Published online Nov 16, 2015. doi: 10.12998/wjcc.v3.i11.935
Peer-review started: August 26, 2014
First decision: September 28, 2014
Revised: July 28, 2015
Accepted: August 4, 2015
Article in press: August 7, 2015
Published online: November 16, 2015
AIM: To develop a framework for the clinical and health economic assessment for management of Clostridium difficile infection (CDI).
METHODS: CDI has vast economic consequences emphasizing the need for innovative and cost effective solutions, which were aim of this study. A guidance model was developed for coverage decisions and guideline development in CDI. The model included pharmacotherapy with oral metronidazole or oral vancomycin, which is the mainstay for pharmacological treatment of CDI and is recommended by most treatment guidelines.
RESULTS: A design for a patient-based cost-effectiveness model was developed, which can be used to estimate the cost-effectiveness of current and future treatment strategies in CDI. Patient-based outcomes were extrapolated to the population by including factors like, e.g., person-to-person transmission, isolation precautions and closing and cleaning wards of hospitals.
CONCLUSION: The proposed framework for a population-based CDI model may be used for clinical and health economic assessments of CDI guidelines and coverage decisions for emerging treatments for CDI.
Core tip: Current clinical guidelines seldom include cost-effectiveness evaluations. Conclusions are typically based on clinical data only and sometimes referral is made to prices of therapies for justification of the treatment sequence advised. However, the price of a therapy as such is just a single criterion and does not reflect the balance between effectiveness and costs associated with the application of that therapy. This results often in a restricted position of new therapies in the treatment algorithm. Integration of cost-effectiveness using the population-based variant of cost-effectiveness evaluations as an instrument in guidelines for Clostridium difficile infection may be provide better decision making framework.
-
Citation: Nuijten MJ, Keller JJ, Visser CE, Redekop K, Claassen E, Speelman P, Pronk MH. Cost-effectiveness in
Clostridium difficile treatment decision-making. World J Clin Cases 2015; 3(11): 935-941 - URL: https://www.wjgnet.com/2307-8960/full/v3/i11/935.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i11.935
Escalating costs resulting from ageing of the population and an increase of innovative, expensive, medical technologies have become a major concern for health care professionals, decision-makers and the public. In addition to considering clinical benefits and the price of the new treatment, decision makers have taken a broader perspective by including cost-effectiveness evaluations, which also include related costs in the health care system.
Clostridium difficile infection (CDI) is considered a hospital-acquired infection. Diarrhea due to pathogenic Clostridium difficile (C. difficile) can occur if the bowel microbiota (bacterial content of the bowel) of a patient is disturbed, which is usually the result of antibiotic use prior to the CDI. With the increasing use of broad-spectrum antibiotics over the past two decades, the incidence of CDI has risen[1,2] and CDI is responsible for 15%-25% of cases of antibiotic associated diarrhea (AAD)[1]. CDI is usually self-limiting, but severe disease leading to colectomy and intensive care admission may occur. Mortality rates of 2%-7% have been reported[2,3], and seem even higher with the hypervirulent strain polymerase chain reaction (PCR) ribotype 027[4-6]. The chance of contracting CDI increases with a longer hospital stay[7] and both spread of C. difficile between patients as well as auto-re-infection, by this spore forming bacterium, have been demonstrated. In 55% of hospitalized patients with CDI, hospital stay was prolonged to more than 4 wk[8].
Pharmacotherapy of an initial episode of CDI with oral metronidazole or oral vancomycin is the recommended treatment in most guidelines[9-11]. However, following antibiotic treatment of CDI, recurrence and re-infection within 30 d occurs in approximately 15%-35% of patients, while 33%-65% of patients with > 2 previous CDI episodes will recur[12]. Recurrence of CDI is a serious and difficult-to-treat problem, impacting on the length and overall cost of hospitalisation[13]. The guidelines of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) have identified recurrence as being the most important challenge in the treatment of CDI[14].
Recently published guidelines[11-13] have incorporated relatively new treatments strategies with the antibiotic fidaxomicin and fecal microbiota transplantation (FMT or donor feces infusion), although their role is restricted because of the high price of fidaxomicin and the complexity (unconventional and unstandardized nature) of FMT. The latest Netherlands guideline suggests weighing fidaxomycin’s high price versus the advantage of fewer recurrences[10,12,15]. English guidelines also recommends oral metronidazole for initial treatment in non-severe CDI, because it is cheaper than oral vancomycin, and because of concern about the selection of vancomycin resistant enterococci[13]. The median cost to treat a patient with CDI was €33840, showing an almost five fold higher and significant difference compared with the non-infected matched controls[16]. The estimated cost of CDI within the European Union (EU) is about €3 billion per year[17], and may further increase with aging. In most studies, hospitalisation is the main cost driver in patients with CDI[18]. Patients with CDI spend on average an extra 7-21 d in hospital, compared with non-infected controls[16,19,20]. The high rates of treatment failure and high rates of currently recommended antibiotics (metronidazole and vancomycin)[15,21,22] significantly affects costs[23]. The influence on clinical outcome and costs of this limited treatment efficacy is particularly apparent for patient groups with multiple comorbidities and a high risk of recurrence. In addition, minimizing the risk of person-to-person transmission of C. difficile in hospital wards seems of utmost importance. Taken together it is evident there is a large socio-economic and clinical unmet need to evaluate all these different factors in a single decision support model[1].
Classic patient-based cost effectiveness model for infectious diseases tend to ignore the supra-patient social-economic consequences such as, for example, person-to-person transmission and closing of hospital wards due to infectious outbreaks. Preparing for an all-inclusive model an expert procedure was convened considering the clinical and economic issues supposed to have an influence on cost-effectiveness evaluation of preventive and therapeutic measures for CDI. Step one was an extensive literature search on all different aspects of such a model. Subsequently, a base model was constructed and presented to experts. Next, the therapeutic and economic issues that were mentioned by the individual experts were incorporated in the model. This model was the input for a plenary discussion. The most relevant therapeutic and economic issues were defined and discussed. Based on scientific sources (literature and professional guidelines) as well as practice based sources, the issues were validated and a framework for the integration of these relevant issues into cost-effectiveness modeling was finalized. The outcomes of the expert procedure are described below.
Clinical and economic relevant issues are shown in Tables 1 and 2.
| Level | Issues |
| Patient level | Recurrence of CDI is a serious and difficult-to-treat problem[26] |
| Patient groups at high risk of recurrence or those for whom the impact of recurrence would be most dramatic include those with multiple comorbidities, who are immunocompromised, who are receiving certain concomitant antibiotics[26, who have had CDI previously, who are renally impaired, who are aged 65 yr or over, patients awaiting further treatment (for example chemotherapy) or rehabilitation (for example after cerebrovascular event) | |
| Population level | The rate of person-to-person transmission of C. difficile is a complicating problem |
| The risk for development of vancomycin-resistant enterococci or other antibiotic induced resistant bacteria, although it is not a major issue in daily practice |
| Level | Issues |
| Patient level | The cost of recurrence of CDI is high |
| CDI leads to additional costs: extra diagnostic tests, extra antibiotics and other medication, time spent by nurse and physician on the ward | |
| The additional circumstances of these seriously ill patients (e.g., not completing primary therapy, thereby complicating cure or improvement of their disease state) due to CDI should be reflected in the CEA | |
| Population level | The rate of person-to-person transmission of C. difficile is a complicating problem with high costs |
| The increased length and overall cost of hospitalization with CDI, including the costs of measures to isolate the patient and other clinical measures to prevent person-to-person transmission, as well as the costs of closing and cleaning wards | |
| The consequences of developing vancomycin-resistant enterococci or other antibiotic induced resistant enterococci are not integrated in standard cost-effectiveness evaluations |
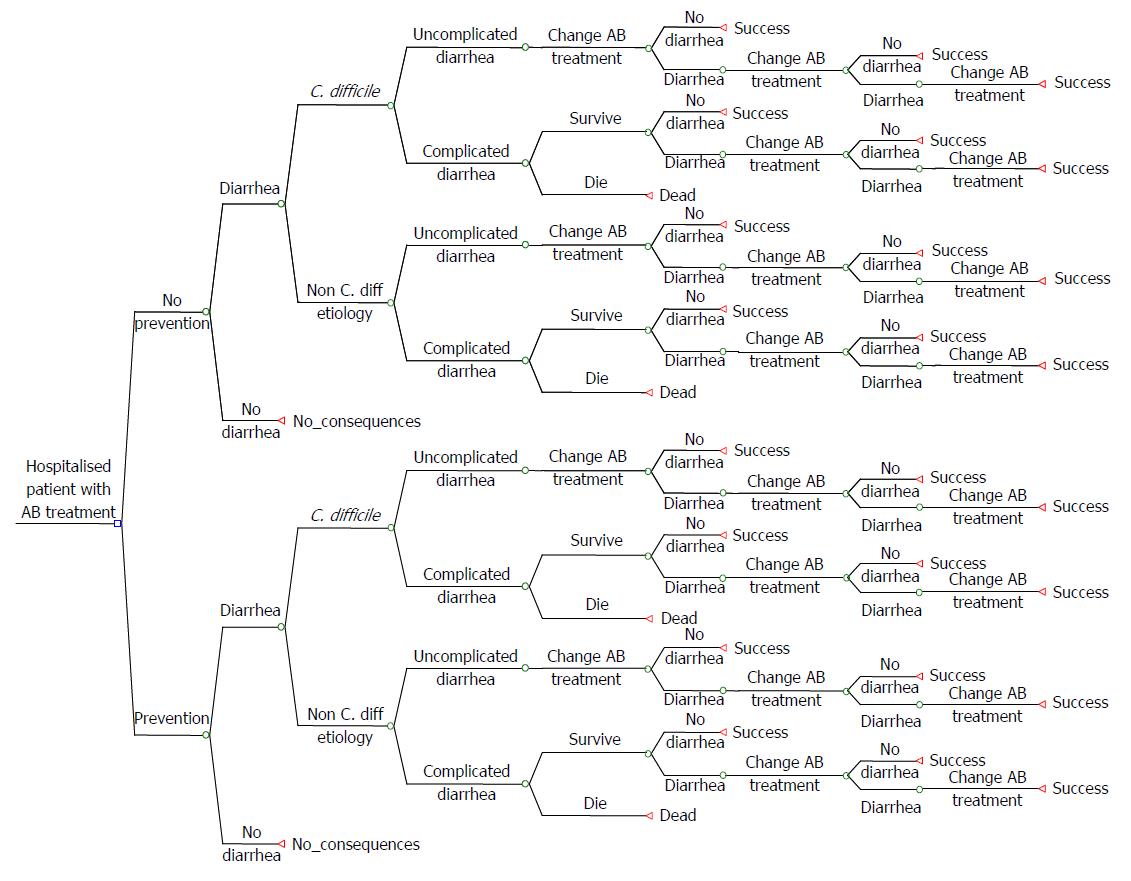
The clinical and economic consequences of CDI in terms of morbidity, survival and costs underline the therapeutic need for innovative cost-effective solutions. Payers require cost-effectiveness analyses when deciding whether or not to reimburse new therapies/approaches for CDI. In such an analysis the first step is typically to develop a patient-based cost-effectiveness model. Such a model for CDI is shown in Figure 1.
Instructions are defined possible different stages for a CDI patient (health states).
Treatment stages hospital setting: (1) In the hospital, discontinuation of the antibacterial therapy that may have precipitated CDI is often not possible; (2) Patients with CDI are usually treated with antibiotics (metronidazole); (3) Patients may die or stay alive and surviving patients may or may not respond to metronidazole; (4) Patients who respond to metronidazole may be cured or experience a recurrence (or re-infection), which may occur during the hospitalization period or after discharge. In both cases the initial treatment with metronidazole is restarted, or vancomycin or fidaxomicin is prescribed instead; (5) If no response to metronidazole is seen, patients may be switched to vancomycin or fidaxomicin; (6) Then again patients may die or stay alive and surviving patients may respond or not respond to vancomycin or fidaxomicin; (7) Patients who respond to vancomycin or fidaxomicin may be cured or experience a recurrence (or re-infection), which may occur during hospitalization or after discharge. In both cases the initial treatment with vancomycin or fidaxomicin is restarted; and (8) Patients not responding will be switched to third-line treatment. For patients failing on third-line treatment, not many treatment options are left. If third line treatment is FMT, this can be repeated several times. Otherwise, patients may have to use vancomycin more or less continuously.
Treatment stages community setting: The treatment stages are similar, as described above, but the difference is that patients may or may not be hospitalized, whereas in the previous section patients are already hospitalized.
The incremental cost per QALY gained is seen as the preferred cost-effectiveness outcome, but is often of limited value in a health economic analysis, which only covers the hospitalization period. The QALY gain is calculated by combining the utility gain (quality of life gain) with the number of life years gained. QALY gained may therefore be the result of longer life expectancy, utility gain, or both. If the cost per QALY gained is not viewed as the most appropriate outcome (for example when transmission and/or ward contamination are a problem), other cost-effectiveness outcomes may be considered, for example, the cost per recurrence avoided, which reflects the additional costs for the prevention of one recurrence. As recurrence is not only a clinical issue, but also may have major economic consequences, this outcome might be relevant for decision makers.
The cost-effectiveness outcome based on the patient-based model only provides a limited health economic outcome in terms of time horizon and perspective and more importantly, disregards the impact on other patients. The relevant economic issues, such as resistance, person-to-person transmission, isolation measures and closing of wards of hospitals, are all supra-patient effects. These consequences of CDI on hospitals, payers and society, that go beyond the individual scope of the patient, are not integrated in standard CEA’s. Therefore, the outcomes of a patient-based cost-effectiveness model should be considered cautiously because they present only a conservative and limited outcome. For all-encompassing cost-effectiveness evaluations of CDI therapies, these supra-patient economic aspects cannot be disregarded. Therefore, we propose performing cost-effectiveness analyses for CDI using a population-based model, which incorporates all of the clinically important elements of the patient-based model as well as the supra-patient therapeutic and economic issues (Table 3).
| Patient-based cost-effectiveness model | Population-based cost-effectiveness model |
| Similarities | |
| Patient-related therapeutic and economic measures for clinical and economic evaluations | |
| Differences | |
| The relevant economic issues, as indicated for CDI like: | |
| increasing incidence of CDI, | |
| person-to person transmission of CDI, | |
| development of vancomycin-resistant enterococci (VRE), | |
| or other antibiotic induced resistant bacteria, | |
| impact for department of microbiology diagnostic testing | |
| isolation measures and | |
| closing of wards of hospitals | |
| other supra-patient effects | |
| Limited health economic outcome in terms of time horizon and perspective | |
| The patient-based cost-effectiveness model only captures the short-term time horizon of the CDI episode within the hospital setting at a patient level | |
The flow diagram (Figure 1) does not contain a particular choice for a specific therapy but serves as a blueprint for cost-effectiveness modeling. Based on the developments in CDI treatment, we suggest applying different treatment sequences for testing the effects on cost-effectiveness outcomes. Other suggestions for application are stratification of the patient population according to potential co-variables, such as risk factors for recurrence (for example, prolonged hospital stay or ICU admission) or underlying diseases (for example, patients after surgery, patients with a malignancy receiving chemotherapy, and renally impaired patients).
Three types of recent therapies could be candidates for comparison using a population-based model: the antibiotic fidaxomicin, fecal microbiota transplantation (FMT), and preventive use of probiotics.
Fidaxomycin is a novel antibiotic with targeted activity against C. Difficile with a similar safety profile as vancomycin. After treatment of an initial episode of CDI, the cure rate after 30 d was increased after fidaxomycin (82%) compared to vancomycin (70%)[16,17,24]. FMT helps restore the normal colonic micro flora in patients with refractory and recurrent CDI[25,26]. The procedure involves single or multiple infusions (e.g., by enema) of a feces based solution from a healthy donor. A recently published randomized trial confirmed the efficacy of FMT in patients with recurrent CDI. For assessment of preventive treatments, the framework (Figure 1) can be used to estimate the costs and benefits of co-prescription of probiotics with antibiotics to prevent CDI. Recently, a patient-based cost-effectiveness evaluation for probiotics showed probiotics “could lead to substantial cost savings”[27]. To further investigate the economic consequences of the use of probiotics to prevent CDI, a population-based model could be applied and although the expected clinical benefit may be limited, total cost savings compared to no preventive treatment, and a predicted (Cochrane) drop in therapy induced side effects, may still be relevant.
Current clinical guidelines seldom include cost-effectiveness evaluations. Conclusions are typically based on clinical data only and sometimes referral is made to prices of therapies for justification of the treatment sequence advised. However, the price of a therapy as such is just a single criterion and does not reflect the balance between effectiveness and costs associated with the application of that therapy. This results often in a restricted position of new therapies in the treatment algorithm.
Among health authorities, it is common to include evidence of cost-effectiveness in decision-making about coverage under the health insurance package. Even though the cost per QALY outcome might fall below the threshold of a country, health authorities might decide to reject coverage based on the high weight they place on the budget impact[28]. This may be considered a paradox, because the cost-effectiveness guidelines were written by the same authorities and payers.
Estimates of the cost-effectiveness of a medicine may only have a limited impact on the use of that medicine within a hospital, as a result of a “silo mentality” found within the hospital as well as within the budget management structure existing at the payer, local and national levels. In that case, a treatment (medication or medical therapy) that is more expensive than existing treatments may exceed the amount of money reserved within the hospital budget or the pharmacy budget.
Another paradox, since exceeding this “local” budget might generate a multiplier and create substantial savings in the total system/hospital.
Achieving changes in the “silo structure” within hospitals as well as the budget management structure by payers depends on the generation of basic information on these cost-effective aspects. We propose that usage of the current flow diagram will generate facts and figures, as well as enable motivated implementation of these facts into guidance documents from professional societies to policy makers and payers (locally or regionally as well as nationally).
Integration of cost-effectiveness using the population-based variant of cost-effectiveness evaluations as an instrument in guidelines for CDI should be considered.
This may help healthcare professionals, patients, hospitals, payers and society to make better decisions about the optimal way to reduce the health and economic impact of CDI.
Current clinical guidelines seldom include cost-effectiveness evaluations. Conclusions are typically based on clinical data only and sometimes referral is made to prices of therapies for justification of the treatment sequence advised. However, the price of a therapy as such is just a single criterion and does not reflect the balance between effectiveness and costs associated with the application of that therapy. This results often in a restricted position of new therapies in the treatment algorithm.
Recent high rates of treatment failure and recurrent infection have vast economic consequences emphasizing the need for innovative and cost effective solutions in Clostridium difficile infections (CDI). The price of new therapies and approaches cannot always compete with the relatively low, generic prices of current standard therapies with metronidazole and vancomycin. The question is then, how should professional societies integrate new and more effective, but also more expensive, remedies into their guidelines and how health authorities make reimbursement decisions.
The cost-effectiveness outcome based on the patient-based model only provides a limited health economic outcome in terms of time horizon and perspective and more importantly, disregards the impact on other patients. The relevant economic issues, such as resistance, person-to-person transmission, isolation measures and closing of wards of hospitals, are all supra-patient effects. These consequences of CDI on hospitals, payers and society, that go beyond the individual scope of the patient, are not integrated in standard cost-effectiveness analyses. Therefore, we developed a guidance model for coverage decisions and guideline development in CDI based on a population-based cost-effectiveness model.
The authors propose performing cost-effectiveness analyses for CDI using a population-based model, which incorporates all of the clinically important elements of the patient-based model as well as the supra-patient therapeutic and economic issues.
CDI is responsible for 15%-25% of cases of antibiotic associated diarrhea (AAD) and is typically seen in elderly hospitalised patients, resulting in significant morbidity and mortality. Pharmacotherapy of an initial episode of CDI with oral metronidazole or oral vancomycin is the mainstay for pharmacological treatment of CDI and is recommended by most treatment guidelines.
This guideline article is interesting and has a high scientific value.
P- Reviewer: Di Lorenzo G, Kirshtein B, Yokoyama Y S- Editor: Tian YL L- Editor: A E- Editor: Wang CH
| 1. | Barbut F, Petit JC. Epidemiology of Clostridium difficile-associated infections. Clin Microbiol Infect. 2001;7:405-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 230] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 803] [Cited by in F6Publishing: 802] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 3. | Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442-2449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1563] [Cited by in F6Publishing: 1499] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 4. | Paltansing S, van den Berg RJ, Guseinova RA, Visser CE, van der Vorm ER, Kuijper EJ. Characteristics and incidence of Clostridium difficile-associated disease in The Netherlands, 2005. Clin Microbiol Infect. 2007;13:1058-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 451] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | Smith A. Outbreak of Clostridium difficile infection in a hospital in south east England linked to hypertoxin-producing strains in Canada and the US. Euro Surveill. 2005;10:E050630.2. [Cited in This Article: ] |
| 7. | Dodek PM, Norena M, Ayas NT, Romney M, Wong H. Length of stay and mortality due to Clostridium difficile infection acquired in the intensive care unit. J Crit Care. 2013;28:335-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Draaiboek Maatregelen ter preventie en bestrijding Clostridium difficile PCR-ribotype 027 - toxinotype III-infectie buiten het ziekenhuis. December 2009 Landelijke Coördinatie Infectieziektebestrijding RIVM - Centrum Infectieziektebestrijding Bilthoven. Available from: http://www.rivm.nl. [Cited in This Article: ] |
| 9. | Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1179] [Cited by in F6Publishing: 1128] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 10. | Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, Sears P, Gorbach S. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 106] [Reference Citation Analysis (0)] |
| 11. | Wilcox MH. Updated guidance on the management and treatment of Clostridium difficile infection. London: Public Health England 2013; . [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Viswanathan VK, Mallozzi MJ, Vedantam G. Clostridium difficile infection: An overview of the disease and its pathogenesis, epidemiology and interventions. Gut Microbes. 2010;1:234-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pépin K, Chouinard D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 844] [Cited by in F6Publishing: 843] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 14. | Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20 Suppl 2:1-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 766] [Cited by in F6Publishing: 763] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 15. | Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1140] [Cited by in F6Publishing: 1097] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 16. | Wilcox MH, Cunniffe JG, Trundle C, Redpath C. Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect. 1996;34:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12 Suppl 6:2-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 617] [Cited by in F6Publishing: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 18. | Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:57-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Commission for Health care Audit and Inspection. Investigation 21 into outbreaks of Clostridium difficile at Stoke Mandeville Hospital. Buckinghamshire: Buckinghamshire Hospitals NHS Trust 2006; . [Cited in This Article: ] |
| 20. | Vonberg RP, Reichardt C, Behnke M, Schwab F, Zindler S, Gastmeier P. Costs of nosocomial Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;70:15-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5:549-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 382] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 23. | McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769-1775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 516] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 24. | European Medicines Agency. EPAR fidaxomicin. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002087/WC500119707.pdf. [Cited in This Article: ] |
| 25. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2582] [Cited by in F6Publishing: 2489] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 26. | van Nood E, Speelman P, Nieuwdorp M, Keller J. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. 2014;30:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Lenoir-Wijnkoop I, Nuijten MJ, Craig J, Butler CC. Nutrition economic evaluation of a probiotic in the prevention of antibiotic-associated diarrhea. Front Pharmacol. 2014;5:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Pronk MH, Bonsel GJ. Out-patient drug policy by clinical assessment rather than financial constraints? The gate-keeping function of the out-patient drug reimbursement system in The Netherlands. Eur J Health Econ. 2004;5:274-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |