Published online Jun 16, 2015. doi: 10.12998/wjcc.v3.i6.484
Peer-review started: January 20, 2015
First decision: January 30, 2015
Revised: February 28, 2015
Accepted: March 30, 2015
Article in press: April 2, 2015
Published online: June 16, 2015
Glucocorticoids remain the cornerstone of medical therapy in giant cell arteritis (GCA) and should be started immediately to prevent severe consequences of the disease, such as blindness. However, glucocorticoid therapy leads to significant toxicity in over 80% of the patients. Various steroid-sparing agents have been tried, but robust scientific evidence of their efficacy and safety is still lacking. Tocilizumab, a monoclonal IL-6 receptor blocker, has shown promising results in a number of case series and is now being tested in a multi-centre randomized controlled trial. Other targeted treatments, such as the use of abatacept, are also now under investigation in GCA. The need for surgical treatment is rare and should ideally be performed in a quiescent phase of the disease. Not all patients follow the same course, but there are no valid biomarkers to assess therapy response. Monitoring of disease progress still relies on assessing clinical features and measuring inflammatory markers (C-reactive protein and erythrocyte sedimentation rate). Imaging techniques (e.g., ultrasound) are clearly important screening tools for aortic aneurysms and assessing patients with large-vessel involvement, but may also have an important role as biomarkers of disease activity over time or in response to therapy. Although GCA is the most common form of primary vasculitis, the optimal strategies for treatment and monitoring remain uncertain.
Core tip: Giant cell arteritis (GCA) is the most common form of primary systemic vasculitis. Treatment with high doses of glucocorticoids should be initiated as early as possible to prevent ischaemic manifestations, such as blindness (occurring in up to 20%). However, glucocorticoid therapy leads to significant toxicity in over 80% of the patients. Various steroid-sparing agents have been tried, but robust scientific evidence of their efficacy and safety is still lacking. Not all patients follow the same course, but there are no valid biomarkers to assess therapy response. The authors review the optimal strategies for treatment and monitoring of patients with GCA.
- Citation: Ponte C, Rodrigues AF, O’Neill L, Luqmani RA. Giant cell arteritis: Current treatment and management. World J Clin Cases 2015; 3(6): 484-494
- URL: https://www.wjgnet.com/2307-8960/full/v3/i6/484.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i6.484
Giant cell arteritis (GCA), also called temporal arteritis, is the most common form of primary systemic vasculitis, with an overall incidence of 15-25 per 100000 per year[1]. It affects large and medium-sized blood vessels with a predisposition for the cranial branches derived from the carotid artery; in approximately 50% of cases, the aorta and its major branches may also be involved[2,3]. It typically affects individuals aged above 50 years and is two to four times more common in women than men[4,5]. Polymyalgia rheumatica (PMR) can be present in up to 50% of the cases, beginning before, simultaneously or after the clinical manifestations of GCA, suggesting they are different spectrums of the same disease process[6].
Due to the intense myointimal proliferation and vessel occlusion, which in up to 20% of the cases may lead to blindness (usually permanent), GCA is considered a medical emergency[7,8]. Treatment with high doses of glucocorticoids should be initiated as early as possible to rapidly control inflammatory symptoms and prevent ischaemic manifestations, such as jaw claudication, visual loss and stroke. However, the burden of high-dose glucocorticoids is considerable, especially in the elderly, with over 80% of the patients experiencing significant treatment related side-effects[9]. Proven et al[10] have reported a high number of major adverse advents related to long-term glucocorticoid use in GCA: posterior subcapsular cataract (41%), bone fractures (38%), infections (31%), hypertension (22%), diabetes mellitus (9%) and gastrointestinal bleeding (4%).
Moreover, the optimal duration and doses of glucocorticoid treatment varies from patient to patient, as well as the need to add other immunosuppressant agents to control disease activity and reduce glucocorticoid toxicity.
The key issues in managing GCA after its diagnosis are prompt institution of correct therapy; recognition and amelioration of the adverse events related to immunosuppressant medications; and rapid identification of disease activity and flares.
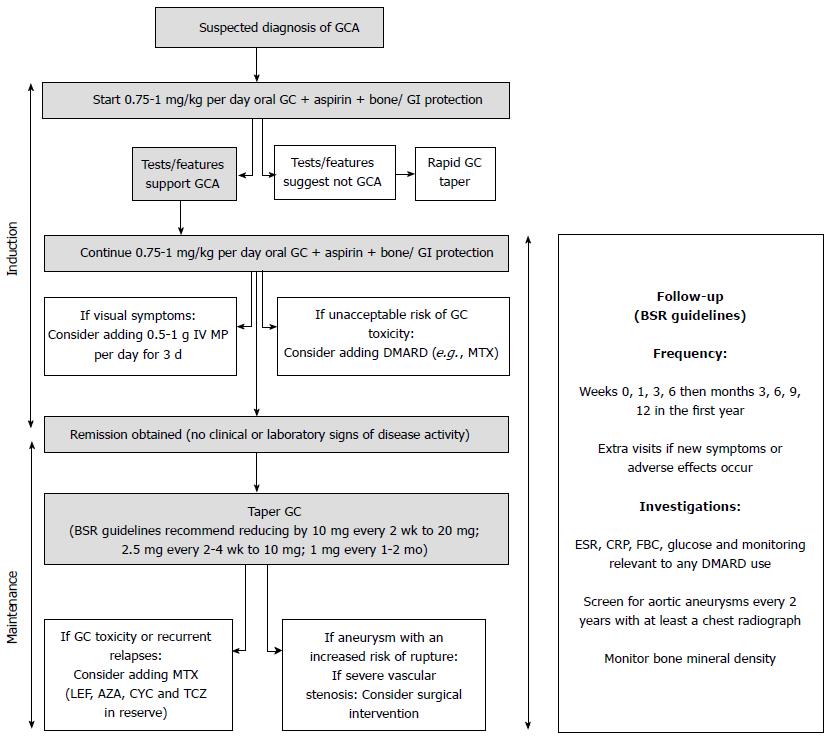
Our purpose is to review the current therapeutic options, guidelines and clinical trials in GCA, as well as to discuss follow-up strategies and potential biomarkers for this condition (Figure 1).
In GCA medical treatment is required for induction and maintenance of remission.
Glucocorticoids: Glucocorticoids remain the cornerstone of treatment in GCA since their discovery in the 1950s[11]. They should be prescribed immediately after the diagnosis of GCA is suspected, and in most cases are able to provide complete symptomatic relief within 24-48 h[12].
Despite their importance, there are no clinical trials comparing different glucocorticoid dosing regimens. Most clinicians will base their practice on personal experience and on the European League Against Rheumatism (EULAR) and British Society for Rheumatology (BSR) guidelines[9,13]. These guidelines were based on an extensive literature review of the available evidence, including published data from randomised controlled trials, and full consensus by expert opinion.
The EULAR guidelines recommend 1 mo of high-dose glucocorticoid therapy (prednisolone 1 mg/kg per day, maximum 60 mg/d) for induction of remission and pulsed intravenous methylprednisolone for patients with early onset of visual symptoms (dose not specified). The BSR guidelines advise prednisolone 40 to 60 mg (at least 0.75 mg/kg) daily until the resolution of symptoms and laboratory abnormalities for patients with uncomplicated GCA (without visual loss or jaw claudication); 500 mg to 1 g of intravenous methylprednisolone per day for 3 d for patients with visual loss or a history of amaurosis fugax; and at least 60 mg prednisolone daily for patients with established visual loss. Daily dosing is more effective than alternate day dosing, but single or divided daily doses have shown comparable results[14].
Induction treatment with high-dose pulsed intravenous (IV) methylprednisolone (15 mg/kg for 3 consecutive days followed by oral prednisone dose of 40 mg/d) has been suggested for all patients with GCA to allow faster tapering and a lower cumulative steroid dose in a double-blind, placebo-controlled, randomized trial involving 27 patients[15]. Although a greater number of patients were able to reduce their oral prednisolone to 5 mg/d by week 36 with this regimen, the small sample size was not sufficient to draw conclusions regarding the differences in steroid-related adverse events; therefore, these results should not be generalized. Larger studies are needed to address this issue[16].
The most frequent type of eye involvement in GCA is anterior ischemic optic neuropathy[17], but other visual manifestations can also occur (Table 1). A degree of controversy exists regarding induction treatment by either high-dose pulsed IV methylprednisolone or oral prednisolone in GCA patients with visual symptoms. There are patients who despite being given high doses of IV methylprednisolone still develop visual loss. This might be explained by the latent period of up to 5 d between starting treatment and controlling the arteritic process in the wall of the posterior ciliary arteries; as well as by the decreased perfusion pressure in the vascular bed of the optic nerve head that makes it very prone to ischaemia due to any minor fall of the systemic blood pressure[18]. Another proposed explanation is that glucocorticoids may have a pro-coagulant effect by enhancing platelet activation[19], but this needs further confirmation. Although conflicting data exist[18,20-22], most clinicians, especially ophthalmologists, will prescribe IV steroids when presented with a patient with GCA with acute visual impairment.
| Anterior ischemic optic neuropathy |
| Posterior ischemic optic neuropathy |
| Arterial occlusion (central retinal artery, branch retinal artery or cilioretinal vessels) |
| Amaurosis fugax |
| “Cotton-wool spots” (microinfarcts of the retinal nerve fiber layer) |
| Diplopia (involvement of muscles, cranial nerves, or brainstem) |
| Ocular ischaemic syndrome (hypotension, ischaemic iritis) |
Other immunosuppressive therapy: The key to successful induction therapy is to initiate glucocorticoids as quickly as possible, given their rapid onset of action. Other immunosuppressive treatments prescribed at presentation of the disease have been tried, particularly with the aim of allowing a faster withdrawal of steroids or help controlling severe manifestations of the disease; however, results have been conflicting and generally disappointing[23-25].
Nevertheless, when a patient has an unacceptable high-risk of glucocorticoid-related side effects, such as concomitant severe osteoporosis and poorly controlled high blood pressure or diabetes mellitus, it might be feasible to add another immunosuppressive (e.g., methotrexate[26]) at the onset of the disease to allow a safer and faster tapering of glucocorticoids.
Glucocorticoids: Glucocorticoid reduction should be considered only in the absence of clinical symptoms, signs and laboratory abnormalities suggestive of active disease. The tapering regimen is variable; it is highly dependent on the clinician’s personal experience, disease severity and response to treatment, use of concomitant immunosuppressive agents, the patient’s compliance and the occurrence of steroid related toxicity. Nevertheless, the BSR[13] has proposed a standard tapering scheme after 1 mo of treatment: reducing by 10 mg of prednisolone every 2 wk to 20 mg, then another 2.5 mg every 2-4 wk to 10 mg, followed by a decrease of 1 mg every 1-2 mo.
During steroid tapering, flares occur in up to 50% of patients, requiring escalation of glucocorticoids and a more prolonged treatment course. An increase of 5-10 mg/d of prednisolone is usually sufficient to treat a common relapse; however, in the presence of ocular or neurological symptoms an increase to the original induction dose (0.75-1 mg/kg per day) should be considered.
There are no reliable predictors to determine treatment duration. Hernández-Rodríguez et al[27] have suggested that the intensity of the systemic inflammatory response at baseline may influence the number of disease relapses and time needed to safely withdrawal steroids. Different studies have shown different treatment durations[28-30], but typically 2 to 3 years are necessary for the patients to be weaned off glucocorticoids without any clinical features of active disease. In some cases, particularly when the disease is recurrent or there is secondary adrenal insufficiency, the treatment duration may exceed 5 years[31,9].
Other immunosuppressive therapy: The search for an effective disease-modifying agent for the treatment of GCA has proven elusive. Few clinical trials have been performed; the number of patients enrolled is limited and the duration of follow up is often short. A number of drugs have been studied, with disappointing results to date.
However, given the significant burden of morbidity associated with long term glucocorticoid treatment, current BSR guidelines for the management of GCA recommend consideration of the early introduction of methotrexate or alternative immunosuppressant therapy following a relapse[13] and EULAR guidelines for the management of large vessel vasculitis recommend that an immunosuppressant agent should be considered for use in large vessel vasculitis as adjunctive therapy[9].
Of the limited available evidence, methotrexate may be of benefit in the management of GCA. Three prospective randomised double blind placebo controlled trials have addressed this issue. In 2001, Spiera et al[23] randomised 21 patients with newly diagnosed GCA to high dose corticosteroids plus methotrexate (up to 20 mg once weekly) or placebo. At study completion there were no differences between the methotrexate and placebo groups with regard to cumulative steroid dose, relapse or adverse events[24]. In a similar study, in the same year, Jover et al[26] randomised 42 patients to high dose corticosteroid and methotrexate (10 mg per week) or placebo. Significant differences were reported in terms of reduction in relapse (one relapse P = 0.02, multiple relapses P = 0.004) and lower mean cumulative steroid dose (P = 0.0009) for the methotrexate treated group. Adverse events were similar in the two groups. In 2002, Hoffman et al[32] randomised 98 patients with newly diagnosed GCA to glucocorticoids plus either methotrexate (up to a maximum of 15 mg once weekly) or placebo. No differences were observed in cumulative steroid exposure or in the number of adverse events between the methotrexate and placebo treated groups. One patient in the methotrexate treated group relapsed vs five patients in the placebo group. A subsequent meta-analysis of individual patient data from these three trials demonstrated a modest reduction in relapse and glucocorticoid exposure in the methotrexate treated groups. However, adverse events remained similar between the groups[33].
Azathioprine is often considered as a potential steroid- sparing agent in GCA. Evidence supporting its use is limited. Only one double blind randomised placebo controlled trial was performed, which included 31 patients with GCA or PMR, or both (not differentiated) randomly assigned in a double blind fashion to either a standard glucocorticoid treatment schedule plus placebo or standard treatment plus azathioprine (150 mg once daily). At week 52 the treatment arm had a statistically significant reduction in steroid requirements (P < 0.05). However, the maintenance steroid dose was low in both arms at week 52, and the differences observed (1.9 ± 0.84 mg vs 4.2 ± 0.58 mg) while statistically significant are in practical terms of limited clinical significance[34].
Two small cases series have suggested that leflunomide has a steroid sparing effect in patients with PMR and GCA[35,36]. Adizie et al[36] demonstrated the efficacy of leflunomide in patients with difficult to treat disease. However, these results require replication in prospective randomised placebo controlled trials.
A number of retrospective studies have looked at cyclophosphamide use in patients with GCA, particularly in those who were steroid resistant, dependent or toxic and who had failed either methotrexate or azathioprine. Overall a significant sustained response was demonstrated (in up to 80%), with lowering of steroid doses. However, substantial treatment related adverse events observed limits it routine use[37-40].
Neither hydroxychloroquine nor cyclosporine have demonstrated any benefit in clinical trials in the management of GCA[41-43].
TNF-α is upregulated in GCA with elevated serum levels in active disease and increased expression of TNF-α in the temporal artery wall of patients with GCA[44]. However TNF-α inhibition in GCA has proven disappointing. Three randomised double blind placebo controlled trials of TNF-α inhibitors (infliximab, adalimumab and etanercept) have failed to show promise in the treatment of patients with GCA. Neither the infliximab nor the adalimumab studies met their primary endpoints and the infliximab trial was stopped prematurely following the interim analysis[25,45]. Nevertheless, patients on etanercept did have a statistically significant lower cumulative steroid dose after 1 year; however, given that only 17 patients in total were enrolled in this study, firm conclusions cannot be made[46].
More recently, treatment with tocilizumab, a monoclonal IL-6 receptor blocker, has shown potential in a number of case studies and case series in the treatment of patients with PMR and GCA in terms of improvement of clinical symptoms and reduction in the acute phase response[47-50]. GiACTA is a multicentre, randomised, double-blind, placebo controlled trial designed to test the ability of tocilizumab to maintain disease remission in GCA and is currently ongoing[51].
Antiplatelet agents: The use of antiplatelet agents in GCA is controversial. There are no randomised controlled trials that have evaluated the use of aspirin as an adjuvant treatment in GCA. However, in addition to its antiplatelet effects, aspirin may have a disease modifying effect in GCA. One of the signature cytokines driving vascular inflammation in GCA is Interferon gamma (IFNγ). IFNγ is produced by Th1 cells and its production is relatively steroid resistant with very high doses of glucocorticoid required for effective suppression.
Using a SCID mouse chimera model, Weyand et al[52] have demonstrated that IFNγ production by T cells was suppressed by high dose aspirin. Nesher et al[53] in a retrospective review of 175 patients with GCA in 2004 found a significantly increased risk of ischaemic events in patients who were not on aspirin prior their diagnosis of GCA (29% vs 8%). Similar results were reported by Lee et al[54] with 16% of patients who were on aspirin at the time of their diagnosis having an ischaemic event vs 48% of those who were not on aspirin. Other studies have not demonstrated any clinical benefit from the use of aspirin[55,56]. Some of the discrepant findings may be explained by a higher burden of ischaemic heart disease in some cohorts, which is of itself associated with a higher risk of developing a subsequent ischaemic event. Nevertheless, the use of low-dose aspirin (75-150 mg/d) is routinely recommended for patients with GCA in the absence of contraindications[13].
Statins: Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, the most powerful class of lipid lowering drugs to date, widely used in medical practice. Apart from their lipid lowering effect, additional pleotropic effects have been discovered, which include anti-inflammatory and immunomodulatory properties. Statins are capable of the following actions: suppressing the expression of major histocompatibility complex class II antigen induced by IFNγ in various cells; decreasing T-cell activation and proliferation; down-regulating endothelial adhesion molecules; and reducing circulating inflammatory molecules and cytokines such as IL-6, IL-8, IL-1β, TNF-α, as well as acute phase proteins (CRP). Moreover they restore endothelial cell function and decrease muscle cell proliferation in the vessel wall, which in turn prevents intimal hyperplasia[57,58]. Given the pathophysiology of the disease, statins could influence the inflammatory process in GCA, since some of the inflammatory pathways may be shared with atherosclerosis. Narváez and colleagues conducted a retrospective follow-up study with 121 patients with GCA treated with or without statins, which found no significant benefit from their use[59]. By contrast, in another retrospective study of 594 patients (GCA and controls), patients receiving statins were less likely to develop GCA; however, these drugs did not appear to modify the clinical presentation or disease course in patients who actually developed GCA[60]. To date there are no formal recommendations on the use of statins in patients with GCA.
Bone protection: The treatment of GCA requires both long-term and high dose glucocorticoid therapy. Oral glucocorticoid treatment with the equivalent of > 5 mg prednisone daily can lead to a reduction in bone mineral density and a rapid dose-dependent increase in the risk of fracture[61,62]. Calcium in isolation appears to have little effect in preventing bone loss in patients starting glucocorticoids[63] although when combined with vitamin D, it is an appropriate adjunctive treatment[64]. Bisphosphonates are indicated in accordance with local guidelines. For example, the BSR advise weekly bisphosphonate therapy for all patients with GCA[13], but American College of Rheumatology recommends a stratified approach according to the FRAX score[65].
Gastrointestinal protection: Given the high doses and long term duration of glucocorticoid therapy in patients with GCA, gastrointestinal protection is recommended with proton pump inhibitors, especially if concomitant risk factors are present such as NSAID use, and older age[13,66]. However, in clinical practice it is often advisable to discontinue NSAID use (apart from low dose aspirin) whilst the patient is receiving glucocorticoids.
Advances in immunology have revealed that a number of molecules are important modulators in the pathophysiology of GCA. Patients with GCA, refractory to glucocorticoids, have a higher expression of pro-inflammatory cytokines such as s IL-1β, TNF-α, and IL-6. Mice lacking the interleukin 1 receptor antagonist (IL-1ra) gene developed large vessel vasculitis[67], suggesting that IL-1 inhibition could be a therapeutic option in patients with GCA. Ly et al[68] have reported three patients with GCA, refractory to glucocorticoids, who were successfully treated with anakinra, showing improvement in clinical and/or inflammatory markers (two patients also showed radiologic improvement). However, randomized control trials are needed to determine the true efficacy and safety of this drug. Activated T-cells are believed to have a critical role in the development of large-vessel vasculitis. Abatacept, a signal modulator of T-cell activation, is being evaluated in a randomized study of patients with active GCA or Takayasu’s Arteritis[69].
Extracranial involvement in GCA, or large-vessel GCA (LV-GCA), has been described in 30%-80% of cases, varying according to the imaging modality performed[70,71]. However, most patients with LV-GCA improve with medical treatment alone, making the need for surgical interventions uncommon.
The risk of aortic aneurysm is higher in patients with GCA when comparing with the general population (twofold increased risk in the United Kingdom[72]), and the aneurysms are more likely to occur late in the disease course. Given there are no validated guidelines on surgical repair of aneurysms in patients with GCA, most strategies are based on the recommendations of atherosclerosis-related aneurysms. Surgical intervention should be considered in case of symptomatic aneurysm; ascending aorta aneurysm >5 cm in diameter; descending aorta aneurysm > 6 cm; abdominal aorta aneurysm > 5.5 cm; and an aneurysm which has grown > 0.5 cm within a 6 mo period[73].
In addition, revascularization procedures (e.g., angioplasty, stenting or bypass surgery) due to artery stenosis are rarely required. Although narrowing of important arteries, such as the subclavian artery, may compromise distal tissue viability, the development of extensive collateral circulation over time is usually sufficient to maintain adequate tissue viability, even when ischaemic symptoms, such as limb claudication or loss of large vessel pulses, are observed. There have been some case reports of successful revascularization surgery, but with common restenosis[74-76]. When necessary, surgical treatment should be performed in the quiescent phase of the disease and in experienced centres[9].
Aortic structural damage in GCA is associated with a trend towards increased mortality (of any cause)[77]; however, the comparison between surgical outcomes in GCA and other causes of aortic disease has not been evaluated.
Not all patients with GCA respond to therapy in the same way, but there are no valid biomarkers to assess treatment response. Several potential molecular and imaging biomarkers have been investigated.
Changes in the conventional inflammatory markers (CRP and ESR) do not consistently reflect disease activity[78]; however, they are still the laboratory tests used routinely to monitor the effects of therapy. Serum levels of IL-6 have been found to be more sensitive than ESR for indicating disease activity in untreated and treated patients with GCA[79]. Additionally, circulating pentraxin 3 (PTX3) and vascular endothelial growth factor (VEGF) levels have been recognized to be significantly increased in patients with very recent optic nerve ischaemia[80], with VEGF levels responding well to treatment[81]. Antibodies against ferritin have also been suggested as potential activity markers for GCA, particularly in patients without cranial artery involvement[82]. However, further studies with larger series are warrant to understand the potential role of these serum markers in the assessment of GCA.
Imaging techniques, especially for patients with extracranial involvement, have an important role in monitoring patients with GCA.
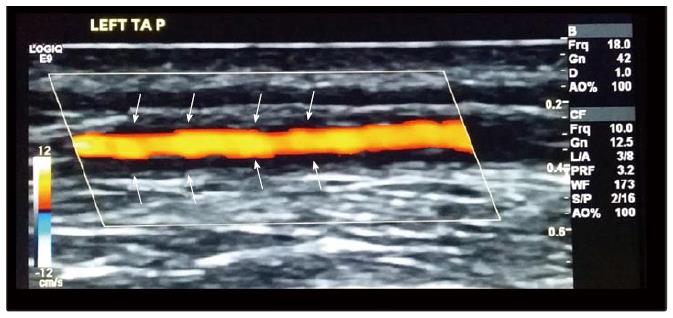
Ultrasound: Three meta-analyses have reported the high value and validity of ultrasound in diagnosing GCA[83-85], and we have recently completed patient recruitment for a large multicentre study looking at ultrasound as a diagnostic tool for GCA - TABUL study (Temporal Artery Biopsy vs ULtrasound in diagnosis of GCA)[86]; however, the role of ultrasound as a measure of disease activity is still unclear. In small case series and case reports, abnormal ultrasound appearances have been reported to resolve within 2 d of starting glucocorticoids[87] or alternatively, to persist for 11 wk despite treatment[88], allowing correlation of imaging changes with clinical response. In the TABUL study, we performed a cross-sectional analysis of 131 patients with GCA and positive ultrasound halo (dark area around arterial wall); the size of the halo was found to be smaller in patients who had received more days of glucocorticoid treatment, as well as correlating with the presence of ischaemic symptoms, supporting the early use of ultrasound as a potential prognostic marker and monitoring tool[89]. In addition, Czihal et al[90] documented that the clinical pattern of patients with extracranial GCA and cranial GCA, all identified by ultrasound, substantially differs, with visual impairment inversely correlated to the frequency of extracranial GCA (Figure 2).
Magnetic resonance imaging/magnetic resonance angiography: Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) can demonstrate the presence of increased wall thickness, oedema; mural contrast enhancement is highly suggestive of vascular inflammation. These imaging modalities can be used in patients with GCA, not only to verify extra-cranial involvement, but also to evaluate temporal arteries. High-resolution MRI of the cranium has been reported to detect biopsy-positive GCA with high sensitivity[91,92], but future research is needed to validate this technique for diagnosis of cranial GCA.
There are still controversies regarding the use of MRI/MRA to monitor patients with extracranial GCA. Although it has great value for assessing aortitis and potential associated aneurysms and stenoses, MRI has failed to correlate well with clinical measures of disease activity[93-95]. In addition, false-positives may occur due to increased mural contrast enhancement as a result of vascular remodelling.
Because MRI/MRA are not invasive and do not involve ionizing radiation, they are widely used for monitoring GCA, despite their limitations.
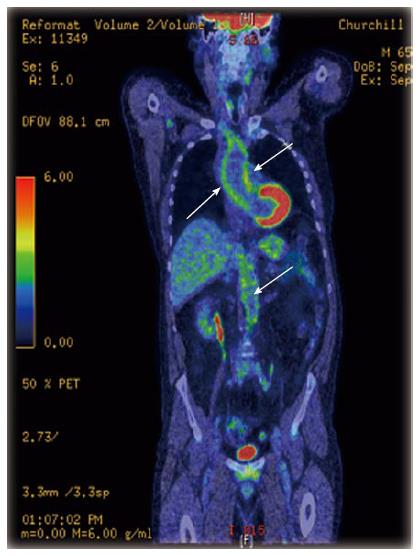
18F-Fluorodeoxyglucose positron emission tomography: 18F-Fluorodeoxyglucose positron emission tomography (18F-FDG PET) is a functional imaging technique which is very sensitive for diagnosing inflammatory changes in vessels with a diameter greater than 4 mm[96] (Figure 3). When compared to MRI, it appears to be more sensitive in detecting early vascular inflammation and correlates better with changes in disease activity over time[93,97,98]. However, like MRI, the relationship between PET findings and the prediction of disease activity or relapse is also inconsistent[93,97], which can be partly explained by the lack of standardized and validated criteria for disease activity in large vessel vasculitis.
The main limitations of this modality are: the lack of a uniform definition of vascular inflammation based on the FDG uptake; inability to provide information regarding wall structure or luminal flow; inability to visualize the temporal arteries; the use of large amounts of ionizing radiation (typically 15-20MSv per scan); and the limited access to this technique by most health institutions.
Computerised tomography/computerised tomography angiography: In GCA the main role of computerised tomography/computerised tomography angiography is to assess large vessel involvement or late complications of the disease, such as vessel stenosis, occlusions or aneurysms. This imaging modality has been proposed to evaluate response to treatment in Takayasu patients[99], but to our knowledge the same has not been considered in GCA.
Chest radiograph: The main use for regular chest radiographs in patients with GCA is to monitor for potential aortic aneurysms. Although the BSR recommends its performance at least every 2 years[100], we have recently demonstrated that the risk of aneurysm development as a result of GCA is actually quite low[72]; if an aneurysm is suspected, more advanced imaging modalities (described above) should additionally be obtained in order to confirm the diagnosis and evaluate possible treatment measures.
The frequency for patient follow-up should be guided by their clinical manifestations and adverse advents. The BSR recommends follow-up during the first year at weeks 0, 1, 3, 6, then months 3, 6, 9, 12 and if new symptoms or adverse effects occur[13]. At each visit bloods tests for ESR, CRP, full blood count, glucose as well as monitoring relevant to any DMARD use should be performed. In practice, this is often not achievable in secondary care and therefore involvement by the patient’s primary care physician is usually required. Screening for aortic aneurysms and monitoring bone density may be indicated in high risk individuals (e.g., older male smokers have the highest risk of aortic aneurysm).
Despite the severe consequences of untreated GCA, such as blindness, there is no consensus on the optimal therapeutic strategies for this disease. Early initiation of glucocorticoid treatment is essential; however, the value of additional steroid-sparing synthetic or biologic agents to avoid the common glucocorticoid adverse effects or obtain quicker remission is still uncertain. We do not know how many and which synthetic DMARDs should be used before considering a biologic agent, because there are no valid and specific biomarkers to assess therapy response in GCA. Potential biomarkers which require further validation include circulating levels of IL-6 and VEGF as well as imaging assessments, such as ultrasound. Further investigation is needed to establish the role of these biomarkers, which can assist in the development and testing of innovative targeted therapies whose effects can be more reliably measured.
P- Reviewer: Kim ST, Trohman RG, Yokoyama Y S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Schmidt WA. Role of ultrasound in the understanding and management of vasculitis. Ther Adv Musculoskelet Dis. 2014;6:39-47. [PubMed] [Cited in This Article: ] |
| 3. | Jennette JC, Falk RJ. Nosology of primary vasculitis. Curr Opin Rheumatol. 2007;19:10-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Warrington KJ, Matteson EL. Management guidelines and outcome measures in giant cell arteritis (GCA). Clin Exp Rheumatol. 2007;25:137-141. [PubMed] [Cited in This Article: ] |
| 5. | Gonzalez-Gay MA, Martinez-Dubois C, Agudo M, Pompei O, Blanco R, Llorca J. Giant cell arteritis: epidemiology, diagnosis, and management. Curr Rheumatol Rep. 2010;12:436-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372:234-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 481] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 7. | Yates M, Loke YK, Watts RA, MacGregor AJ. Prednisolone combined with adjunctive immunosuppression is not superior to prednisolone alone in terms of efficacy and safety in giant cell arteritis: meta-analysis. Clin Rheumatol. 2014;33:227-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ghosh P, Borg FA, Dasgupta B. Current understanding and management of giant cell arteritis and polymyalgia rheumatica. Expert Rev Clin Immunol. 2010;6:913-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallenberg CG. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68:318-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 399] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Birkhead NC, Wagener HP, Shick RM. Treatment of temporal arteritis with adrenal corticosteroids; results in fifty-five cases in which lesion was proved at biopsy. J Am Med Assoc. 1957;163:821-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 117] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Chatterjee S, Flamm SD, Tan CD, Rodriguez ER. Clinical diagnosis and management of large vessel vasculitis: giant cell arteritis. Curr Cardiol Rep. 2014;16:498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Dasgupta B, Borg FA, Hassan N, Alexander L, Barraclough K, Bourke B, Fulcher J, Hollywood J, Hutchings A, James P. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford). 2010;49:1594-1597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 14. | Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med. 1975;82:613-618. [PubMed] [Cited in This Article: ] |
| 15. | Mazlumzadeh M, Hunder GG, Easley KA, Calamia KT, Matteson EL, Griffing WL, Younge BR, Weyand CM, Goronzy JJ. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310-3318. [PubMed] [Cited in This Article: ] |
| 16. | Langford CA, Hoffman GS. Should induction therapy with high-dose glucocorticoids be the standard treatment for all patients with giant cell arteritis? Nat Clin Pract Rheumatol. 2007;3:132-133. [PubMed] [Cited in This Article: ] |
| 17. | Ness T, Bley TA, Schmidt WA, Lamprecht P. The diagnosis and treatment of giant cell arteritis. Dtsch Arztebl Int. 2013;110:376-385; quiz 386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology. 2003;110:1204-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Conn DL, Tompkins RB, Nichols WL. Glucocorticoids in the management of vasculitis--a double edged sword? J Rheumatol. 1988;15:1181-1183. [PubMed] [Cited in This Article: ] |
| 20. | Chevalet P, Barrier JH, Pottier P, Magadur-Joly G, Pottier MA, Hamidou M, Planchon B, El Kouri D, Connan L, Dupond JL. A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: a one year followup study of 164 patients. J Rheumatol. 2000;27:1484-1491. [PubMed] [Cited in This Article: ] |
| 21. | Chan CC, Paine M, O’Day J. Steroid management in giant cell arteritis. Br J Ophthalmol. 2001;85:1061-1064. [PubMed] [Cited in This Article: ] |
| 22. | González-Gay MA, Blanco R, Rodríguez-Valverde V, Martínez-Taboada VM, Delgado-Rodriguez M, Figueroa M, Uriarte E. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: predictors and response to treatment. Arthritis Rheum. 1998;41:1497-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 23. | Spiera RF, Mitnick HJ, Kupersmith M, Richmond M, Spiera H, Peterson MG, Paget SA. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol. 2001;19:495-501. [PubMed] [Cited in This Article: ] |
| 24. | Rüegg S, Engelter S, Jeanneret C, Hetzel A, Probst A, Steck AJ, Lyrer P. Bilateral vertebral artery occlusion resulting from giant cell arteritis: report of 3 cases and review of the literature. Medicine (Baltimore). 2003;82:1-12. [PubMed] [Cited in This Article: ] |
| 25. | Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, Salvarani C, Xu W, Visvanathan S, Rahman MU. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621-630. [PubMed] [Cited in This Article: ] |
| 26. | Jover JA, Hernández-García C, Morado IC, Vargas E, Bañares A, Fernández-Gutiérrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:106-114. [PubMed] [Cited in This Article: ] |
| 27. | Hernández-Rodríguez J, García-Martínez A, Casademont J, Filella X, Esteban MJ, López-Soto A, Fernández-Solà J, Urbano-Márquez A, Grau JM, Cid MC. A strong initial systemic inflammatory response is associated with higher corticosteroid requirements and longer duration of therapy in patients with giant-cell arteritis. Arthritis Rheum. 2002;47:29-35. [PubMed] [Cited in This Article: ] |
| 28. | Hachulla E, Boivin V, Pasturel-Michon U, Fauchais AL, Bouroz-Joly J, Perez-Cousin M, Hatron PY, Devulder B. Prognostic factors and long-term evolution in a cohort of 133 patients with giant cell arteritis. Clin Exp Rheumatol. 2001;19:171-176. [PubMed] [Cited in This Article: ] |
| 29. | Lundberg I, Hedfors E. Restricted dose and duration of corticosteroid treatment in patients with polymyalgia rheumatica and temporal arteritis. J Rheumatol. 1990;17:1340-1345. [PubMed] [Cited in This Article: ] |
| 30. | Andersson R, Malmvall BE, Bengtsson BA. Long-term corticosteroid treatment in giant cell arteritis. Acta Med Scand. 1986;220:465-469. [PubMed] [Cited in This Article: ] |
| 31. | Masson C. Therapeutic approach to giant cell arteritis. Joint Bone Spine. 2012;79:219-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, Cohen P, Calabrese LH, Dickler H, Merkel PA. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309-1318. [PubMed] [Cited in This Article: ] |
| 33. | Mahr AD, Jover JA, Spiera RF, Hernández-García C, Fernández-Gutiérrez B, Lavalley MP, Merkel PA. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789-2797. [PubMed] [Cited in This Article: ] |
| 34. | De Silva M, Hazleman BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis. 1986;45:136-138. [PubMed] [Cited in This Article: ] |
| 35. | Diamantopoulos AP, Hetland H, Myklebust G. Leflunomide as a corticosteroid-sparing agent in giant cell arteritis and polymyalgia rheumatica: a case series. Biomed Res Int. 2013;2013:120638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Adizie T, Christidis D, Dharmapaliah C, Borg F, Dasgupta B. Efficacy and tolerability of leflunomide in difficult-to-treat polymyalgia rheumatica and giant cell arteritis: a case series. Int J Clin Pract. 2012;66:906-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 37. | Henes JC, Mueller M, Pfannenberg C, Kanz L, Koetter I. Cyclophosphamide for large vessel vasculitis: assessment of response by PET/CT. Clin Exp Rheumatol. 2011;29:S43-S48. [PubMed] [Cited in This Article: ] |
| 38. | Loock J, Henes J, Kötter I, Witte T, Lamprecht P, Schirmer M, Gross WL. Treatment of refractory giant cell arteritis with cyclophosphamide: a retrospective analysis of 35 patients from three centres. Clin Exp Rheumatol. 2012;30:S70-S76. [PubMed] [Cited in This Article: ] |
| 39. | de Boysson H, Boutemy J, Creveuil C, Ollivier Y, Letellier P, Pagnoux C, Bienvenu B. Is there a place for cyclophosphamide in the treatment of giant-cell arteritis? A case series and systematic review. Semin Arthritis Rheum. 2013;43:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Fytili C, Bournia VK, Korkou C, Pentazos G, Kokkinos A. Multiple cranial nerve palsies in giant cell arteritis and response to cyclophosphamide: a case report and review of the literature. Rheumatol Int. 2015;35:773-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Sailler L, Lapeyre-Mestre M, Geffray L, Letellier P, Liozon E, De La Roque PM, Hamidou M. Adding Hydroxychloroquine To Prednisone Does Not Improve The Outcome In Giant Cell Arteritis: A Double Blind Randomized Controlled Trial. Arthritis Rheum. 2009;60:1972. [DOI] [Cited in This Article: ] |
| 42. | Schaufelberger C, Andersson R, Nordborg E. No additive effect of cyclosporin A compared with glucocorticoid treatment alone in giant cell arteritis: results of an open, controlled, randomized study. Br J Rheumatol. 1998;37:464-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Schaufelberger C, Möllby H, Uddhammar A, Bratt J, Nordborg E. No additional steroid-sparing effect of cyclosporine A in giant cell arteritis. Scand J Rheumatol. 2006;35:327-329. [PubMed] [Cited in This Article: ] |
| 44. | Visvanathan S, Rahman MU, Hoffman GS, Xu S, García-Martínez A, Segarra M, Lozano E, Espígol-Frigolé G, Hernández-Rodríguez J, Cid MC. Tissue and serum markers of inflammation during the follow-up of patients with giant-cell arteritis--a prospective longitudinal study. Rheumatology (Oxford). 2011;50:2061-2070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Seror R, Baron G, Hachulla E, Debandt M, Larroche C, Puéchal X, Maurier F, de Wazieres B, Quéméneur T, Ravaud P. Adalimumab for steroid sparing in patients with giant-cell arteritis: results of a multicentre randomised controlled trial. Ann Rheum Dis. 2014;73:2074-2081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 46. | Martínez-Taboada VM, Rodríguez-Valverde V, Carreño L, López-Longo J, Figueroa M, Belzunegui J, Mola EM, Bonilla G. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann Rheum Dis. 2008;67:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 47. | Macchioni P, Boiardi L, Catanoso M, Pulsatelli L, Pipitone N, Meliconi R, Salvarani C. Tocilizumab for polymyalgia rheumatica: report of two cases and review of the literature. Semin Arthritis Rheum. 2013;43:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Christidis D, Jain S, Das Gupta B. Successful use of tocilizumab in polymyalgic onset biopsy positive GCA with large vessel involvement. BMJ Case Rep. 2011;2011:pii: bcr0420114135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Vinit J, Bielefeld P, Muller G, Besancenot JF. Efficacy of tocilizumab in refractory giant cell arteritis. Joint Bone Spine. 2012;79:317-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Unizony S, Arias-Urdaneta L, Miloslavsky E, Arvikar S, Khosroshahi A, Keroack B, Stone JR, Stone JH. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken). 2012;64:1720-1729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 51. | Tocilizumab for Patients with Giant Cell Arteritis. ClinicalTrials.gov Identifier: NCT01450137. Available from: http://clinicaltrials.gov/ct2/show/NCT01450137. [Cited in This Article: ] |
| 52. | Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum. 2002;46:457-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick M. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum. 2004;50:1332-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 54. | Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum. 2006;54:3306-3309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 55. | Narváez J, Bernad B, Gómez-Vaquero C, García-Gómez C, Roig-Vilaseca D, Juanola X, Rodriguez-Moreno J, Nolla JM, Valverde J. Impact of antiplatelet therapy in the development of severe ischemic complications and in the outcome of patients with giant cell arteritis. Clin Exp Rheumatol. 2008;26:S57-S62. [PubMed] [Cited in This Article: ] |
| 56. | Salvarani C, Della Bella C, Cimino L, Macchioni P, Formisano D, Bajocchi G, Pipitone N, Catanoso MG, Restuccia G, Ghinoi A. Risk factors for severe cranial ischaemic events in an Italian population-based cohort of patients with giant cell arteritis. Rheumatology (Oxford). 2009;48:250-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Arnaud C, Mach F. Potential antiinflammatory and immunomodulatory effects of statins in rheumatologic therapy. Arthritis Rheum. 2006;54:390-392. [PubMed] [Cited in This Article: ] |
| 58. | Veillard NR, Mach F. Statins: the new aspirin? Cell Mol Life Sci. 2002;59:1771-1786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Narváez J, Bernad B, Nolla JM, Valverde J. Statin therapy does not seem to benefit giant cell arteritis. Semin Arthritis Rheum. 2007;36:322-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Schmidt J, Kermani TA, Muratore F, Crowson CS, Matteson EL, Warrington KJ. Statin use in giant cell arteritis: a retrospective study. J Rheumatol. 2013;40:910-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 766] [Cited by in F6Publishing: 753] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 62. | van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2006;79:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 63. | Amin S, Lavalley MP, Simms RW, Felson DT. The comparative efficacy of drug therapies used for the management of corticosteroid-induced osteoporosis: a meta-regression. J Bone Miner Res. 2002;17:1512-1526. [PubMed] [Cited in This Article: ] |
| 64. | Richy F, Ethgen O, Bruyere O, Reginster JY. Efficacy of alphacalcidol and calcitriol in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int. 2004;15:301-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, Curtis JR, Furst DE, McMahon M, Patkar NM. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010;62:1515-1526. [PubMed] [Cited in This Article: ] |
| 66. | Duru N, van der Goes MC, Jacobs JW, Andrews T, Boers M, Buttgereit F, Caeyers N, Cutolo M, Halliday S, Da Silva JA. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2013;72:1905-1913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 67. | Shepherd J, Nicklin MJ. Elastic-vessel arteritis in interleukin-1 receptor antagonist-deficient mice involves effector Th1 cells and requires interleukin-1 receptor. Circulation. 2005;111:3135-3140. [PubMed] [Cited in This Article: ] |
| 68. | Ly KH, Stirnemann J, Liozon E, Michel M, Fain O, Fauchais AL. Interleukin-1 blockade in refractory giant cell arteritis. Joint Bone Spine. 2014;81:76-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Abatacept for treating Adults With Giant Cell Arteritis and Takayasu’s Arteritis. ClinicalTrials.gov; Identifier: NCT00556439. Available from: http://clinicaltrials.gov/ct2/show/NCT00556439. [Cited in This Article: ] |
| 70. | Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55:131-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 411] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 71. | Diamantopoulos AP, Haugeberg G, Hetland H, Soldal DM, Bie R, Myklebust G. Diagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res (Hoboken). 2014;66:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 72. | Robson JC, Kiran A, Maskell J, Hutchings A, Arden N, Dasgupta B, Hamilton W, Emin A, Culliford D, Luqmani RA. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis. 2015;74:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 73. | Bongartz T, Matteson EL. Large-vessel involvement in giant cell arteritis. Curr Opin Rheumatol. 2006;18:10-17. [PubMed] [Cited in This Article: ] |
| 74. | Monte R, González-Gay MA, García-Porrúa C, López-Alvarez MJ, Pulpeiro JR. Successful response to angioplasty in a patient with upper limb ischaemia secondary to giant cell arteritis. Br J Rheumatol. 1998;37:344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Dellaripa PF, Eisenhauer AC. Bilateral percutaneous balloon angioplasty of the axillary arteries in a patient with giant cell arteritis and upper extremity ischemic symptoms not responsive to corticosteroids. J Rheumatol. 1998;25:1429-1433. [PubMed] [Cited in This Article: ] |
| 76. | Both M, Aries PM, Müller-Hülsbeck S, Jahnke T, Schäfer PJ, Gross WL, Heller M, Reuter M. Balloon angioplasty of arteries of the upper extremities in patients with extracranial giant-cell arteritis. Ann Rheum Dis. 2006;65:1124-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | García-Martínez A, Arguis P, Prieto-González S, Espígol-Frigolé G, Alba MA, Butjosa M, Tavera-Bahillo I, Hernández-Rodríguez J, Cid MC. Prospective long term follow-up of a cohort of patients with giant cell arteritis screened for aortic structural damage (aneurysm or dilatation). Ann Rheum Dis. 2014;73:1826-1832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Tse WY, Cockwell P, Savage CO. Assessment of disease activity in systemic vasculitis. Postgrad Med J. 1998;74:1-6. [PubMed] [Cited in This Article: ] |
| 79. | Weyand CM, Fulbright JW, Hunder GG, Evans JM, Goronzy JJ. Treatment of giant cell arteritis: interleukin-6 as a biologic marker of disease activity. Arthritis Rheum. 2000;43:1041-1048. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Baldini M, Maugeri N, Ramirez GA, Giacomassi C, Castiglioni A, Prieto-González S, Corbera-Bellalta M, Comite GD, Papa I, Dell’antonio G. Selective up-regulation of the soluble pattern-recognition receptor pentraxin 3 and of vascular endothelial growth factor in giant cell arteritis: relevance for recent optic nerve ischemia. Arthritis Rheum. 2012;64:854-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 81. | Morlet J, Mahoney D, Zarei A, Singh S, Manhas V, Sharma V, Sabokbar A, Luqmani RA. Serum vascular endothelial growth factor is selectively upregulated in patients with biopsy-proven giant cell arteritis. Rheumatology. 2014;53 Supple 1:i188. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 82. | Baerlecken NT, Linnemann A, Gross WL, Moosig F, Vazquez-Rodriguez TR, Gonzalez-Gay MA, Martin J, Kötter I, Henes JC, Melchers I. Association of ferritin autoantibodies with giant cell arteritis/polymyalgia rheumatica. Ann Rheum Dis. 2012;71:943-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 83. | Ball EL, Walsh SR, Tang TY, Gohil R, Clarke JM. Role of ultrasonography in the diagnosis of temporal arteritis. Br J Surg. 2010;97:1765-1771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 84. | Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11:44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 85. | Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med. 2005;142:359-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 86. | Temporal Artery Biopsy vs ULtrasound in Diagnosis of GCA (TABUL). ClinicalTrials.gov Identifier: NCT00974883. Available from: https://clinicaltrials.gov/ct2/show/NCT00974883. [Cited in This Article: ] |
| 87. | Santoro L, D’Onofrio F, Bernardi S, Gremese E, Ferraccioli G, Santoliquido A. Temporal ultrasonography findings in temporal arteritis: early disappearance of halo sign after only 2 days of steroid treatment. Rheumatology (Oxford). 2013;52:622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | De Miguel E, Roxo A, Castillo C, Peiteado D, Villalba A, Martín-Mola E. The utility and sensitivity of colour Doppler ultrasound in monitoring changes in giant cell arteritis. Clin Exp Rheumatol. 2012;30:S34-S38. [PubMed] [Cited in This Article: ] |
| 89. | Serafim AS, Singh S, Piper J, Hutchings A, Bradburn M, Ponte C, Schmidt WA, McNally E, Diamantopoulos A, Luqmani RA. Early Halo Sign Features on Ultrasound Examination of Treated Patients with Giant Cell Arteritis. Arthritis Rheumatol. 2014;66:S349. [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Czihal M, Zanker S, Rademacher A, Tatò F, Kuhlencordt PJ, Schulze-Koops H, Hoffmann U. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol. 2012;41:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Bley TA, Weiben O, Uhl M, Vaith P, Schmidt D, Warnatz K, Langer M. Assessment of the cranial involvement pattern of giant cell arteritis with 3T magnetic resonance imaging. Arthritis Rheum. 2005;52:2470-2477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Rhéaume M, Rebello R, Pagnoux C, Carette S, Clements-Baker M, Cohen-Hallaleh V, Doucette-Preville D, Jackson BS, Salama S, Ioannidis G. Accuracy of High Resolution MRI of Scalp Arteries for the Diagnosis of Giant Cell Arteritis: Results of a Prospective Study. Arthritis Rheumatol. 2014;66:S390-S391. [Cited in This Article: ] |
| 93. | Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K, Conrad M, Vosshenrich R. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30:730-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 332] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 94. | Both M, Ahmadi-Simab K, Reuter M, Dourvos O, Fritzer E, Ullrich S, Gross WL, Heller M, Bähre M. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis. 2008;67:1030-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Scheel AK, Meller J, Vosshenrich R, Kohlhoff E, Siefker U, Müller GA, Strutz F. Diagnosis and follow up of aortitis in the elderly. Ann Rheum Dis. 2004;63:1507-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Blockmans D, Bley T, Schmidt W. Imaging for large-vessel vasculitis. Curr Opin Rheumatol. 2009;21:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 97. | Pipitone N, Versari A, Salvarani C. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology (Oxford). 2008;47:403-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 98. | Andrews J, Al-Nahhas A, Pennell DJ, Hossain MS, Davies KA, Haskard DO, Mason JC. Non-invasive imaging in the diagnosis and management of Takayasu’s arteritis. Ann Rheum Dis. 2004;63:995-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 99. | Paul JF, Fiessinger JN, Sapoval M, Hernigou A, Mousseaux E, Emmerich J, Piette JC. Follow-up electron beam CT for the management of early phase Takayasu arteritis. J Comput Assist Tomogr. 2001;25:924-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Dasgupta B. Concise guidance: diagnosis and management of giant cell arteritis. Clin Med. 2010;10:381-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |