Published online Oct 16, 2015. doi: 10.12998/wjcc.v3.i10.880
Peer-review started: February 2, 2015
First decision: July 6, 2015
Revised: July 26, 2015
Accepted: September 16, 2015
Article in press: September 18, 2015
Published online: October 16, 2015
Processing time: 257 Days and 19.2 Hours
AIM: To retrospectively compare previous-day vs split-dose preparation in terms of bowel cleanliness and polyp detection in patients referred for polypectomy.
METHODS: Fifty patients underwent two colonoscopies: one diagnostic in a private clinic and a second for polypectomy in a University Hospital. The latter procedures were performed within 12 wk of the index ones. Examinations were accomplished by two experienced endoscopists, different in each facility. Twenty-seven patients underwent screening/surveillance colonoscopy, while the rest were symptomatic. Previous day bowel preparation was utilized initially and split-dose for polypectomy. Colon cleansing was evaluated using the Aronchick scale. We measured the number of detected polyps, and the polyp miss rates per-polyp.
RESULTS: Excellent/good preparation was reported in 38 cases with previous-day preparation (76%) vs 46 with split-dose (92%), respectively (P = 0.03). One hundred and twenty-six polyps were detected initially and 169 subsequently (P < 0.0001); 88 vs 126 polyps were diminutive (P < 0.0001), 25 vs 29 small (P = 0.048) and 13 vs 14 equal or larger than 10 mm. The miss rates for total, diminutive, small and large polyps were 25.4%, 30.1%, 13.7% and 6.6%, respectively. Multivariate analysis revealed that split-dose preparation was significantly associated (OR, P) with increased number of polyps detected overall (0.869, P < 0.001), in the right (0.418, P = 0.008) and in the left colon (0.452, P = 0.02).
CONCLUSION: Split-dose preparation improved colon cleansing, enhanced polyp detection and unmasked significant polyp miss rates.
Core tip: Colonoscopy and polypectomy are currently considered as the gold standard to prevent colorectal cancer. However, a significant proportion of precancerous lesions are missed during the procedure, limiting its efficacy and giving rise to interval cancers. Adequate bowel cleanliness represents a major factor with regards to colonoscopy quality. This study demonstrates that split-dose bowel preparation results to significantly better mucosal cleansing compared to previous-day preparation. Moreover, we showed that preparation with the split-dose regimen significantly enhanced polyp detection, especially of the diminutive ones. Finally, better inspection of the colonic epithelium unmasked a notable polyp miss rate.
- Citation: Papanikolaou IS, Sioulas AD, Magdalinos N, Beintaris I, Lazaridis LD, Polymeros D, Malli C, Dimitriadis GD, Triantafyllou K. Improved bowel preparation increases polyp detection and unmasks significant polyp miss rate. World J Clin Cases 2015; 3(10): 880-886
- URL: https://www.wjgnet.com/2307-8960/full/v3/i10/880.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i10.880
Colonoscopy is currently regarded as the modality of choice, in order to reduce the incidence of colorectal cancer (CRC) and its associated mortality[1]. The rationale behind this is its ability to detect and remove polyps that represent precancerous lesions[2,3]. However, interval CRC, namely cases that are diagnosed between screening and post-screening surveillance examinations, do exist[4,5]. The majority of them are thought to originate from missed polyps during colonoscopy. Polyp and adenoma miss rates reach 28% and 24%, respectively, in several studies reducing colonoscopy preventive efficacy against CRC[6,7].
Numerous technical-, patient- and endoscopist-related factors influence the detection of polyps during colonoscopy[8-11]. In this setting, international associations of endoscopy include adenoma detection rate (ADR) among principal colonoscopy quality indicators[12,13]. Poor bowel preparation is regarded as an impediment to the detection of both small and large polyps[14]. Therefore, multiple interventions have been proposed to improve bowel cleansing and thus increase the quality of colonoscopy[15-17].
Using a tandem colonoscopic evaluation we investigated the impact of different timing of purgative administration in colon cleansing and polyp detection. Polyp miss rates, as well as variables affecting polyp detection were also assessed.
This retrospective study was performed on a consecutive series of patients from January to December 2012. All patients were diagnosed with colon polyps during colonoscopy in a small private clinic on an island near Athens and were referred for polypectomy in the Endoscopy Unit of “Attikon” University General Hospital. Exclusion criteria included: (1) age less than 18 or more than 80 years; (2) history of bowel resection; (3) history of inflammatory bowel diseases; (4) suspicion of polyposis syndrome; (5) incomplete colonoscopy (in one of the two examinations); (6) poor bowel preparation as assessed with the Aronchick scale; and (7) ongoing anticoagulation treatment.
Prior to the index colonoscopies, patients received the full dose of a 4-L polyethylene glycol (PEG) regimen (Fortrans, Ipsen, Athens, Greece) in the previous day. On the other hand, split dosing (3 L on the previous and 1 L on the same day) was preferred for the subsequent colonoscopies. In all cases patients were advised to maintain a low-fiber diet during the day preceding the examinations.
The quality of bowel cleansing was evaluated by the performing endoscopists using the Aronchick scale. This assesses the preparation quality of the entire colon as excellent (a small volume of clear liquid or greater than 95% of the surface seen), good (a large volume of clear liquid covering 5% to 25% of the surface but greater than 90% of the surface was seen), fair (some semisolid stool that could be suctioned or washed away, but greater than 90% of the surface was seen), poor (semisolid stool that could not be suctioned or washed away and less than 90% of the surface was seen), or inadequate (repeat preparation and colonoscopy was needed)[18]. The evaluations of bowel cleanliness were further summarized as adequate (excellent/good) and inadequate (fair/poor).
Two equally experienced endoscopists with experience of more than 5000 colonoscopies each did all the examinations. Specifically, one endoscopist conducted the diagnostic examinations using uniquely previous-day preparation and the other performed the second series with split-dose preparation. The endoscopist who performed the polypectomies was not aware of the number, size and location of polyps detected during the first colonoscopies and had no data regarding the quality of bowel preparation during index colonoscopies. Procedures were done using olympus CF-Q145L standard-definition white-light colonoscopes (Olympus Corporation, Tokyo, Japan). Polypectomies were accomplished by means of forceps, snares or endoscopic mucosal resection, as needed.
All patients signed a standard informed consent form prior to the exam. Institutional ethics committee approval for our study was not needed, since all patients received the standard-of-care without reference to any study.
During the examinations, pulse rate, arterial blood pressure, oxygen saturation and consciousness level were monitored. Supplemental oxygen was routinely delivered via nasal catheters at 2 L/min. Intravenous conscious sedation and analgesia including midazolam (Dormicum, Roche Hellas, Athens, Greece) and pethidine hydrochloride (Petidina cloridrato, Molteni Farmaceutici Cilteni, Scandicci, Firenze, Italy) was administered depending on patient’s willingness along with comorbidities and baseline vital signs assessment. Reversal agents including flumazenil (Anexate, Roche Hellas, Athens, Greece) and naloxone (Naloxon, B. Brown Melsungen AG, Melsungen, Germany) were available in case of sedation-related complications. No antispasmodics were administered.
In the first colonoscopies, the colonoscopes were advanced to the cecum and polyps were identified during both insertion and withdrawal, counted, but not removed. In the second examinations, all detected polyps were resected and sent for histologic evaluation. Adenomas larger than 1 cm and/or with high-grade dysplasia or a villous component more than 25% were defined as advanced adenomas. To note, numerous tiny hyperplastic polyps in the rectosigmoid area were not subject to assessment.
For each procedure eligible for analysis, the following data were collected: (1) patients’ characteristics (age, gender, American Society of Anesthesiologists-ASA grade); (2) indication for colonoscopy; (3) sedation and oxygen administration; (4) bowel preparation quality; (5) polyp features (size, location, shape); and (6) other findings. According to their size, polyps were categorized as diminutive (≤ 5 mm), small (6-9 mm) and large (≥ 10 mm). Polyp size was determined by comparison with opened biopsy forceps. All colonoscopies were performed between 8:00 a.m. and 2:00 p.m.
Polyps per patient were calculated as number of detected polyps/number of patients. Polyp miss rates were calculated as: number of missed polyps/total number of missed polyps + total number of polyps on initial examination and presented as percentages. Both parameters were calculated overall and within strata of polyp size and location. Ideally, a third gold-standard preparation methodology against which comparisons regarding polyp miss rates were applied should be available. Since that was not the case in our retrospective trial we decided to use as reference the type of bowel preparation that showed better results regarding colon cleanliness. Therefore, no OR (95%CI) were calculated in the univariate analysis.
Continuous variables were presented as means or medians and standard deviations, while categorical ones were expressed as absolute values and percentages. Differences in the number of detected colon polyps (overall, right- and left-sided) between the two endoscopic procedures were examined using non-parametric related samples (Wilcoxon Signed Rank Test) tests.
A multivariate linear regression analysis model was constructed to examine variables associated with the number of polyps (overall, right- and left-sided) detected at colonoscopies (dependent variable). Independent variables include: patients’ age; sex (male vs female), ASA grade (1 vs 2), indication for colonoscopy (screening/surveillance vs symptoms evaluation) and the quality of bowel preparation (adequate vs inadequate). The OR (95%CI) and the level of significance were calculated. A P value of less than 0.05 indicated statistical significance.
Statistical analysis of data was carried out by international business machines corporation (IBM) SPSS Statistics Client for Trial 32. bit 22.0 Microsoft Windows Multilingual (IBM, New York, USA).
A total of 50 patients (28 male) completed both examinations; 4 patients were excluded. Reasons for exclusion were poor bowel preparation (n = 3) and failure to complete the second colonoscopy secondary to sedation-related hypoxemia (n = 1). Mean age was 58.4 ± 11.1 years. Indication for the index colonoscopies were: screening (n = 22), blood in stool (n = 7), abdominal pain (n = 12), family history of CRC (n = 2), altered bowel habits (n = 4) and post-polypectomy surveillance (n = 3). Median interval period between the two exams was 6 wk (range: 1-12).
Bowel preparation according to the Aronchick scale in the 2 series of colonoscopies was described as excellent in 17 (34%) vs 24 (48%) patients, good in 21 (42%) vs 22 (44%) patients and fair in 12 (24%) vs 4 (8%) patients, respectively. When the evaluations of bowel cleanliness were classified as adequate (excellent/good) and inadequate (fair/poor), the second group of colonoscopies showed a significant increased rate of adequate preparations (92% vs 76%, P = 0.03).
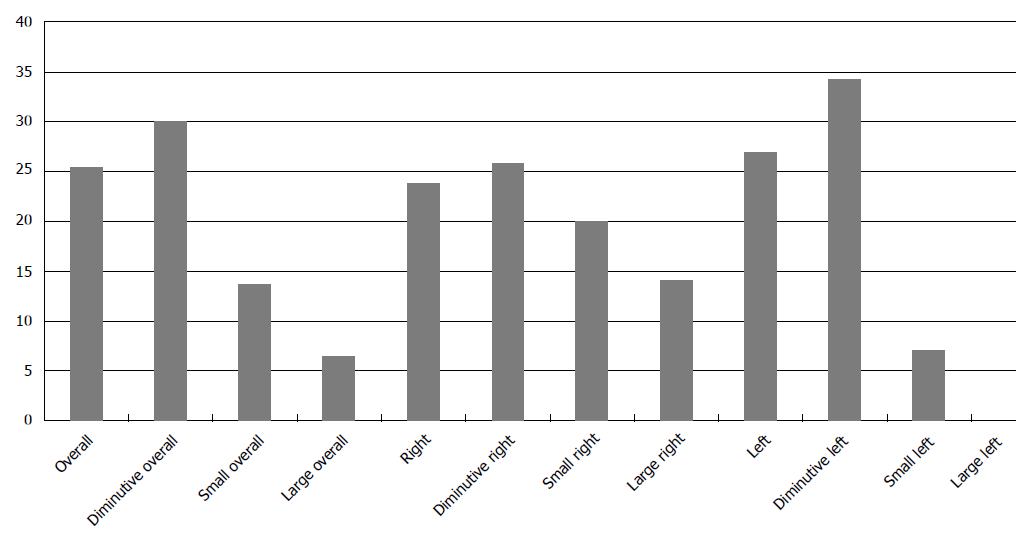
One hundred and twenty-six polyps were detected during the first examinations. Of those, 88 were diminutive, 25 small and 13 large; 43 additional polyps were identified during the tandem colonoscopies divided in 38 diminutive, 4 small and 1 large. Importantly, better colonic cleansing with the split-dose preparation contributed to significantly increased numbers of identified overall, right- and left-sided polyps (P < 0.0001). Significantly more diminutive polyps were detected throughout the colon (P < 0.001), while a marginal increase in the number of small polyps was also revealed (Table 1).
| Previous day | Split-dose | P value | |
| Overall | 126 | 169 | < 0.001 |
| Diminutive overall | 88 | 126 | < 0.001 |
| Small overall | 25 | 29 | 0.046 |
| Large overall | 13 | 14 | 0.317 |
| Right | 64 | 84 | < 0.001 |
| Diminutive right | 46 | 62 | < 0.001 |
| Small right | 12 | 15 | 0.083 |
| Large right | 6 | 7 | 0.317 |
| Left | 62 | 85 | < 0.001 |
| Diminutive left | 42 | 64 | < 0.001 |
| Small left | 13 | 14 | 0.317 |
| Large left | 7 | 7 | 1.000 |
The calculated miss rates regarding overall, diminutive, small and large polyps were 25.4%, 30.1%, 13.7% and 6.6%, respectively. The overall miss rates for polyps located in the right colon (cecum, ascending and transverse colon) was 23.8% compared with 27% in the left colon (distal to splenic flexure). Based on size, the miss rates for right-sided diminutive, small and large polyps were 25.8%, 20% and 14.2%, respectively, in comparison to 34.3%, 7.1% and 0%, respectively, for left-sided ones (Figure 1).
Linear regression analysis revealed that increased patients’ age and split-dose bowel preparation were the only variables associated with the number of polyps detected overall. Split-dose bowel preparation entered the model first [OR = 0.869 (95%CI: 0.456-1.283); P < 0.001], followed by increased age [OR = 0.054 (95%CI: 0.017-0.092); P = 0.005]. The same variables were also associated with the number of polyps detected in the right colon. Split-dose bowel preparation entered the model first [OR = 0.418 (95%CI: 0.111-0.724); P = 0.008], followed by increased age [OR = 0.032 (95%CI: 0.004-0.060); P = 0.024]. Split-dose bowel preparation was the only variable associated with the number of polyps detected in the left colon [OR = 0.452 (95%CI: 0.076-0.828); P = 0.02].
A total of 169 polyps were found and resected in 50 patients during the second series of colonoscopies. Histologic examination of resected polyps revealed tubular adenomas (n = 110), advanced adenomas (n = 18), serrated lesions (n = 7), hyperplastic polyps (n = 51) and adenocarcinoma (n = 1). Of note, 9 advanced adenomas and 4 serrated lesions were detected in the right colon, while 9 and 3 respectively, similar lesions, were located in the left colon.
This study demonstrates that split-dose bowel preparation results to significantly better mucosal cleansing compared to previous-day preparation. Moreover, we showed that better preparation with the split-dose regimen significantly enhanced overall, right- and left-sided polyp detection, especially referring to diminutive ones. Furthermore, improved view of the colonic epithelium unmasked a noteworthy polyp miss rate, inversely linked to their size.
Colonoscopy is currently considered as the gold standard for the detection of colonic neoplasia. However, emerging data demonstrate that a significant proportion of precancerous lesions are missed during the procedure, limiting its efficacy and leading to interval cancers[19].
It is established that variations in colonoscopy quality reflect differences in numerous patient-, procedure- and endoscopist-related parameters. Taking that into consideration, a great body of interventions has been conducted aiming to decrease colonoscopy’s native imperfections, including internal audits and feedback to individual endoscopists, education in quality indicators, implementation of mandatory withdrawal times, bowel preparation modifications, discussion with poor-performers, introduction to emerging technologies, routine sedation administration, repeat attempts for cecal intubation and report card utilization[20-22].
In terms of pre-colonoscopy bowel preparation, numerous interventions have been suggested. These include dietary modifications and various purgatives alone or combined with adjunctive agents (e.g., prokinetics, enemas, simethicone). Timing of bowel preparation administration has been tested in several randomized controlled trials focusing on bowel cleanliness and lesion detection. Recently, the European Society of Gastrointestinal Endoscopy adopted the results of a meta-analysis recommending split dose preparation for morning colonoscopies[10,23,24]. In line with this, our study highlights the significantly better colon cleansing achieved with split-dose preparation, as well as its contribution to increase polyp detection. However, our splitting of PEG dose was 3:1, in contrast to the recommended 2:2. Additionally, we did not collected data with respect to patients’ satisfaction, impact on daily activities and willingness to repeat the same bowel preparation in the future, if indicated.
Our data supports the importance of better bowel preparation in the detection of additional polyps. This finding is in line with the results of Gurudu et al[21] demonstrating improved polyp detection rates (PDR) and ADR with split-dosing. Unfortunately, we cannot provide information for possible differences in adenoma detection in the present study, as the index series of colonoscopies was diagnostic. However, PDR and ADR seem to correlate well, at least in segments proximal to the splenic flexure[25].
Miss rates for total, diminutive, small and large polyps were 25.4%, 30.1%, 13.7% and 6.6% respectively. These results indicate that the smaller the polyp size, the higher the polyp miss rate, which is in accordance to findings of previous studies[6,26]. Location did not affect the polyp miss rates similarly to a recent study conducted by Ahn et al[27]. Interestingly, other data suggests that the risk of missing a polyp is related to left colon location[28]. However, it should be clearly stated that no gold-standard bowel preparation method against which our studied alternatives (i.e., previous-day vs split-dose preparation) were compared in terms of polyp miss rates was available. Therefore, we favored split-dose preparation’s findings to serve as comparator given that it yielded significantly better results as regards colon cleanliness. This reflects the current knowledge that the risk of missing polyps and adenomas during colonoscopy is affected by bowel preparation quality[29]. Nevertheless, our assumption encompasses a disadvantage of this study and weakens its conclusions.
As obvious, this study bears several limitations. First, we enrolled a small number of patients, which limits the power of our results. Second, we used as as reference methodology the results of the split-dose examination to calculate miss rates, as presented above. Third, we did not assess the inter-observer agreement considering bowel preparation status evaluation. Our results could have been affected by a possible significant discrepancy between the two examiners in rating preparation quality. Fourth, we could not provide data regarding histological features of polyps identified in the first series of colonoscopies (as they were not removed). Fifth, no reports of patients’ preference in terms of timing of purgatives administration and comfort during the examinations were collected (the majority of patients had received sedation). Sixth, we did not captured data regarding withdrawal times which seem to influence ADRs. Additionally, we did not utilize validated scales such as Boston or Ottawa scales to assess the quality of cleansing in each bowel segment, as the Aronchick scale is closer to what an endoscopy unit uses in its “normal” -outside a study-practice, which was what we actually wanted to assess. Finally, we are not aware of the true polyp miss rate, since we considered the second colonoscopy as the gold standard.
In conclusion, our results support that split-dose bowel preparation improves the quality of colonoscopy in terms of mucosal cleanliness and polyp detection. However, future efforts to identify barriers and develop interventions aiming to further enhance colonoscopy effectiveness in the prevention of CRC are also necessary, as there are many factors that contribute to a high-quality examination.
Several factors influence colonoscopy quality and affect its potential to decrease colorectal cancer incidence. Quality of bowel preparation represents one of the most studied ones. In this setting, numerous regimens, combinations and administration timings have been tested. Apart from rating bowel cleanliness achieved, polyp and adenoma detection seems to improve in parallel to the quality of preparation. This retrospective study assesses two different schedules of preparation regimen administration in terms of bowel cleansing and polyp detection.
In this study it is suggested that splitting preparation regimen results in better quality of colon cleanliness than that achieved by previous-day dosing and leads to improved polyp detection.
The authors’ 3:1 splitting of polyethylene glycol (PEG) regimen is shown to significantly improve the adequacy of bowel preparation and increase the number of detected polyps in both entire and colon segments. A remarkable polyp miss rate is substantially unmasked.
The results of this study serve as additional evidence aiming to improve colon cleanliness and polyp detection rates in every day clinical practice.
Polyps per patient: number of detected polyps/number of patients. Polyp miss rates: number of missed polyps/total number of missed polyps + total number of polyps on initial examination. PEG is an osmotic laxative containing PEG, water and added electrolytes that is used in bowel preparation prior to colonoscopy and surgery.
The manuscript “Improved bowel preparation increases polyp detection and unmasks significant polyp miss rate“ is clear and well-written. The manuscript reports on the comparison of two methodologies, full dose vs spilt dose, in colonoscopy and concludes with the report, that split-dose regimen enhanced polyp detection and reduced polyp miss rate.
P- Reviewer: Berkemeyer S, Sidiropoulou Z S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 1059] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3126] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 3. | Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 5. | Huang Y, Gong W, Su B, Zhi F, Liu S, Jiang B. Risk and cause of interval colorectal cancer after colonoscopic polypectomy. Digestion. 2012;86:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 7. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1048] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 8. | Benson ME, Reichelderfer M, Said A, Gaumnitz EA, Pfau PR. Variation in colonoscopic technique and adenoma detection rates at an academic gastroenterology unit. Dig Dis Sci. 2010;55:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Long MD, Martin C, Sandler RS, Herfarth HH, Shaheen NJ, Dellon ES. Reduced polyp detection as endoscopy shift progresses: experience with screening colonoscopy at a tertiary-care hospital. J Clin Gastroenterol. 2011;45:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Kilgore TW, Abdinoor AA, Szary NM, Schowengerdt SW, Yust JB, Choudhary A, Matteson ML, Puli SR, Marshall JB, Bechtold ML. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011;73:1240-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Lee TJ, Rees CJ, Blanks RG, Moss SM, Nickerson C, Wright KC, James PW, McNally RJ, Patnick J, Rutter MD. Colonoscopic factors associated with adenoma detection in a national colorectal cancer screening program. Endoscopy. 2014;46:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 384] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Rembacken B, Hassan C, Riemann JF, Chilton A, Rutter M, Dumonceau JM, Omar M, Ponchon T. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy. 2012;44:957-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 14. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 559] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 15. | Brahmania M, Ou G, Bressler B, Ko HK, Lam E, Telford J, Enns R. 2 L versus 4 L of PEG3350 + electrolytes for outpatient colonic preparation: a randomized, controlled trial. Gastrointest Endosc. 2014;79:408-416.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | de Leone A, Tamayo D, Fiori G, Ravizza D, Trovato C, De Roberto G, Fazzini L, Dal Fante M, Crosta C. Same-day 2-L PEG-citrate-simethicone plus bisacodyl vs split 4-L PEG: Bowel cleansing for late-morning colonoscopy. World J Gastrointest Endosc. 2013;5:433-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Park JJ, Lee SK, Jang JY, Kim HJ, Kim NH. The effectiveness of simethicone in improving visibility during colonoscopy. Hepatogastroenterology. 2009;56:1321-1325. [PubMed] |
| 18. | Aronchick CA. Bowel preparation scale. Gastrointest Endosc. 2004;60:1037-1038; author reply 1038-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1467] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 20. | Taber A, Romagnuolo J. Effect of simply recording colonoscopy withdrawal time on polyp and adenoma detection rates. Gastrointest Endosc. 2010;71:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Gurudu SR, Ramirez FC, Harrison ME, Leighton JA, Crowell MD. Increased adenoma detection rate with system-wide implementation of a split-dose preparation for colonoscopy. Gastrointest Endosc. 2012;76:603-8.e1. [PubMed] |
| 22. | Imperiali G, Minoli G, Meucci GM, Spinzi G, Strocchi E, Terruzzi V, Radaelli F. Effectiveness of a continuous quality improvement program on colonoscopy practice. Endoscopy. 2007;39:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, Grosso B, Jimenez A, Ortega J, Quintero E. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;12:6161-6166. [PubMed] |
| 24. | Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 25. | Boroff ES, Gurudu SR, Hentz JG, Leighton JA, Ramirez FC. Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroenterol. 2013;108:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 27. | Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The Miss Rate for Colorectal Adenoma Determined by Quality-Adjusted, Back-to-Back Colonoscopies. Gut Liver. 2012;6:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | Leufkens AM, van Oijen MG, Vleggaar FP, Siersema PD. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012;44:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | Hong SN, Sung IK, Kim JH, Choe WH, Kim BK, Ko SY, Lee JH, Seol DC, Ahn SY, Lee SY. The Effect of the Bowel Preparation Status on the Risk of Missing Polyp and Adenoma during Screening Colonoscopy: A Tandem Colonoscopic Study. Clin Endosc. 2012;45:404-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |