INTRODUCTION
Bacillus anthracis (B. anthracis), the causative organism of anthrax is a Gram-positive spore forming bacillus commonly found in soil of endemic areas. Anthrax is a zoonotic disease which is mainly associated with herbivores and domestic animals. The disease occurs regularly in countries where widespread vaccination of animals is not practiced. Human anthrax is less common and usually spreads to human populations through close occupational proximity to infected livestock by handling infected domestic animals including cattle and goats or their products like skin, meat, hides and bones. This bacterium can infect humans by cutaneous, gastrointestinal, or respiratory routes[1]. B. anthracis exists in two forms, vegetative cells (inside the host) and spores for persistence in the soil or environment[2]. In the soil, B. anthracis is generally found in endospore form where it can remain viable for decades in this form. As B. anthracis forms spores that can be aerosolized and sprayed to spread disease, the potential use of this bacterium as a bioterrorism agent has long been suspected. However, the events in 2001 have confirmed that bioterrorism is no longer a threat but a reality[3]. Owing to its highly pathogenic nature and spore forming capability, B. anthracis is considered as one of the most important biological warfare agents[4,5].
There are two major virulence factors in B. anthracis, poly-γ-D-glutamic acid capsule and a tripartite toxin[6]. Pathogenic B. anthracis bacteria produce capsule which mimics the immune system of host by masking the bacteria from macrophages[7]. The tripartite toxin of anthrax consists of three independently secreted proteins, i.e., protective antigen (PA), lethal factor (LF) and edema factor (EF)[8,9]. Anthrax toxin is a binary A-B toxin, where PA acts as the binding (B) domain and LF and EF act as active (A) domains individually to form the binary toxins lethal toxin (LTx) and edema toxin (ETx), respectively[10]. After ingestion or coming in contact with skin lesions, bacteria multiply and within a few days or weeks cause the death of human or animal host.
Anthrax is not a major issue of health in developed countries as only a few incidences are reported from such countries. However, for developing countries whose economy is mainly agriculture dependent, cutaneous anthrax is still a major concern of health. India ranks first in having the world’s largest livestock population. Therefore, animal anthrax is common in several regions in India. However, only a few intermittent cases of human anthrax are reported from the Southern states[11]. Human cutaneous anthrax is a concern of public health in some states like Orissa and Andhra Pradesh[12].
HISTORY OF ANTHRAX
Anthrax, caused by B. anthracis is a highly contagious and fatal. Anthrax has a long association with human history and was known in Europe (1190-1491 BC) and China (3000 BC). Anthrax was described in the early literature of the Greeks, Romans and Hindus. The name anthrax was derived from the Greek word “anthrakis” which means coal because coal black skin lesions are formed in cutaneous form of anthrax. The description of fifth plague of Egypt, an epidemic of ancient Egypt in the book of Genesis (1491 BC), which exterminated the Egyptian livestock including cattle, sheep, goats, camels, horses and donkeys without affecting the Israelites livestock, may be due to anthrax. The disease described by Virgil (29 BC) in his third Georgics (selection of poems on agriculture and animal husbandry) seems to be anthrax in domestic and wild animals as it was an economically important agricultural disease in Europe during the 16th to the 18th centuries[13].
In 19th century, research on anthrax led to a lot of medical developments. In 1850, Pierre Rayer first described filiform bodies (small rods, about half the length of a red blood corpuscle) in the blood of sheep that had died due to anthrax. Casimir-Joseph Davaine in 1863 suggested that the “corpuscles” were the etiology of anthrax that could be transmitted to sheep, horses, cattle, guinea pigs, and mice by subcutaneous inoculation of infected blood[14]. Tiegel and Klebs in 1864 demonstrated that anthrax-infected blood, if filtered through a clay candle (bacterial filter), lost its infectivity, while the deposit on filter remained infective[15]. These observations in absence of culture of the organism strongly supported the concept that the causative agent of anthrax was a living organism that multiplied in the body, invaded the blood stream, and produced death by septicaemia. Robert Koch derived his three postulates for germ theory of disease considering anthrax as prototype. In 1876, he conclusively proved that B. anthracis was the etiological agent of anthrax by applying his postulates for the first time during his research in Wollstein, Germany[16]. Thus, anthrax was the first disease whose causative agent was established as microbial agent. After isolating the anthrax bacteria from skin lesions of sheep, he obtained the pure cultures by growing the bacilli on the aqueous humor of ox’s eye, and injected the bacteria into healthy sheep. He performed another experiment by growing pure cultures of rods from the aqueous humor of an ox’s eye. By studying, drawing and photographing these cultures, he recorded the multiplication of the bacilli and found that under unfavourable environmental conditions, especially under conditions of oxygen deprivation, they produced round spores within themselves. The spores return to bacilli when growth conditions are favourable, proving the spore formation as self-protective mechanism of B. anthracis. Thus, by now it was clear how certain pastures or agriculture areas became dangerous. When any animal dies from anthrax infection, the infected blood and body fluids comes out in soil from the natural orifices of animal. The bacteria, which are in the vegetative form in the blood, convert into spores on exposure to air. These spores are extremely resistant to natural conditions and could remain dormant in the soil for decades. These spores remain available to cause new infections among susceptible animals that graze in the field.
Pasteur et al[17] proved the buried cadavers of anthrax infected animals as important origin of new infections. They further revealed that spores from buried soil could be transported to the upper surface by the activities of earthworms[18]. He also confirmed Koch’s discovery of the anthrax germ. He found that chickens were immune to anthrax, and postulated that it was because of high body temperature (43 °C-44 °C) of chickens. On lowering the body temperature to 37 °C, chickens became susceptible to anthrax. For vaccination, Pasteur heated the anthrax germs and inoculated 25 sheep. He used the heated anthrax bacteria to inoculate sheep and found that all sheep survived (only one pregnant sheep died due to some other complications), whereas all un-inoculated sheep died after one or two days of challenge with virulent B. anthracis. Pasteur proved that the weakened anthrax lost its virulence but still could confer immunity and this technique was termed as “vaccination”. Thus, first live bacterial vaccine was developed for anthrax by Pasteur et al[17]. During 1876-1877, a devastating anthrax outbreak affected several sheep and cattle in France’s livestock. By that time, rod-shaped B. anthracis was established as the causative agent of anthrax by Robert Koch. However, still many people believed that instead of bacterium itself, some toxic substance produced by B. anthracis was causing the disease. But, Pasteur finally proved that anthrax was caused by living B. anthracis and not by some toxic substance.
Anthrax was also known as woolsorters’ disease. Prior to 1837, no specific disease had been associated with wool. However, after that a large number of cases occurred in and around Bradford, England and the name Bradford disease became synonymous with woolsorters’ disease. In 1879, Bell proved that woolsorters’ disease (now inhalational anthrax) was due to anthrax[19]. This led to institution of Bradford rules which in 1897 became law. Consequently, incidences of inhalation anthrax among sorters decreased significantly. In 1913, Eurich found that blood contamination was the important factor in woolsorters’ disease[20]. Blood seemed to serve as a glue to bind anthrax spores to the raw product. Washing of wool removed soil, dried serum and blood but anthrax spores remained adherent. Elimination of inhalation anthrax as an industrial hazard followed passage of the Anthrax Prevention Act in 1919. This law mandated the construction of a decontamination station in Liverpool whereby all dangerous wool and hair products entering England were disinfected with formaldehyde[21].
During 1979-1980, the world’s largest ever recorded outbreak of anthrax occurred in Zimbabwe during the civil war. In a two-year period, over 9400 cutaneous anthrax cases, including 182 fatalities were reported. Before the war, anthrax was endemic in Zimbabwe and only a few cases of anthrax were reported. Number of human anthrax cases increased significantly during this period because lack of food due to civil war in country forced people to handle and eat anthrax infected animals. Anthrax being a zoonotic disease, it first appeared in cattle and then spread in human population in all the affected areas of Zimbabwe.
Anthrax has been supposed to be developed for use as a bioweapon during world war-1 and world war-II. Recently, in 2001, envelopes containing the B. anthracis organism were sent through the mail to different dignitaries in United States affecting 22 people. This was considered as an act of bioterrorism[3].
BIOLOGY OF B. ANTHRACIS
B. anthracis is a Gram positive, rod-shaped, aerobic, facultative anaerobic, sporulating, capsulated bacterium. It measures 1-1.2 μm in width and 3-5 μm in length. Under microscope, it appears as chain like structure. Though an aerobic organism, yet B. anthracis can survive in anaerobic environment because of its property of sporulation. In fact, it can survive for several years in soil, air and water in the form of spores. Unaffected to harsh environment, spores are resistant to high temperature, pressure, pH, chemicals, UV and deficiency of nutrients[22-24]. The capsule is composed of γ-linked poly-D-glutamic acid which gives mucoid appearance to the colony. Formation of capsule decides the virulence of bacteria. The capsule itself is non-toxic and doesn’t provoke immune system of the host. However, it contributes significantly in establishing the infection, once the organism escapes phagocyte action, later phase of disease is controlled by anthrax toxin[25].
Pathogenic strains of B. anthracis harbour two virulent plasmids[26]. Plasmid pXO1 carries toxins encoding genes and plasmid pXO2 carries capsule encoding genes. Size of pXO1 is 184.5 kb that harbours three structural genes, pag (coding for PA), lef (coding for lethal factor) and cya, coding edema factor[1]. Plasmid pXO1 also encodes atxA gene which regulates the expression of gene encoded on pXO1 and pXO2. Another plasmid pXO2 is 95.3 kb in size and carries the genes for capsule production, degradation and regulation. Genes capB, capC and capA code for capsule synthesis, and gene dep codes for its degradation[1]. A gerX operon is also present on plasmid pXO1 and its deletion affects the germination of spores in macrophages. The operon codes for three proteins GerXA, GerXB and GerXC. These proteins are supposed to form a receptor, which specifically detects germinant within the host[27].
POTENCY OF BACILLUS ANTHRACIS AS BIOWARFARE AGENT
Anthrax was linked to soil contamination long before the identification of B. anthracis as its causative agent[14,16]. Spores can resist prolonged exposure to stress as desiccation, solvents and extreme temperature, pressure, pH, ultraviolet and ionizing radiation[28,29]. Spores of Bacillus genus are known to have a half life of about 100 years[30]. Spores are dormant form of the bacterium which returns into vegetative form on receiving the signals for germination. The surprisingly resistant spores have earned the status of potential bio-terror weapon for anthrax. The possibility to create aerosol from spores makes B. anthracis a lethal biological weapon. All the attributes of spores: high resistance to temperature, pressure, pH, ionizing radiations and half life of 100 years make them a suitable bio-terror agent. After production and purification, anthrax spores can be stored in a dry form which remains viable for decades. Spores may survive in the water, soil and on surface for several years. Inhalation of spores causes inhalational anthrax which is the most dangerous form of disease. Inhalational anthrax is dangerous for obvious reasons as initial symptoms resemble to that of flu, making its early diagnosis difficult; by the time disease is correctly recognized it’s too late.
The use of microorganisms as a means of waging war or as bioterror agents is becoming a real possibility now around the world. Any biological agent from a large gamut of human infection causing pathogens could be considered a potential biological weapon. However, only a small number of these agents fulfil the desirable criteria like ease of cultivation and dispersal or dissemination for recognition as possible biological weapons. Anthrax spores pose the biggest bioterrorism threat because it is easier to produce and preserve them. Anthrax spores have already been used in United States and in future also it is most likely preferable agent to be used for biothreat because of high case fatality rates, rapid transmission by aerosol and its stability in the environment. The release of any bio-warfare agent by a militant or miscreant would likely be silent and untraceable or nearly so. Therefore, of the recognized possible biological weapons, anthrax bacilli are rated the most lethal.
Naturally, anthrax is a zoonotic disease, which primarily occurs in animals and then spreads to human. Several animal species like cattle, goat and sheep are susceptible to this disease. A major public health preparedness challenge is increasing the importance of recognition of individual, potential sentinel cases of biothreat agent disease. According to CDC norms, B. anthracis is placed in high priority- Category A due to its ease of dissemination, high mortality rates, epidemic potential and special preparedness it requires. In 2001, mails deliberately contaminated with B. anthracis spores were used to terrorize people and subsequently research for the development of anthrax vaccine speeded up. Moreover, each category A biothreat agent has its unique clinical and diagnostic features and no single system can meet the challenges of all the agents. Besides, anthrax is still a concern of human as well as veterinary public heath in several states of country like India. Bioterrorism itself is an emerging problem for public health. Hence, it is not possible to look into bioterrorism and public health separately. Rather, it is the need of time to give more emphasis on such diseases which have both the potential.
DOSE-RESPONSE RELATIONSHIP
The information on dose-response relationship is prerequisite for assessment of risk of any biothreat agent. The LD50 of human inhalational anthrax is not known, but has been estimated from the animal studies and disease outbreaks. After conducting experiments on 1236 cynomolgus monkeys (Macaca fascicularis), Glassman estimated the median lethal dose to be 4130 spores with 95%CI range of 1980-8630[31]. Further, he suggested that LD25 was associated with a 10-fold decrease in dose i.e., 413 spores. In 1957 in Manchester, 16 susceptible workers were exposed to B. anthracis in a goat hair processing mill and 4 persons were infected. Based on the 8-h inhaled dose, LD50 of B. anthracis was estimated to be 6200-22000 spores[32]. The infectious dose for inhalational anthrax in 50% of susceptible human population (ID50) was estimated to be 8000-50000 spores by biodefense experts from the United States Army Institute of Infectious Diseases (USAMRIID, Fort Detrick, MD)[33]. In 1998, a panel of seven subject matter experts on anthrax calculated the ID10, ID50 and ID90 as 1000-2000 spores, 8000 to 10000 spores and 50000 to 100000 spores, respectively[34]. Another group extrapolated the lethal dose (LD50) values of 4100 spores[31] and 8000 spores[33] and suggested an LD10 of 50 or 98; an LD5 of 14 or 28, an LD2 of four or seven, and an LD1 of one or three spores[35]. Although they did not establish the validity of extrapolation, yet they cautioned about the low number of spores.
Theoretically, even a single spore of B. anthracis can cause anthrax. However, in the low dose range, there is high uncertainty between the dose-response relationships of aerosolized B. anthracis for human. Recently, on the basis of experimental data on primates and epidemiological data of human anthrax, a new quantitative model known as Exposure-Infection-Symptomatic illness-Death (EISD) has been suggested for the dose-response as well as time course of pulmonary anthrax in human[36]. According to this model, the ID50, ID10 and ID1 of B. anthracis spores were 11000 (95%CI: 7200-17000), 1700 (1100-2600) and 160 (100-250), respectively. The ID50 (7200-17000) and ID10 (1100-2600) confidence ranges produced by this model were remarkably consistent with the corresponding ranges produced by an expert panel surveyed in 1998, i.e., 8000-10000 and 1000-2000, respectively[34]. The confidence range of ID1 from 100-250 spores as suggested by this model indicates that a threshold of 600 B. anthracis spore to human infection is underestimated and infection by even a single spore is overestimated in the literature. This model also suggested the median incubation time from exposure to onset of symptoms. For exposure with ID50 of B. anthracis spores, it was 9.9 d with 95%CI of 7.7 to 13.1 d, where as for ID10 and ID1, it was 11.8 (95%CI: 9.5-15) d and 12.1 (95%CI: 9.9-15.3) d, respectively.
DIFFERENT STRAINS OF BACILLUS ANTHRACIS
Three well known strains of B. anthracis are Ames, Sterne and Vollum. Ames is a well studied, highly virulent strain containing both plasmids, i.e., pXO1 and pXO2. Originally it was isolated from a dead cow in Texas in 1981. Its geographic region is United States and United Kingdom. Another isolate of Ames strain is Florida which was first isolated from a victim of anthrax attack in 2001[37,38]. B. anthracis Sterne is a toxigenic but avirulent strain as it carries the anthrax toxin plasmid pXO1 but lacks the capsule forming plasmid pXO2[39]. This strain is generally used for vaccine development for animals. Its geographic region is in Canada[37,38]. In contrast to Sterne, Pasteur strain carries pXO2 plasmid but not pXO1. Vollum is low virulent strain used in research studies and is found in the United Kingdom, Spain and Zimbabwe[37]. Along with Vollum and Sterne, strain V770 is also used for toxin production and various research related studies.
B. anthracis belongs to Bacillus cereus sensu lato group, shared by six other species including B. cereus, Bacillus mycoides, Bacillus pseudomycoides, Bacillus thuringiensis, Bacillus weihenstephanensis, and Bacillus cytotoxicus[40]. B. cereus primarily causes foodborne illness. Besides, B. cereus is considered as an opportunistic pathogen that can cause wound infections, endocarditis and urinary tract infections in humans. Recent studies indicate that a Bacillus species other than B. anthracis can cause anthrax-like disease and a few B. cereus strains have been found to be associated with “anthrax like” infections in human[41,42]. In India, a B. cereus strain TF5 was isolated from the tissue fluid of cutaneous anthrax-like skin lesions of a human patient from an anthrax endemic area in India[43,44]. The strain harboured a PA gene, however, the pXO1 or pXO2-like plasmids were not present. Exoproteome analysis exhibiting qualitative and quantitative differences between the two strains indicated an altered regulatory mechanism and putative role of S-layer protein and sphingomyelinase in the pathogenesis of strain TF5[43].
EPIDEMIOLOGY OF ANTHRAX
B. anthracis bacteria are very fragile and susceptible to disinfectant or exposure to moderate temperature. However, B. anthracis vegetative cells convert into spores on exposure to air. These spores are highly resistant to heat and to most of the disinfectants. Therefore, post-mortem of anthrax infected animals is never recommended to avoid the exposure of bacteria to oxygen. A peculiar feature of anthrax infection in animals is that blood does not clot and drains from the natural orifices like nose, mouth and bowl. This results in contamination of soil and water with bacteria which ultimately transform into spores[45]. As much as 109B. anthracis bacteria may be present in the oozing blood[46]. Even the processed parts and products like leather, hides, wool, etc., of an anthrax infected animal can carry spores for year. The spores can remain viable for a prolonged period in the soil, especially when deposited 15 cm below the upper soil levels.
Environmental and climatic factors have a great influence on the ecology of anthrax[47]. Climatic factors like rainfall and temperature play a pivotal role in incidences of anthrax cases[48]. However, it is not easy to understand the anthrax occurrence and its epidemiology due to large variations in timing of different outbreaks and associated deaths of a particular species even within a single ecosystem[49]. It has been hypothesized that some soil factors like alkaline pH, high organic content, moisture, and ambient temperature (in excess of 15.5 °C) favor the germination of B. anthracis spores into vegetative bacteria, which ultimately results into amplification of number of spores[22]. It has been observed that high pH and high contents of calcium in soil contribute to maintain the spores viable for a longer time. These soil spores cause new infections when come into contact of a suitable new host[22,50,51]. Therefore, alkaline pH of soil, high moisture and organic contents, precipitation and ambient temperature in excess of 15 °C are deciding factors for triggering a large anthrax outbreak and can be considered to predict exposure and infection risk of anthrax in a particular area[48]. During grazing, herbivores animals are most likely to be exposed to B. anthracis spores by inhalation or ingestion during grazing. It has been observed that B. anthracis bacteria need specific nutrients (animal blood, viscera) and physiological conditions and therefore it is very difficult to survive outside a viable host and convert into spores. Moreover, the vegetative cells of B. anthracis are poor competitor and are easily killed by other bacterial species outside the host in environment. Moreover, virulence of B. anthracis is reduced when grown outside the host and bacteria with reduced virulence will not lead to an outbreak[22].
According to an estimate, every year about 2000 to 20000 human anthrax cases occur globally. Apart from India and Pakistan, anthrax has also been reported from Bangladesh, Zimbabwe, United States, South Africa, Iran, Iraq and Turkey. In India, southern states are more prone to anthrax. Reports of anthrax appear almost every year from Andhra Pradesh, Tamil Nadu and Karnataka but exact figures are not available. In 1980s, there were only 2000 cases reported worldwide most of them were of cutaneous anthrax. Most of the anthrax cases recorded were from the persons involved in industrial occupations related to processing of animal parts and products like meat packing, bone meal processing, tanning of leather and sorting of hair wool[52]. Several outbreaks have been recorded in recent history. Anthrax outbreaks in animals are more prominent and common than humans. From 1991 to 1996, a total of 1612 anthrax outbreaks occurred in India. In Nepal, a total of 222 animals were affected during 19 different outbreaks in 1996[53]. In 1996, about 1570 cases of ruminant anthrax were reported in China. The death of 204 livestock in Australia was reported in 1997[54]. From 1984 to 1989, thousands of wild animals were killed in an anthrax epidemic in Namibia and South Africa[53]. In Iran, about one million sheep were killed during an anthrax outbreak in 1945. In Manchester, United States, a large anthrax epidemic occurred in 1957 in a goat hair processing plant resulting in four fatalities and nine cases[55]. In Russia during 1979, an unusual, accidental anthrax outbreak in a Soviet military laboratory of Sverdlovsk killed 68 persons out of 79 infected[56]. In Zimbabwe, 10000 cases occurred between 1979 and 1980 leading to 182 deaths. In Tibet, 507 anthrax cases resulted in 162 deaths in 1989 and in China, 898 and 1210 anthrax cases were recorded in 1996 and 1997, respectively. Between 1991 and 1995, a relatively large number of anthrax incidences was observed in Spain[49,57], Central America[57] and Africa[53]. In most of the cases, exposure was through cutaneous route which accounts for a total of 95% cases. The inhalational route accounts for 5% anthrax cases reported, while gastrointestinal anthrax is quite rare[58,59]. In 2007, a few animal and human cases of anthrax were reported from Orissa and West Bengal, India[60]. The most recent anthrax cases were found in 2010 in Bangladesh. More than 600 peoples were killed in the outbreak due to consumption of infected cattle meat[61].
As India stands first in having the largest population of livestock in the world, therefore anthrax is endemic in several regions. Based on the epidemiological study from 1991 to 2010 by National Animal Disease Referral Expert System (NADRES) in India, anthrax was found one of the ten major diseases causing deaths in livestock[62]. During 1991-2010, anthrax was reported in eighteen states of India viz., Andhra Pradesh, Assam, Bihar, Chhattisgarh, Gujrat, Himachal Pradesh, Jammu and Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Manipur, Meghalaya, Odisha, Rajasthan, Tamil Nadu, and West Bengal. Although several regions are endemic for anthrax, yet seasonal fluctuation in the number of anthrax outbreaks has been observed. Most of the anthrax outbreaks are reported in post-monsoon season, i.e., from July to September and November to January in different parts of India. Anthrax epidemics are generally reported between July to September and also in November and January, coinciding with the post monsoon months across the country. Several Southern states such as Andhra Pradesh, Tamil Nadu, Kerala, Karnataka and Orissa are common endemic regions with sporadic human anthrax cases reported time to time. From the Union Territory of Pondicherry, 28 cases of anthrax were detected in 1999 and 2000[45]. Both, animal as well as human anthrax cases are reported usually from certain anthrax endemic districts like Chittoor, Cuddapah, Guntur, Prakasam and Nellore of Andhra Pradesh[63]. In 2006, some cases were noticed near Narsinghpur, Madhya Pradesh also. In 2007, 20 people were affected in two cutaneous anthrax outbreaks in Murshidabad district, West Bengal. These anthrax outbreaks were caused due to slaughtering of sick cattle and subsequently handling of meat without taking proper preventive measures[64]. An increase in number of animal and human anthrax cases has been observed in this area in recent past[65]. During a tenure of 10 years, anthrax outbreak were reported at least 61 times from Orissa affecting 750 people[65]. The anthrax outbreak is a common phenomenon in this area because tribal population mainly depends on forest for livelihood. Most of the human anthrax cases occur in agricultural workers due to handling of meat or hides of diseased animal. An anthrax outbreak was reported in Orissa, India in 2013 where several people died due to consumption of infected goat meat[66]. Recently, nine cutaneous anthrax cases were reported from the tribal population of Midnapur, West Bengal in India[67].
VIRULENCE OF B. ANTHRACIS
Anthrax, being a disease of mainly herbivorous is generally prevalent in those areas where animals like cattle, horse, sheep, goat, etc., graze. Several animal species like pigs, dogs, cats, rats and chicken are fairly resistant to anthrax. Many scavenging birds like vultures which feed on dead animals have a natural resistance to anthrax. However, such birds may disseminate the anthrax spores from infected animals through claws, beaks or feathers.
The spores of B. anthracis that can remain in the environment for a prolonged time become the infectious form of anthrax. For causing anthrax, spores first germinate, i.e., lose their dormancy and resistance properties, regain metabolism and start vegetative growth[68,69]. After getting favourable environmental and nutritional growth condition, spores convert into vegetative bacteria and result in further multiplication. Human skin generally does not permit spores to invade; however, spores find access through small cuts or abrasion in skin to cause cutaneous anthrax. After entry into host, B. anthracis remains in the capillaries of invaded organs and produce lethal and edema toxins which cause the local and fatal effects of infection.
TOXINS OF B. ANTHRACIS
In soil, B. anthracis is found in its highly resistant en-dospore form and therefore, can remain live for a very long period in this state. Spores of B. anthracis can find entry in the body through lungs, skin lesion or gastrointestinal route and germinate to yield vegetative form. In case of cutaneous infections, B. anthracis comes into contact with a skin lesion, or cut. In inhalational cases, herbivorous and sometimes humans are infected after inhalation of spores. After inhalation, these spores reach alveoli of lungs through air passages. Generally, herbivores get gastrointestinal anthrax infection during grazing or browsing an anthrax spore infested agricultural field having spiky or rough vegetation. Gastrointestinal tract of animals probably gets wounds due to eating of spiky vegetation which facilitates the entry of spores into tissues and resulting in gastrointestinal anthrax.
The virulence of B. anthracis is attributed to a tripartite anthrax toxin and a poly-D-glutamic acid capsule. After entry into the host through ingestion or skin wounds, B. anthracis multiply inside the tissues of animal or human host, spread in the lymphatic system and undergo rapid multiplication. This results in production of anthrax toxin inside the body and causes death of host within a few days or weeks.
Capsule formed by the virulent B. anthracis vegetative cells helps the bacterium to evade the host immune system by impeding the ability of macrophages to engulf and destroy the bacteria[7]. Three non-toxic proteins namely PA, LF and EF of anthrax tripartite toxin co-assemble to produce a series of free or cell-bound toxic complexes[8,9,70]. Two of the toxins, LF and EF, are enzymes that modify substrates within the cytosolic compartments of host cells[71]. PA binds on the receptors of host cells and makes a pore for transportation of LF and EF to the cytosol[72]. Thus, anthrax toxin is an A-B type toxin, where PA acts as B subunit and it combines with the LF and EF, which act as A subunits to form the edema toxin and lethal toxin, respectively[10,17].
Anthrax PA is an 83 kDa precursor polypeptide consisting of 735 amino acids which binds to anthrax toxin receptors. There are two distinct toxin cell receptors, ANTXR1 (TEM8, Tumor endothelial marker 8) and ANTXR2 (CMG2, Capillary morphogenesis protein 2) which are widely expressed in cells[73,74]. Cleavage of PA by cellular proteases of the furin family, or by serum proteases generates a nicked 20 kDa fragment (PA20) at N-terminal and a 63 kDa fragment (PA63) at C-terminal[75,76]. The 63 kDa fragment self-associates to form a prepore which is a heptameric ring and can bind up to three copies of EF and/or LF molecules[6]. A smaller population of PA octamers (20%-30% of oligomers) is also formed, which binds up to four molecules of EF and/or LF and this structure is more stable than heptamer[77]. These hetero-oligomeric complexes are endocytosed and brought to an acidic environment, where the PA prepore makes a translocase channel after inserting into the membrane[78]. This channel is used for translocation of LF and EF into the cytosol, where by enzymatic activities they disrupt the host cell[79]. Both, LF and EF toxins reach the late endosomal compartment, where EF remains associated with the late endosomal membranes that surrounds the nucleus forming a perinuclear necklace and LF is ejected into the cytoplasm[80,81].
LF is a zinc dependent metalloprotease which inac-tivates the members of mitogen-activated protein kinase kinase family (MAPKK)[82-84]. Inactivation of three major MEKs i.e., extracellular signal regulated kinases, c-Jun N-terminal kinases and p38 MAPKs results in impairment of various cellular processes like cell division, cell differentiation, cellular response to different types of stress and ultimately apoptosis[17].
Another protein EF is has adenylate cyclase activity. It is produced in an inactive form by the bacterium and needs calcium modulated protein (calmodulin, CaM) for its activity[71]. CaM, which acts as Ca2++ sensor has two Ca2++ binding sites on each of the C- and N-terminal domain. CaM binds with helical domain of EF using its N-terminal domain. EF is a highly active and its adenylate cyclise activity is almost equal to that of most active known cyclase. Activity of EF is also regulated by intracellular level of Ca++ in a biphasic manner. Resting or little high levels of Ca++ activate the EF via CaM, whereas high levels of Ca2++ reduce its activity due to competition between Ca++ and Mg++ ion in the EF active site[85]. Because EF is associated with the perinuclear later endosomal membrane, therefore, a cAMP gradient decreasing from the nucleus to plasma membrane is generated[80,81,86]. Contrary, endogenous host adenylate cyclises generate a cAMP gradient in opposite orientation (decreasing from plasma membrane to nucleus) because these are localized on plasma membrane[86,87]. In anthrax infection, these two toxins are responsible for immune system failure and ultimate death of host[9].
PATHOGENESIS OF B. ANTHRACIS
Human anthrax is mainly of two types, agriculture related anthrax that occurs in a seasonal pattern, and occupation related that can occur at any time. On the basis of route of infection, there are three clinical forms of anthrax viz., cutaneous (skin), gastrointestinal (ingestion) and pulmonary (through inhalation of spores)[88]. Recently, another type of anthrax has been identified among the heroin injecting drug users Europe[89,90]. The term injectional anthrax was then coined to describe this new mode of infection. A few anthrax cases have been reported due to insect bites also, which could probably be due to feeding of insect on an anthrax infected animal[91,92]. Once inside the mammalian host, the high nutrient content of the body triggers germination of spores, although there may be host-specific germination factors as well[93]. Sporulation does not appear to occur inside the host[94]; perhaps because once the available nutrients are depleted in the dead or dying host, the oxygen tension is too low for Sporulation[95] or possibly due to the repression of sporulation by the virulence gene regulator AtxA[96]. Spores infect macrophages at the site of entry, germinate into vegetative cells and proliferate into the tissues and start producing anthrax toxin within 3 h of spore germination[93].
Cutaneous anthrax infection starts with a small itching papule resembling an insect bite at the site of infection on skin. In a day or 2, this papule enlarges and transforms into a painless ulcer with a depressed necrotic centre and a raised and round edge. Generally, such lesions are formed with 2-5 d at the site of spore entry on skin. Finally, after 7-10 d, a black eschar, surrounded by edema is formed and this leaves permanent scar after anthrax cure[97]. Regional lymph nodes draining the infected area may be swollen and enlarged. Cutaneous anthrax infection mostly remains painless and limited to dermis. However, in certain cases it can become systemic when bacteria enter into blood stream causing bacterimia. Hemorrhagic lesions can be developed on any part of body and can be fatal in bacteremic anthrax.
Gastrointestinal (GI) anthrax occurs by eating the food contaminated with anthrax spores (most often contaminated meat). After ingestion, spores germinate and can cause lesions anywhere in the body. Based on the lesions, GI anthrax is of two types, abdominal and oropharyngeal. In abdominal GI anthrax, lesions are formed mainly in the ileum and cecum. The incubation period is generally 3-7 d. The symptoms of abdominal GI anthrax include nausea, bloody vomiting, diarrhea, abdominal pain, headache, loss of appetite and massive ascites. Another variant of intestinal anthrax is oropharyngeal anthrax where lesions are formed mainly in the oral cavity and resemble the lesions of cutaneous anthrax. Symptoms include throat pain, problem in swallowing and swelling in neck due to edema and cervical lymphadenopathy[97].
Pulmonary or inhalational anthrax occurs by inh-alation of spores into lungs. This is the most severe form of anthrax. Alveolar macrophages ingest the spores and transport to lymph nodes in mediastinum. Initially, symptoms of inhalation anthrax are like cold or flu-like with mild chest discomfort, shortness of breath, nausea and finally severe respiratory collapse. Pulmonary anthrax doesn’t cause pneumonia, but causes hemorrhagic mediastinitis and pulmonary edema. Historical, mortality was 92%, but, it can be reduced significantly if treated early as only 45% mortality was observed during the 2001 anthrax attack in United States.
Symptoms of anthrax caused by injection remain the same as in cutaneous anthrax, but there may be infection deep under the skin or in the muscle where the drug is injected. Sometimes there is redness at the area of injection. Injectional anthrax is difficult to diagnose because several other common bacteria can cause skin and injection site infections. Therefore, it is hard to treat injectional anthrax as it spreads throughout the body very fast.
There are two basic stages in the systemic anthrax infection, a prodromal and fulminant. The prodromal stage is mainly asymptomatic and generally lasts 2-4 d[98]. In this stage, macrophages engulf the spores and release to lymph nodes near the port of entry. Behaviour of macrophages and phagocytic cells is changed due to action of anthrax toxins resulting in the apoptosis and release and germination of spores into vegetative bacteria. In the fulminant stage, bacteria multiply and are distributed to different organs through bloodstream[99,100]. In human inhalation anthrax, treatment is started after the onset of fulminant stage because prodromal stage is largely asymptomatic. The symptoms at fulminate stage are flu-like and include labored breathing, chest pain, hypotension, headache and disorientation[55,99-102]. Bacteria secrete anthrax toxins which affect functioning of different organs like spleen, lymph nodes, liver, kidney, heart and brain. It becomes very difficult to cure the disease by antibiotic therapy at this stage and action of anthrax toxins ultimately leads to septic shock and death of host in 1-2 d.
LIFE CYCLE OF B. ANTHRACIS
B. anthracis is found in two forms, vegetative cells and spores. Adverse environmental conditions induce the sporulation and endospores are released from the mother vegetative cells. The endospores are dormant, well organized and highly resistant to various stress conditions. Therefore, these endospores can remain viable for a prolonged time in the environment and can germinate into vegetative bacteria after getting the suitable envir-onmental and nutritional requirements. During both the processes, i.e., sporulation and germination, a lot of metabolical as well as morphological changes are observed. For spore formation, B. anthracis bacterium is divided asymmetrically by a septum into forespore (smaller portion) and mother cells (larger portion). Each portion gets a single copy of DNA. After the asymmetric division, forespore is engulfed by the mother cell with a double-membrane system. The mother cell DNA material is degraded and forespore DNA material is surrounded an inner membrane. Two peptidoglycan layers known as primordial germ cell wall (inner thin layer) and the cortex (outer thick layer) are synthesized between the inner and the outer membrane of forespore[103,104]. The outer membrane of forespore gets deposited by various proteins to form the coat. Thickness of spore coat varies among different species of Bacillus. In B. anthracis and B. cereus, the spore coat is compact whereas it can be distinguished in B. subtilis[105,106]. During spore maturation, spore acquires resistance for temperature and UV radiations and becomes dormant. Thus, spore coat imparts important functions to protect cortex and DNA of spore from various adverse conditions like environmental stress, chemicals and peptidoglycan lysing enzymes.
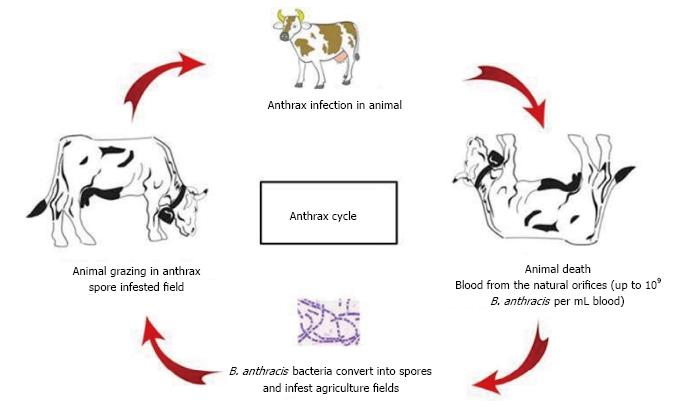
The life cycle of B. anthracis has been shown in Figure 1. Animals get infected by uptake of anthrax endospores present in the agriculture fields. Inside the mammalian host, endospores find the favourable conditions like aqueous environment with sufficient nutrients and therefore, start germination[107]. During anthrax pathogenesis, transformation of spore into vegetative cell is a crucial step, because it is the vegetative form of bacterium only which forms the virulent factors, i.e., capsule and tripartite toxin[27]. The poly-γ-D glutamic acid capsule of B. anthracis makes a complex surface of the bacterium and is surrounded by peptidoglycan layer and S-layers[108]. The capsule evades the host immune system and thus is a crucial factor for the survival of the bacteria in the host. On death, the capsulated bacteria are released with blood into the environment through natural orifices. On coming into contact with oxygen, the vegetative bacteria convert into spores and thus again infest the agriculture fields for subsequent anthrax infection in grazing animals.
Figure 1 Life cycle of B.
anthracis. B. anthracis: Bacillus anthracis.
DIAGNOSIS OF ANTHRAX
As various outbreaks are reported time to time from different areas, there is a great need of an early diagnosis of the disease to save human and animal life. Besides, requirement of rapid and reliable detection, identification and diagnosis systems for anthrax has been emphasized by recent bioterrorism events. The early monitoring of the disease requires the detection of anthrax spores and infection both at environmental and clinical levels.
Cutaneous anthrax is diagnosed clinically employing traditional microbiological methods like gram-staining, capsule staining from the smear of the lesion or culturing of B. anthracis[109,110]. Several methods have been reported for isolation and identification of B. anthracis. However, on sheep Blood agar (5%) and other routine culture media, almost all Bacillus species grow well[111]. A selective media containing polymyxin-B, lysozyme, EDTA and thallous acetate was used for isolation of B. anthracis from contaminated and suspected samples[112]. Another media (bicarbonate agar) is used to induce capsule formation for subsequent identification of B. anthracis. However, there is very little utility of these selective growth media because several closely related bacteria of B. anthracis like B. cereus and B. subtilis also grow well on these media. Another undesirable feature is that it takes 18-24 h for B. anthracis to grow for characterization by various biochemical tests like catalase, oxidase, nitrate reduction, haemolysis, citrate utilization, urease[113]. Sometimes, microbiological methods like culture and Gram staining of B. anthracis do not hold good for patients who have already taken antibiotics before the sample[114]. Immunoflorescence has also been used for direct identification of B. anthracis spores[115].
Serodiagnosis is important for surveillance and confirmation of anthrax infection in animals and human. Anthrax toxin consists of PA, LF and EF. Antibodies response against these toxin components is used as a diagnostic tool for determination of past infection or vaccination.
It is well established that PA is the most important protein of anthrax tripartite toxin and it becomes the major component of anthrax vaccines including anthrax vaccine adsorbed. Therefore, antibody (IgG) levels against PA in human and animals are determined to study the host immune response to B. anthracis infection and anthrax vaccine[116,117]. In United States, a total of 22 individuals were identified with bioterrorism-related inhalation or cutaneous anthrax, 11 patients for each type from 4th October to 20th November 2001[118]. In 16 of 17 confirmed or suspected clinical anthrax patients, anti-PA IgG antibody could be detected after 11 d of onset of symptoms or probably 15 d after the exposure to B. anthracis. Antibodies against PA could be detected up to 8-16 mo in all the cases of inhalation anthrax and 7 out of 11 surviving cutaneous anthrax patients[118]. For serodiagnosis of cutaneous anthrax, an enzyme-linked immunosorbent assay was developed in India for determination of anti-PA IgGs with 99.4% specificity and 100% sensitivity[119]. A field based qualitative visual ELISA for anti-PA IgG was also developed for serodiagnosis of anthrax[120]. Results of sensitivity and specificity of visual ELISA were found compatible with the results obtained from standard ELISA measuring OD values. Likewise, a quantitative ELISA was developed for measurement of the anti-PA IgG level in human serum samples[121]. The minimum detection limits and lower limits of quantification of the assay for anti-PA IgG were 3.2 μg/mL and 4 μg/mL, respectively. The serum samples collected from the anthrax infected patients were found to have anti-PA IgG concentrations of 5.2 to 166 μg/mL[121]. CDC, United States has developed a lateral flow immunochromatographic device using colloidal gold nanoparticles for determination of anti-PA IgG in serum or whole blood[122].
However, animal studies with anthrax vaccine revealed that LF evokes higher IgG response in comparison to PA in animals[123]. In patients of natural cutaneous anthrax, immune response to LF is higher and faster than the antibody response to EF and PA, which is lower and delayed[124]. Anti-LF IgG antibodies appeared in patients just after 4 d of onset of anthrax symptoms, whereas anti-LF and anti-PA IgG could be detected after 6 d and 13 d, respectively. In a study of human cutaneous anthrax, 11 of the 17 patients had measurable IgGs against one of the three toxin components. Anti-LF IgG was found in 65% patients, while anti-PA and anti-EF response could be found only in 18% and 24% patients. The anti-LF IgG titre in all the infected patients was higher than the titre of anti-PA or anti-EF IgG. After two weeks of infection, the mean anti-LF IgG titre in all infected patients was 69.3 μg/mL, which was twice the tire of anti-EF IgG (37.4 μg/mL) and thrice the titre of anti-PA IgG (22.6 μg/mL)[124]. It was also observed that in anthrax cases, class switching of antibody from IgM to IgG occurs faster. Anti-PA IgG could be detected just after 11 d of onset of symptoms in patients with inhalation anthrax, while no anti-PA IgG response was found till 21-34 d in patients with cutaneous anthrax[117]. Therefore, it is evident that LF evokes a faster and stronger host immune response in comparison to the other two anthrax toxins, i.e., PA or EF. Therefore, detection of anti-LF IgG in human serum can be a good marker for serodiagnosis of anthrax. For detection of anti-LF antibodies, an indirect ELISA was developed for serodiagnosis of cutaneous anthrax in human[125]. The vaccinated and cases of natural anthrax infection can be differentiated by the anti-LF ELISA because PA is the principal component in anthrax vaccine.
Rapid diagnosis of anthrax at an early stage of infection i.e., before the appearance of symptoms can be very useful for proper medical treatment to stop the further spread of infection and accumulation of toxins. For early diagnosis, detection of anthrax toxin in serum or plasma can be a reliable marker of infection[126]. An ultra sensitive immunoassay known as European Nanoparticle Immuno Assay (ENIA) has been developed using European nanoparticle for the detection of PA in sera, which has been found 100 times more sensitive than ELISA[85]. ENIA showed good linearity for detection of PA in the range of 10 pg/mL to 100 pg/mL, whereas range of PA detection in ELISA was 1-100 ng/mL. An engineered sandwich capture ELISA was also reported for the detection of both PA as well as LF[127]. In the sandwich ELISA for PA detection, anti-PA high affinity single chain fragment antibody or receptors for anthrax toxin (ANTXR2) were used for capturing the analyte (PA), and rabbit anti-PA polyclonal serum was used for revealing antibodies. The detection sensitivity of PA by was as low as 1 ng/mL in serum. The detection sensitivity of sandwich ELISA for LF, where PA63 was used for capturing of analyte was 20 ng/mL. Surface Plasmon Resonance (SPR) has also been found a very good technique for detection of PA from serum samples of human[128]. The SPR assay could detect 1 pg/mL of the purified PA and 10 pg/mL of PA in human serum[128].
Recently, a new method utilizing genetically modified phages has been developed for detection of pathogenic B. anthracis from clinical sources[129]. The reporter phage displays species specificity by its inability, or significantly reduced ability, to detect members of the closely related B. cereus group and other common bacterial pathogens.
Nucleic acid based detection methods have also been developed for detection of anthrax. These techniques make use of nucleic acid sequences unique to B. anthracis. The technique has gained enormous popularity for its specificity. Polymerase chain reaction (PCR) or real-time PCR amplify the specific chromosomal markers or virulence plasmids present in the B. anthracis. Such new rapid detection and diagnostic tests are important for clinicians for early identification of infection.
CONCLUSION
Anthrax, caused by B. anthracis is still an important endemic disease of public health importance in several countries of Asia, Africa and Europe. It is re-emerging in some western countries due to political unrest or changing life style (use of intravenous drugs) as evident from the recent outbreaks. In country like India, anthrax is a concern of public health as clandestinely encountering in several states like Andhra Pradesh, Kerala and Karnataka, Orissa and West Bengal. Although anthrax can be cured by prompt antibiotic therapy, yet it is fatal in several cases because of lack of proper diagnosis well in time. Among the three clinical forms of anthrax cutaneous anthrax is most frequent but can be easily cured. The other two forms, gastrointestinal and inhalational anthrax are less common but difficult to cure and have high mortality rate. Recently another form of anthrax, i.e., injectional anthrax is also posing threats for early diagnosis and treatment. However, active surveillance, proper animal immunization and awareness can help to curb the disease. Rapid and accurate diagnosis of cutaneous anthrax is crucial for treatment well in time and making strategies for further spread and control of disease. Although a lot of molecular tests are available for anthrax, yet this is difficult to employ these systems keeping in mind the available resources at far off locations where anthrax is endemic. Therefore, rapid, user friendly, inexpensive serodiagnosis tests can be important tools for surveillance of anthrax and active surveillance can help to minimize the agriculture or occupation related anthrax.
ACKNOWLEDGMENTS
We are thankful to Director, Defence Research and Development Establishment (DRDE), Defence Research and Development Organization (DRDO), Ministry of Defence, Gwalior for providing necessary facilities and funds for this research work.