Published online Oct 16, 2015. doi: 10.12998/wjcc.v3.i10.900
Peer-review started: January 21, 2015
First decision: March 6, 2015
Revised: April 18, 2015
Accepted: August 10, 2015
Article in press: August 11, 2015
Published online: October 16, 2015
Processing time: 269 Days and 9.2 Hours
Acute hepatitis is a very rare, but potentially fatal, adverse effect of intravenous amiodarone. We present a case of an 88-year-old man with history of ischemic dilated cardiomyopathy and severely depressed left ventricular function that was admitted to our coronary care unit with diagnosis of decompensated heart failure and non-sustained ventricular tachycardia. A few hours after the beginning of intravenous amiodarone he developed an acute hepatitis. There was a completely recovery within the next days after amiodarone withdrawn and other causes of acute hepatitis have been ruled out. This case highlights the need for close monitoring of hepatic function during amiodarone infusion in order to identify any potential hepatotoxicity and prevent a fatal outcome. Oral amiodarone is, apparently, a safe option in these patients.
Core tip: We report a rare case of acute hepatitis induced by intravenous amiodarone in a patient with non-sustained ventricular tachycardia. The physiopathology of this adverse effect is still unclear. Close monitoring of hepatic function during amiodarone infusion is essential to avoid any potential hepatotoxicity.
- Citation: Fonseca P, Dias A, Gonçalves H, Albuquerque A, Gama V. Acute hepatitis after amiodarone infusion. World J Clin Cases 2015; 3(10): 900-903
- URL: https://www.wjgnet.com/2307-8960/full/v3/i10/900.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i10.900
Long-term oral amiodarone therapy is associated with many extracardiac adverse effects, such as thyroid dysfunction, photosensitivity, corneal microdeposits and pulmonary and hepatic toxicities. The frequency of most adverse effects is related to the total drug exposure. Hepatic toxicity in these patients ranges from an asymptomatic elevation of serum aminotransferases (in approximately 25%) that is usually transient and resolves after dose reduction or withdrawal, to severe liver disease (1%-3%)[1]. Acute hepatic toxicity during intravenous amiodarone has been rarely described[2-6].
An 88-year-old man with history of ischemic dilated cardiomyopathy and severely depressed left ventricular function presented at Emergency Department due to progressive worsening of dyspnea, orthopnea and peripheral edema during the previous week. He was on long-term treatment with aspirin, ramipril, furosemide, transdermal nitroglycerin, sinvastatine and pantoprazole. He had no history of alcohol abuse or chronic acetaminophen intake.
On physical examination, blood pressure was 110/60 mmHg and percutaneous peripheral oxygen saturation was 87% on air. He had bilateral basilar rales on pulmonary auscultation and moderate lower leg edema. Arterial gasometry confirmed type 1 respiratory failure and blood tests were unremarkable, including liver function. Electrocardiogram monitoring revealed periods of non-sustained ventricular tachycardia (NSVT).
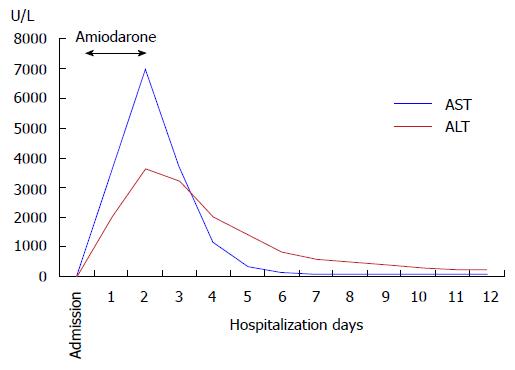
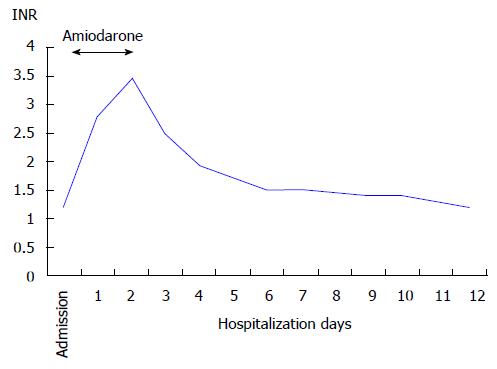
He was admitted to our coronary care unit with the diagnosis of acute decompensated heart failure and NSVT. He was started on intravenous amiodarone with a bolus dose of 300 mg followed by a continuous infusion of 900 mg over 24 h. Control blood tests performed 18 h after starting amiodarone showed an abrupt elevation of aminotransferases (aspartate aminotransferase 3398 U/L, alanine aminotransferase 1964 U/L), lactate dehydrogenase (2127 U/L), direct bilirubin (2.47 mg/dL) and international normalized ratio (2.79). Gamma-GT and alkaline phosphatase were normal. Despite hemodynamic and ventricular electric stability, he evolved with worsening of hepatic function associated with thrombocytopenia, metabolic acidosis and acute kidney injury. Abdominal ultrasonography showed a liver with normal appearance and excluded hepatic artery and vein thrombosis and any bile duct abnormalities. Viral hepatitis serologies (hepatitis B and C, cytomegalovirus, Epstein-Barr and herpes zoster viruses) and autoimmune markers (antinuclear antibody, anti-smooth muscle antibody, anti-liver/kidney microsomal antibody type 1) were negative.
Drug-induced liver injury secondary to amiodarone was the main diagnostic hypothesis and amiodarone was withdrawn about 40 h after its beginning (total dose of 1800 mg). Since then, he improved gradually with progressive normalization of renal and hepatic function (Figures 1 and 2). At day 4, he restarted amiodarone in oral form, at loading doses of 200 mg three times daily, without any additional liver injury. There was no recurrence of VT and he was discharged on day 12 with nearly normal hepatic tests.
This report describes a severe acute hepatitis induced by intravenous amiodarone. Among the few cases reported in literature of idiosyncratic reactions to intravenous amiodarone, some had a fatal outcome[4-6].
American College of Gastroenterology guidelines recommends that the causality assessment in patients with drug-induced hepatic injury should rely primarily on consensus expert opinion following a thorough evaluation for competing etiologies[7]. The causal relationship between intravenous amiodarone exposure and acute hepatitis has been established based on the following principles: (1) Sudden hepatic tests abnormalities within 24 h after starting amiodarone administration; (2) Presence of a pattern of hepatocellular injury with peak aminotransferases levels of more than 50 times the upper limit of normal; (3) Rapid improvement after amiodarone withdrawal; and (4) Exclusion of other causes.
It’s often difficult to distinguish between this entity and acute hepatic ischemia since many of these patients on intravenous amiodarone present hemodynamic instability. In this case, the patient was under invasive monitoring and maintained mean arterial pressure superior to 75 mmHg, which makes the diagnosis of ischemic hepatitis unlikely. He also didn’t have a severe congestive heart failure that could possibly explain a congestive hepatopathy. Thrombocytopenia and acute kidney injury were assumed to be secondary to acute hepatic injury. Because of his favorable evolution, we did not perform a hepatic biopsy.
According to the Council for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method scale[8] this case fulfilled the criteria of a highly probable amiodarone adverse effect.
The physiopathology of this adverse effect is still unclear. Different potential mechanisms have been proposed, including an immunologically mediated mechanism[9,10], a free radical mechanism, in which formation of free radicals leads to peroxidative injury of membrane lipids and necrosis[11,12] and a mechanism based in increased expression of the PPAR-α gene secondary to disrupted hepatic lipid homeostasis[13]. The mechanism of oral amiodarone induced hepatotoxicity seems to be different from that induced by intravenous amiodarone. Some reports, including our own, showed that introduction of oral amiodarone in these patients did not result in any additional liver injury. Based on this observation, Rhodes et al[14] proposed that polysorbate 80, the solvent of intravenous formulation of amiodarone, could be involved in this adverse effect since it is present in the intravenous but not in the oral form of amiodarone. Polysorbate 80 has been implicated in the E-ferol syndrome, which has been described in infants after intravenous administration of E vitamin with this component[15]. The E-ferol syndrome shows significant similarities to the cases of liver toxicity due to amiodarone[14]. In addition, polysorbate 80 has a short plasma half life, which could justify the rapid recover of hepatic failure after discontinuation of intravenous amiodarone. In 2008 Food and Drug Administration approved a polysorbate-free formulation of amiodarone (Nexterone, Baxter Healthcare Corporation, Deerfield, IL), however it’s still not available in several hospitals.
In conclusion, acute hepatotoxicity is a rare, but potentially fatal, adverse effect of intravenous amiodarone. This case highlights the need for close monitoring of hepatic function during amiodarone infusion in order to identify any potential hepatotoxicity and prevent a fatal outcome. If available, it should be considered the use of polysorbate-free formulation of intravenous amiodarone. Oral amiodarone is, apparently, a safe option in these patients.
An 88-year-old man admitted with acute decompensated heart failure and non-sustained ventricular tachycardia underwent intravenous amiodarone.
The patient developed severe acute hepatitis induced by intravenous amiodarone.
Acute viral hepatitis, autoimmune hepatitis, ischemic liver injury, congestive hepatopathy.
Aspartate aminotransferase 3398 U/L, alanine aminotransferase 1964 U/L, lactate dehydrogenase 2127 U/L, direct bilirubin 2.47 mg/dL and international normalized ratio 2.79; viral hepatitis serologies and autoimmune markers were negative.
Abdominal ultrasonography showed a liver with normal appearance and excluded hepatic artery and vein thrombosis and any bile duct abnormalities.
Hepatic biopsy was not performed due to favorable evolution after amiodarone withdrawal.
The treatment was mainly supportive after amiodarone withdrawal.
Few cases of acute hepatic injury after intravenous amiodarone have been reported in literature. Some of them had a fatal outcome.
Idiosyncratic reactions are unpredictable adverse drug reactions that do not occur in most patients, but can be life-threatening.
This case highlights the need for close monitoring of hepatic function during amiodarone infusion in order to identify any potential hepatotoxicity and prevent a fatal outcome. Oral amiodarone is, apparently, a safe option in these patients.
This is a well written case report on a serious complication following iv amiodarone administration.
P- Reviewer: De Ponti F, Ghinolfi D, Lankarani KB S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Lewis JH, Ranard RC, Caruso A, Jackson LK, Mullick F, Ishak KG, Seeff LB, Zimmerman HJ. Amiodarone hepatotoxicity: prevalence and clinicopathologic correlations among 104 patients. Hepatology. 1989;9:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 158] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Rizzioli E, Incasa E, Gamberini S, Savelli S, Zangirolami A, Tampieri M, Manfredini R. Acute toxic hepatitis after amiodarone intravenous loading. Am J Emerg Med. 2007;25:1082.e1-1082.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Chen CC, Wu CC. Acute Hepatotoxicity of Intravenous Amiodarone: Case Report and Review of the Literature. Am J Ther. 2014;Sep 24; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Kalantzis N, Gabriel P, Mouzas J, Tiniakos D, Tsigas D, Tiniakos G. Acute amiodarone-induced hepatitis. Hepatogastroenterology. 1991;38:71-74. [PubMed] |
| 5. | MacFadyen RJ, Palmer TJ, Hisamuddin K. Rapidly fatal acute amiodarone hepatitis occurring in the context of multiple organ failure. Int J Cardiol. 2003;91:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Chan AL, Hsieh HJ, Hsieh YA, Lin SJ. Fatal amiodarone-induced hepatotoxicity: a case report and literature review. Int J Clin Pharmacol Ther. 2008;46:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966; quiz 967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 513] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 8. | Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1064] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 9. | Breuer HW, Bossek W, Haferland C, Schmidt M, Neumann H, Gruszka J. Amiodarone-induced severe hepatitis mediated by immunological mechanisms. Int J Clin Pharmacol Ther. 1998;36:350-352. [PubMed] |
| 10. | Lupon-Rosés J, Simó-Canonge R, Lu-Cortez L, Permanyer-Miralda G, Allende-Monclús H. Probable early acute hepatitis with parenteral amiodarone. Clin Cardiol. 1986;9:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Sarma JS, Pei H, Venkataraman K. Role of Oxidative Stress in Amiodarone-induced Toxicity. J Cardiovasc Pharmacol Ther. 1997;2:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kaufmann P, Török M, Hänni A, Roberts P, Gasser R, Krähenbühl S. Mechanisms of benzarone and benzbromarone-induced hepatic toxicity. Hepatology. 2005;41:925-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | McCarthy TC, Pollak PT, Hanniman EA, Sinal CJ. Disruption of hepatic lipid homeostasis in mice after amiodarone treatment is associated with peroxisome proliferator-activated receptor-alpha target gene activation. J Pharmacol Exp Ther. 2004;311:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Rhodes A, Eastwood JB, Smith SA. Early acute hepatitis with parenteral amiodarone: a toxic effect of the vehicle? Gut. 1993;34:565-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Balistreri WF, Farrell MK, Bove KE. Lessons from the E-Ferol tragedy. Pediatrics. 1986;78:503-506. [PubMed] |