
- Citation: Jideh B, Keegan A, Weltman M. Lymphocytic esophagitis: Report of three cases and review of the literature. World J Clin Cases 2016; 4(12): 413-418
- URL: https://www.wjgnet.com/2307-8960/full/v4/i12/413.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i12.413
Lymphocytic esophagitis (LyE) is an uncommon histologic phenotype of esophagitis first described by Rubio et al[1]. Its histology is characterised by a high numbers of intraepithelial lymphocytes mainly in a peripapillary distribution with the absence of significant granulocytes (neutrophils and eosinophils), along with intercellular edema (“spongiosis”). The clinical significance and natural history of LyE is unknown. In this case series we highlight the clinical, endoscopic, histopathologic features and outcome of three patients with lymphocytic esophagitis followed by a review of the current literature. This includes the associations of LyE with other disease entities including gastroesophageal reflux disease (GERD), Crohn’s disease and most recently primary esophageal dysmotility; the endoscopic features which may range from normal mucosa to features observed in eosinophilic esophagitis (EoE); the management of LyE and its natural history.
An 85-year-old lady presented with progressive dysphagia to solids. Background history included proton pump inhibitor (PPI)-refractory GERD and chronic airway limitation with recurrent exacerbations requiring corticosteroids and antibiotics. Upper endoscopy revealed a 5 cm sliding hiatus hernia with otherwise normal appearing oesophagus; there was no macroscopic evidence of esophagitis or strictures. The stomach and duodenum were unremarkable. Biopsies from the middle and lower esophagus revealed prominent basal cell hyperplasia with intercellular edema and a heavy intra-epithelial lymphocytic infiltrate (up to 60 per high power fields) in a peripapillary distribution without granulocytes. A diagnosis of LyE was made. Therapy including escalating doses of PPI, a histamine receptor antagonist and a course of corticosteroids provided minimal symptomatic relief.
A 51-year-old gentleman presented with 12 mo of dysphagia to solids and a recent episode of food bolus impaction that cleared spontaneously. He was on no regular medications. A barium swallow revealed transient hold-up of a marshmallow bolus suggesting dysmotility but no stricture. An upper endoscopy demonstrated decreased motility of the esophageal body with macroscopically normal esophageal mucosa. The stomach and duodenum appeared unremarkable. Mid esophageal mucosal histology revealed spongiosis and a heavy lymphocytic infiltrate with occasional neutrophils and eosinophils, consistent with LyE. Gastric biopsies did not reveal any abnormalities. The patient reported spontaneous resolution of his dysphagia with no further episodes of food bolus impaction without the need for further intervention.
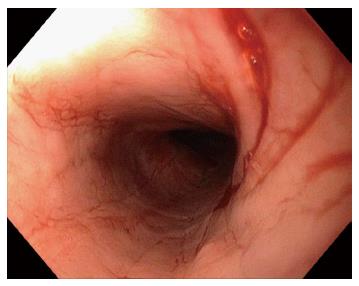
A 59-year-old lady presented with fluctuating dysphagia to solids and globus sensation over the past few months. There were no other symptoms reported. Past medical history included irritable bowel syndrome and hypothyroidism. Upper endoscopy revealed linear furrows and a tight benign appearing esophageal stricture in the proximal esophagus (25 cm from the incisors) that was dilated (Figure 1). There was no macroscopic evidence of esophagitis. The stomach and examined duodenum appeared normal. Biopsies from the mid esophagus demonstrated mucosal spongiosis with significant intraepithelial lymphocytic infiltrate and minimal eosinophils or neutrophils, consistent with LyE. Biopsies of the stomach and duodenum were unremarkable. Patient reported immediate improvement of symptoms following esophageal dilatation, but has required repeat dilatations over the years at approximately 12 mo intervals. This is in addition to high doses of acid suppression therapy (proton pump inhibitor). On subsequent esophageal biopsies the histopathological findings remained unchanged.
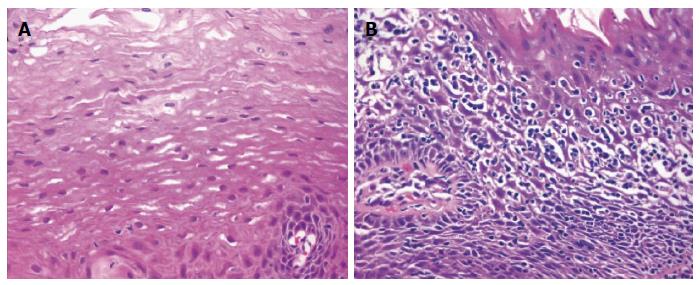
LyE is an uncommon and poorly defined histological subset of esophagitis first described by Rubio et al[1] in 2006. Whilst small numbers of intraepithelial lymphocytes are normal constituents of the esophageal mucosa[2], the histological hallmarks of LyE noted by Rubio et al[1] includes increased number of intraepithelial lymphocytes with few or no associated intraepithelial granulocytes (neutrophils and eosinophils) in addition to intercellular edema (“spongiosis”) (Figure 2)[3]. The intraepithelial infiltrate most densely in peripapillary fields and express CD3, CD4 and CD8 markers. This is in contrast with the distribution of intraepithelial lymphocytes in GERD, radiation and Candida albicans esophagitis where the number of interpapillary intraepithelial lymphocytes highly exceeds that recorded in peripapillary areas.
Various thresholds for the number of intraepithelial lymphocytes required for the diagnosis of LyE have been proposed[4] contributing to differences in reported incidence of LyE. The original work of Rubio et al[1] proposed at least greater than 20 lymphocytes per high power field. In the study by Haque et al[5] it was believed that stipulating a required minimum lymphocyte count could be potentially misleading and would not necessarily increase the specificity of the diagnosis. In their analysis of 129252 esophageal biopsies, LyE was detected in approximately one in a thousand patients when LyE was defined histopathologically as the presence of dense lymphocytic infiltrate (without a minimum number required) in the peripapillary esophageal squamous mucosa and marked spongiosis without significant number of eosinophils or neutrophils.
LyE appears to be localised to the esophagus when biopsies from other sites of the gastrointestinal tract have been concurrently analysed. In the study by Rubio et al[1], none of the biopsy specimens obtained from sites other than the esophagus showed intraepithelial lymphocytes in any of the 20 patients studied. Similarly, in a separate study by Purdy et al[6], the most common finding was normal mucosa when concurrent biopsies were taken from sites other than the esophagus including the stomach, small bowel and colon. This finding is true for all three cases in the present series.
The clinical presentation of LyE is inconsistent across different studies. Cohen et al[7] in their study cohort of 81 patients found the presentation of LyE was highest in females in the 6th deciles of life and most commonly presenting with symptoms of dysphagia, chest discomfort, heartburn, and less frequently food impaction. This was corroborated in a recent study by Xue et al[8]. However, this was not true in an earlier study by Purdy et al[6] that compared 42 patients with LyE to 34 control patients and no differences were found between the two groups in the rates of the above reported symptoms. In our case series, all three patients presented with dysphagia to solids.
The association between GERD and LyE has been of particular interest with conflicting reports in the literature. In GERD, none to small numbers of intraepithelial lymphocytes admixed with neutrophils and/or eosinophils has been the traditional observation[2]. In some studies, no association was found between LyE and symptoms or endoscopic findings consistent with GERD[4]. This was supported by Haque et al[5] who reviewed biopsy specimens from 129252 patients and found symptoms of GERD were significantly less frequent (18%) in patients with LyE compared to patients with normal esophageal biopsies (37%). This finding however was contradicted by Cohen et al[7] who reported GERD to be the most common association (49%) in their study cohort of 81 patients with LyE. In an assessment of the histologic importance of lymphocytes in EoE and reflux esophagitis, there were similar number of patient with GERD and intraepithelial lymphocytosis[9] prompting some to postulate that perhaps LyE is an extreme spectrum of GERD[10]. In support of this view, a recent study by Conner et al[11] described LyE in patients with Barrett esophagus and more severe reflux disease. Only one of our three cases described above complained of GERD symptoms, and on endoscopy there was no evidence of esophagitis noting that the patient was on high doses of PPI.
An interesting association between LyE and Crohn’s disease was first suggested by Rubio et al[1] in their initial description of LyE. In their study cohort of 20 patients (9 adults and 11 paediatric), 42% of the patients had Crohn’s disease. This prompted subsequent studies in both adult and paediatric patient populations. In the adult cohort, various studies the most recent by Xue et al[8] found no association of LyE with Crohn’s disease. This is similar to other reports describing adult patients including subjects with advanced Crohn’s disease[4-6]. In contrast, an association between LyE and Crohn’s disease in children is supported by several studies that demonstrated 12% to 28% prevalence of LyE in children with Crohn’s disease compared with 4% to 5% in children without Crohn’s disease[1,12,13].
LyE has most recently been associated with primary esophageal dysmotility disorder. In the study by Xue et al[8], primary esophageal motility abnormalities evident on esophageal manometry or barium swallow were found in 91% (10/11) of patients with LyE that had no identifiable granulocytes, and in 60% (6/10) of patients with LyE that had few granulocytes. This was compared to the control group of 28 patients with active esophagitis consistent with reflux disease and overtly increased intraepithelial lymphocytes. In those that had motility testing, 54% (6/11) were found to have primary esophageal motility abnormalities. In this study lymphocyte subsets were analysed, and the prevalence of primary motility abnormalities was significantly higher in patients with CD4-predominant esophagitis than in patients with CD8-predominant esophagitis from all groups. This led the authors to postulate that a distinctive type of LyE with CD4-predominant lymphocytes is associated with primary esophageal dysmotility, and there is good clinical utility in evaluating lymphocytes subsets in LyE. Further studies are needed to characterise this possible distinct clinicopathologic entity. In one of our cases (case 2), there was an impression of esophageal dysmotility at both upper endoscopy and barium swallow, but it was not further assessed with manometry as the patient’s symptoms resolved spontaneously.
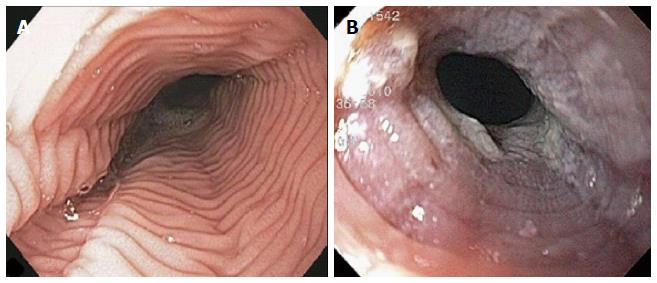
The endoscopic findings in LyE have been described in only a few reports and they can often mimics EoE[1,5,14,15]. Features include esophageal rings, linear furrows, whitish exudates, and esophageal strictures/stenosis (Figure 3). Normal esophageal mucosa is found in 23%-55% of cases on conventional endoscopy[1,5]. Attempts have been made to characterise the endoscopic features of both LyE and EoE using narrow band imaging magnifying endoscopy (NBI-ME). In the study by Tanaka et al[14], three features were identified on NBI-ME in their cohort of 21 patients (11 patients with LyE, 10 patients with EoE): (1) beige coloured mucosa (normal mucosa has light green colour); (2) increased and dot-shaped congested intrapapillary capillary loop; and (3) invisibility of submucosal vessels (normal mucosa has cyna-coloured vessels). In the LyE group, at least one of the above finding was evident in 91% (10/11) of patients, and all three findings were present in 82% (9/11) of patients. In the group with EoE, all patients had all three abnormalities. Compared with the control group with GERD, at least one of the three abnormal findings was seen in 30% (3/10) patients and none had all three features. The authors therefore proposed that coexistence of these three NBI-ME findings may indicate suspicion of LyE or EoE. Larger studies are required to further evaluate the diagnostic value of these criteria.
The management of LyE has not been addressed by any studies in the literature thus far. This may be in part due to the low prevalence of LyE. In the study by Cohen et al[7] most patients had an improvement in their symptoms with a PPI. However, it is unclear if the symptom improvement associated with PPI therapy was due to treatment of LyE or related to treatment of concomitant GERD. Haque et al[5] found widespread use of PPIs in their study subjects and therefore were unable to make any associations. However, it would not be unreasonable to initiate a trial course of PPI or to increase the dose when a diagnosis of LyE is made as it may provide symptomatic benefits. Topical corticosteroids (e.g., fluticasone) is another proposed course of treatment[16]. This is because corticosteroids are beneficial in other gastrointestinal lymphocytic and autoimmune diseases[17-19], and swallowed fluticasone in particular has been shown to induce histological remission in EoE[18]. Prospective studies are required to assess for appropriate and effective therapy for LyE. In our case series, one patient failed multiple lines of therapy, one patient had spontaneous resolution of symptoms, and the other patient required repeat esophageal dilatation for structuring disease.
The natural history of LyE has been specifically assessed in one study by Cohen et al[7] who found it to be a relatively benign disease in their retrospective study cohort of 29 patients who had follow-up surveys. In 59% (17/29) of patients, there was improvement in symptoms with escalating medical therapy, most commonly with a PPI and there was no significant adverse effect on their health-related quality of life. The highest rates of symptomatic improvement were seen in patients with dysphagia and the lowest rates were in patients with chest pain. In this study, follow-up endoscopy was performed in 22 patients, of which 9 had LyE on repeat biopsies. This finding suggests that the histological changes seen in LyE can potentially normalise, which was also observed in another study by Purdy et al[6]. However, these were retrospective studies with small numbers of study patients. A case report of spontaneous esophageal perforation attributed to LyE has been described[20], although the strength of the association is uncertain. Larger studies are necessary to clarify the natural history and disease course of LyE.
In conclusion, LyE is a rare histologic subset of esophagitis with uncertain clinical significance and associations. Primary esophageal dysmotility is a newly postulated association. Large prospective studies are required to further characterise this disease entity including an assessment of appropriate and effective treatment strategies.
The authors report three cases of esophageal dysphagia that had a histological diagnosis of lymphocytic esophagitis.
The case presentations were non-specific raising a number of possible aetiologies including gastroesophageal reflux disease (GERD), eosinophilic esophagitis, esophageal stricture, esophageal dysmotility, and esophageal tumour.
The case presentations were non-specific raising a number of possible aetiologies including GERD, eosinophilic esophagitis, esophageal stricture, esophageal dysmotility, and esophageal tumour.
The first case had evidence of a hiatus hernia on upper endoscopy with an otherwise normal appearing esophagus. The second case revealed decreased esophageal motility on upper endoscopy with a macroscopically normal mucosa; a barium swallow demonstrated transient hold-up of a marshmallow suggesting esophageal dysmotility. The third case had evidence of a tight benign-appearing esophageal stricture in the proximal esophagus that was dilated.
Lymphocytic esophagitis in all three cases.
First case did not respond to multiple medical therapies including acid suppression, histamine receptor antagonist and corticosteroids; the second case had spontaneous resolution of symptoms; and the third case required repeat esophageal dilatations in addition to acid suppressive therapy.
There have been various case reports of lymphocytic esophagitis with a lack of large prospective studies on this rare disease entity.
Management of lymphocytic esophagitis can be difficult in the absence of evidence-based diagnostic criteria and management algorithms.
This is a nice and well-structured manuscript describing the new entity among esophageal diseases. In addition there are descriptions of three patients with lymphocytic esophagitis. The topic is very interesting for readers.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Homan M, Quigley E, Romano C S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Rubio CA, Sjödahl K, Lagergren J. Lymphocytic esophagitis: a histologic subset of chronic esophagitis. Am J Clin Pathol. 2006;125:432-437. [PubMed] [Cited in This Article: ] |
| 2. | Geboes K, De Wolf-Peeters C, Rutgeerts P, Janssens J, Vantrappen G, Desmet V. Lymphocytes and Langerhans cells in the human oesophageal epithelium. Virchows Arch A Pathol Anat Histopathol. 1983;401:45-55. [PubMed] [Cited in This Article: ] |
| 3. | Dunbar KB, Ayyar B, Spechler SJ, Genta R, Melton S. Clinical, endoscopic and histological features of patients with lymphocytic esophagitis compared to patients with GERD. Poster presentation at the 45th Annual Digestive Disease Week; Chicago, IL, 2014. Abstract Mo1846. Available from: http://www.gastrojournal.org/article/S0016-5085(14)62437-9/pdf. [Cited in This Article: ] |
| 4. | Basseri B, Vasiliauskas EA, Chan O, Wang HL, Basseri RJ, Pimentel M, Soffer E, Conklin JL. Evaluation of peripapillary lymphocytosis and lymphocytic esophagitis in adult inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:505-511. [PubMed] [Cited in This Article: ] |
| 5. | Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut. 2012;61:1108-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Purdy JK, Appelman HD, Golembeski CP, McKenna BJ. Lymphocytic esophagitis: a chronic or recurring pattern of esophagitis resembling allergic contact dermatitis. Am J Clin Pathol. 2008;130:508-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 7. | Cohen S, Saxena A, Waljee AK, Piraka C, Purdy J, Appelman H, McKenna B, Elmunzer BJ, Singal AG. Lymphocytic esophagitis: a diagnosis of increasing frequency. J Clin Gastroenterol. 2012;46:828-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 8. | Xue Y, Suriawinata A, Liu X, Li Z, Gabbard S, Rothstein R, Lacy B, Lisovsky M. Lymphocytic Esophagitis With CD4 T-cell-predominant Intraepithelial Lymphocytes and Primary Esophageal Motility Abnormalities: A Potential Novel Clinicopathologic Entity. Am J Surg Pathol. 2015;39:1558-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Basseri B, Levy M, Wang HL, Shaye OA, Pimentel M, Soffer EE, Conklin JL. Redefining the role of lymphocytes in gastroesophageal reflux disease and eosinophilic esophagitis. Dis Esophagus. 2010;23:368-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ronkainen J, Walker MM, Aro P, Storskrubb T, Talley NJ, Ahmed ZB, Salter V, Vieth M, Agréus L. Lymphocytic oesophagitis, a condition in search of a disease? Gut. 2012;61:1776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Conner JR, Sanchez CA, Reid BJ, Vaughan TL, Blount PL, Wang HH, Odze RD, Srivastava A. Lymphocytic esophagitis in Barrett’s esophagus: correlation with patient symptoms and risk factors. Mod Pathol. 2014;27:169A. [Cited in This Article: ] |
| 12. | Ebach DR, Vanderheyden AD, Ellison JM, Jensen CS. Lymphocytic esophagitis: a possible manifestation of pediatric upper gastrointestinal Crohn’s disease. Inflamm Bowel Dis. 2011;17:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 13. | Sutton LM, Heintz DD, Patel AS, Weinberg AG. Lymphocytic esophagitis in children. Inflamm Bowel Dis. 2014;20:1324-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 14. | Tanaka K, Rubio CA, Dlugosz A, Truskaite K, Befrits R, Lindberg G, Schmidt PT. Narrow-band imaging magnifying endoscopy in adult patients with eosinophilic esophagitis/esophageal eosinophilia and lymphocytic esophagitis. Gastrointest Endosc. 2013;78:659-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Mandaliya R, Dimarino AJ, Cohen S. Lymphocytic esophagitis mimicking eosinophilic esophagitis. Ann Gastroenterol. 2012;25:355-357. [PubMed] [Cited in This Article: ] |
| 16. | Kasirye Y, John A, Rall C, Resnick J. Lymphocytic esophagitis presenting as chronic dysphagia. Clin Med Res. 2012;10:83-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Chande N, McDonald JW, Macdonald JK. Interventions for treating lymphocytic colitis. Cochrane Database Syst Rev. 2008;CD006096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, Akers R, Cohen MB, Collins MH, Assa’ad AH. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 19. | Miehlke S, Madisch A, Karimi D, Wonschik S, Kuhlisch E, Beckmann R, Morgner A, Mueller R, Greinwald R, Seitz G. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology. 2009;136:2092-2100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Hendy PJ, Wong DS, Florin TH. Spontaneous oesophageal perforation: an unreported complication of lymphocytic oesophagitis. Gut. 2013;62:1668-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |