INTRODUCTION
Eyelid malignancies have been reported with yearly incidence as high as 15.7 cases per 100000 individuals in the United States[1]. The most common type of eyelid carcinoma is basal cell carcinoma (BCC), accounting for 86% to 96% of all cases, followed by squamous cell carcinoma (SCC), 3.4% to 12.6%, sebaceous carcinoma (SebCa), 0.6% to 10.2%, and melanoma, less than 1%[1-3]. Distant metastasis from eyelid carcinoma is not common, reported in up to 6% of squamous cell carcinoma[4]. Furthermore, the integrity of the eyelids are essential for protection and function of the globe making locally advanced disease difficult if not impossible to resect completely without disruption of globe function.
Traditional cytotoxic chemotherapy for treatment of metastatic or unresectable disease targets all rapidly dividing cells, whether cancerous or not. This results in an unfavorable toxicity profile. With the discovery of the common mutations in BCC and SCC, drug therapies targeted against these mutations can potentially block only the growth of cancer cells and thus lead to less wide spread toxicity. In addition, many of these targeted therapies are administered orally rather than by the intravenous route. The ease of administration and lower toxicity generates momentous interest in these new classes of drugs.
EGFR INHIBITION IN SQUAMOUS CELL CARCINOMA
Epithelial growth factor receptor (EGFR), also known as HER-1 or ErbB-1, is a transmembrane protein with an extracellular receptor domain for multiple ligands, including EGF, TGF-alpha and epiregulin, and an intracellular kinase domain with tyrosine autophosphorylation site[5]. Upon activation, the EGFR forms homodimer with another EGFR or heterodimer with another ErbB family receptor. Multiple pathways can be activated by these dimer formations, including RAS/RAF/MEK/MAPK, PI3K/AKT and STAT. The resultant effect of EGFR activation in both normal and malignant human skin is severe epidermal disorganization and invasion[6].
Squamous cell carcinoma has been shown to have overexpression of EGFR. In a series of 13 metastatic SCC of the skin, all showed strong expression of EGFR whereas normal skin close to the tumor had weak EGFR expression that was limited to the basal layer of the epidermis[7]. EGFR overexpression has been found in up to 78% (25 of 32) of cutaneous SCC cells and 62% (13 of 21) of actinic keratoses (AKs)[8]. In conjunctival SCC, EGFR had either moderate or strong expression of EGFR on all (5 out of 5) epithelial cell of conjunctiva[9]. Furthermore, EGFR numerical aberration was found 77% (27 out of 35) SCC and 52% (13 out of 25) of AKs[8]. Analysis of 3 lymph nodes of metastatic SCC all showed amplification of 7p12-13, location of EGFR gene[10].
EGFR expression level is also shown to be associated with metastasis in 45 cases of head and neck SCC[11]. In 25 cases without metastasis, only 9 (36%) showed strong expression for EGFR, compared to 11 of 14 cases (79%) with metastasis[11].
EFFICACY OF EGFR INHIBITORS
Cetuximab (C225, Erbitux) is a chimeric mouse-human IgG1 monoclonal antibody that primarily competitively inhibits EGF binding at the same affinity as the natural ligand[12]. It was approved for use by the Food and Drug Administration (FDA) on March 1, 2006 for locally or regionally advanced head and neck cancer SCC (HNSCC) in combination with radiation therapy or as single agent for recurrent or metastatic HNSCC after failing platinum-based therapy. It was the first EGFR inhibitor studied in clinical trials and to be approved by FDA. In vivo study with A431 model epidermoid carcinoma cells had shown that even in the presence of EGF, cetuximab completely inhibits the activation of EGFR[13]. It is also able to induce dimerization of EGFR and down regulation and further activation by ligands[14]. In a phase 2 study of 36 patients with unresectable (locally advanced or metastatic) SCC of the skin with confirmed strong or moderate expression of EGFR in the primary tumor, the overall tumor response rate was 69% (95%CI: 52%-84%) base on intent-to-treat and 81% (95%CI: 63%-93%) base on actual treatment received[15]. Cetuximab was given as an intravenous infusion at 400 mg/m2 dose followed by weekly 1-hour infusion at a dose of 250 mg/m2. The median number of infusion received during the 48-week study was 15 (range 1 to 47 infusions)[15]. There is currently no published study on the effect of cetuximab compared to or in combination with standard cytotoxic chemotherapy. However, in a study of 424 head and neck squamous cell carcinoma (HNSCC) randomized to high dose radiation with or without cetuximab has shown superior median survival (54 mo vs 28 mo) with the addition of cetuximab[16].
Gefitinib (ZD1839, Iressa) was the first orally administered EGFR inhibitor shown to selectively inhibit EGFR tyrosine kinase activity through blockage of autophosphorylation[17]. Gefitinib was approved in 2003 as monotherapy for non-small cell lung cancer (NSCLC), but the approval was amended to only in patients who had previously benefited from the drug due to lack of survival benefit especially when compared to erlotinib. In vitro incubation of gefitinib with EGF and cutaneous SCC cells showed a dose-dependent reduction in EGFR and MAPK phosphorylation between IC50 of 0.02 and 0.2 μm[18]. In 22 patients with locally aggressive or recurrent cutaneous SCC, neoadjuvant use of gefitinib 250 mg per day showed overall response rate of 45.5% (95%CI: 24.4%-67.8%) and 2-year overall survival of 72.1% (95%CI: 55.4%-93.9%)[19]. Of the 4 patients who showed complete response in this study, all were alive and were disease free at last follow-up[19]. In addition, 3 of these 4 patients with complete response showed pathologic complete response with no evidence of SCC in their resected surgical tissue[19]. There is currently only 1 case report of use of gefitinib as primary treatment for metastatic cutaneous SCC of the foot showed maintained clinical response for 30 mo and an additional 12 mo with the addition of sirolimus[20].
Erlotinib (OSI-744, Tarceva) is similar to gefitinib in that it inhibits EGFR activity through reduction of autophosphorylation causing cell cycle arrest at G1 phase; however, it does this through competitive inhibition with ATP[21]. FDA first approved its use on November 18, 2004, for treatment of patients with locally advanced or metastatic NSCLC after failure of at lease one prior chemotherapy regimen. It was later approved for use in locally advanced or metastatic pancreatic carcinoma in combination with gemcitabine and most recently as first-line therapy for metastatic NSCLC in the presence of EGFR mutation. To our knowledge the use of erlotinib in cutaneous SCC has only been investigated in one phase 1 study of erlotinib plus radiation therapy after surgical resection. The 2-year overall survival was 65% with median time to recurrence of 10.5mo (range 1 to 14 mo) in the 15 Stage III cutaneous SCC patients included in this study[22]. Palliative treatment of metastatic cutaneous SCC with erlotinib has been reported in multiple case reports with initial tumor response[23-25]. In phase 2 studies of locally advanced HNSCC, the addition of erlotinib to cisplatin and 70Gy of radiation showed improved clinical response rate, 52% compared to 40%, however, this was not statistically significant, P = 0.08[26].
USE OF EGFR INHIBITORS FOR ORBITAL AND PERIORBITAL SCC
EGFR expression has been shown in 5 patients with conjunctival SCC as moderate or strongly expressed in both in situ and invasive components of SCC[9]. In contrast, study of periocular sebaceous carcinoma showed lower intensity of EGFR expression with only 2 (11%) of 19 patients showing 3+ intensity of staining[27].
Our group has also reported good clinical and radiological tumor response to EGFR inhibitors in three patients who presented with locally advanced cutaneous SCC of the periocular skin with orbital extension[25,28]. Two of the three patients were treated with cetuximab at the standard dosage of 400 mg/m2 followed by 250 mg/m2 weekly and the third was treated with erlotinib 150 mg daily. One of the patients treated with cetuximab had involvement in the cavernous sinus, Meckel’s cave, trigeminal tract and infraorbital nerve and showed improvement in motility and sensation after 4 weeks of treatment. This patient’s tumor was also analyzed for 182 cancer-related genes and found to have EGFR P753S mutation along with CDKN2A mutation, MYC amplication and TP53 mutation[28].
SIDE EFFECTS OF EGFR INHIBITORS
The most common side effect of EGFR inhibitors is skin toxicity in the form of an acne-like rash, papular and/or pustular follicular eruption, in 32% to 78% of patient with a median time to onset of 14 d[7,19]. Interestingly, the presence rash as a side effect during treatment was significantly correlated with higher overall response rate, median overall survival and progression-free survival in patients treated with erlotinib for NSCLC[29]. The lower incidence of acne-like rash with gefitinib is believed to be due to its attenuation instead of complete blockage of EGFR tyrosine kinase. Instead, toxicity from gefitinib is most commonly diarrhea and fatigue before rash[19]. Erlotinib is also associated with mucositis (87%), esophagitis (40%)[22], and ocular side effects including trichomegaly leading to corneal ulceration[30], conjunctivitis and ectropion[31]. Discontinuation of EGFR inhibitors due to toxicity varied from 9% with gefitinib[19], 11% in cetuximab[7], and 13% in erlotinib[22].
COST OF EGFR INHIBITORS
Cetuximab costs from $2.94 per milligram in Switzerland to $6.73 per milligram in the United States[32]. At the dosage used in the phase 2 clinical trials, the cost would be approximately $28000/m2 or for an average white male the cost of the course of cetuximab would be approximately $37500. Cetuximab is approved in Europe and Asia, specifically Japan, and available in central and south America as well. In comparison, Iressa costs on average $79 per 250 mg tablet[33] and Tarceva cost on average $100 per 150 mg tablet[34], or cost of $2370 and $3000 per month, respectively. Iressa and Tarceva are both available in Europe and Asia in addition to the United States.
PTCH1 GENE AND BASAL CELL CARCINOMA
Basal cell carcinoma (BCC) has been linked to disruption in the Hedgehog (Hh) signaling cascade, a pathway important during embryogenesis but normally inactive in the adult[35,36]. Patched-1 (Ptch-1) is a transmembrane receptor that normally has inhibitory effects on downstream receptor Smoothened (Smo)[37]. When Ptch-1 is activated, upon binding by Hh protein, the constitutive inhibition on Smo is reversed and produces angiogenesis and cellular proliferation in tumorigenesis[38]. Specifically, expression of the downstream product GLI1 in basal cells is proposed to induce formation of BCC[39]. Mutations both in PTCH-1 and Smo have been identified in basal cell nevus syndrome and sporadic basal cell carcinomas[35,36,40,41]. Additionally, continued signaling of the Hh pathway is needed for sustained growth of established BCCs in the mouse model[42].
INHIBITION OF HEDGEHOG PATHWAY IN BCC
Vismodegib (GDC-0449, Erivedge) is a first-in-class small molecule oral Hh signaling pathway inhibitor approved January 2012 for the treatment of locally advanced or metastatic BCC. Vismodegib directly binds to and inhibits Smo[43]. Vismodegib’s inhibition at this point in the cascade prevents formation of GLI1 and therefore targets BCCs related to both constitutively activated Smo mutations and mutations in the upstream PTCH-1. Hedgehog gene expression was reduced by 90% at 1 month after vismodegib treatment in in vivo studies[44]. Biopsy samples from patients treated with vismodegib showed decrease in GLI1 expression by more than two folds after treatment compared to baseline[45].
At a dose of 150mg daily, vismodegib has shown no dose-limiting toxicities in Phase 1 trials[43,45,46]. Initial Phase I trial of 68 patients with advanced BCC and medulloblastoma compared doses of 150 mg/d to 270 mg/d and 540 mg/d and demonstrated no increase in steady state plasma concentrations associated with vismodegib efficacy with higher doses[43,45]. In the cohort of patients from the phase I trial, overall response was shown in 18/33 patients (55%) with locally advanced or metastatic BCC, 2/18 demonstrating complete response and 16/18 demonstrating partial response[45].
A phase 2 trial of 33 patients with metastatic BCC and 63 patients with locally advanced BCC also showed tumor response when given 150 mg of vismodegib daily for a mean duration of 10 mo[47]. Investigators found a 30% objective response rate in the group with metastatic basal-cell carcinoma as defined by a decrease of 30% or more in the dimension or complete resolution of ulceration. The overall objective response rate in the group with locally advanced BCC was 43% with 21% of these patients demonstrating a complete response. By independent review, none of patients in the metastatic cohort had complete response, 10 (30%) patients had partial response and disease progression was noted in 1 (3%) patient with metastatic disease.
Vismodegib has also demonstrated promise in treatment of basal-cell nevus syndrome. Tang et al[44] demonstrated reduction of existing BCC tumor burden and prevention of new BCC in patients with basal-cell nevus syndrome. The vismodegib treatment group had a per-patient average rate of 2 per year while the placebo group had a rate of 29 per patient per year. The investigators also found a greater decrease (-65%) in the size of existing basal cell carcinomas in the vismodegib group compared to the placebo group (-11%). There was no progression of tumors in the treatment group during vismodegib administration and no signs of resistance to the drug during the study. The response was so significant in the treatment arm that the DSMB (Data Safety Monitoring Board) discontinued the study after the first interim analysis. Biopsies sampled from sites of flat-appearing, clinically-regressed basal cell lesions showed residual tumor in 1/6 samples (17%)[44]. Although discontinuation of vismodegib led to expected return of BCC at original sites, significant decrease in the incidence of new surgically eligible BCCs was noted even after cessation for several mo; 0.69 new lesions per month in the treated group compared to 2.4 per month in the placebo group.
VISMODEGIB FOR ADVANCED PERIOCULAR BCC
The use of vismodegib specifically for periocular BCC was first reported in a review by Yin et al [48] in which some of the patients treated with vismodegib in our practice at MD Anderson Cancer Center were highlighted. In this report, a 30-year-old male patient with advanced basal cell nevus syndrome and numerous large BCCs throughout his periocular and facial region including all four eyelids, experienced near complete resolution of his periocular lesions after 16 wk of treatment with durable response during the more than 2 years of follow-up. Subsequently Gill et al[49] highlighted the results of vismodegib in 7 patients with advanced periocular or orbital BCC for a mean duration of 11 wk. With the study’s mean follow-up duration of 7.3 mo, two out of seven patients (29%) had complete regression, 2 out of 7 patients (29%) had partial clinical regression greater than 80%, and 2 out of 7 patients had partial clinical regression less than 35%, and 1 patient progressed.
The histologic effects of vismodegib on a single case of periocular BCC was demonstrated by Kahana et al[50] in a patient with recurrent orbital BCC. After 5 mo of vismodegib, the patient opted for surgical excision due to drug side effects. The surgical specimen showed lack of Ki-67 expression, a marker for proliferation, and mitotic index < 1%.
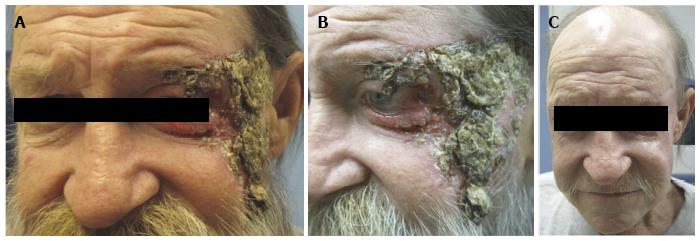
The use of vismodegib in the periocular region can yield impressive responses in patients with locally advanced disease that would otherwise need major disfiguring surgery such as orbital exenteration or would experience loss of major parts of their face during surgical resection. An example of impressive response in a recent such patient in our practice is shown in Figure 1.
Figure 1 A 55 year-old man with locally advanced neglected basal cell carcinoma.
A-B: The lesion involves the left lateral canthus, upper and lower eyelids, left temple and midface; C: After 29 wk of vismodegib 150 mg daily, there is significant resolution of clinically visible tumor. The only surgical intervention that the patient had undergone at this point was reconstructive surgery to repair the left lower lid cicatricial ectropion via a lateral tarsal strip procedure, a lateral tarsorrhaphy and full-thickness skin graft.
SIDE EFFECTS OF VISMODEGIB
Adverse reactions to vismodegib of any grade are reported in 86% to 100% of patients during treatment[46,47,49]. The most common adverse events associated with vismodegib use are mild to moderate nausea, alopecia, dysgeusia, anorexia, and muscle spasms[44-47] In the phase I trial by Tang et al[44] 27% of patients receiving vismodegib had stopped the drug due to these averse events by 8 mo, increasing to 54% cessation a year later. Resolution of dysgeusia, muscle cramps and regrowth of hair was noted within 3 mo after cessation of therapy. No serious adverse events or dose-limiting toxicities have been observed with the recommended 150 mg dosage[43,46].
COST OF VISMODEGIB
The cost of vismodegib (Erivedge) is set by Genetech at $7500 monthly or $250 per capsule[51]. Besides the United States, vismodegib is also approved in Switzerland, United Kingdom, the EU, Australia, Israel, South Korea, Mexico and Ecuador.
CONCLUSION
The treatment of surgically unresectable eyelid and periocular carcinoma is no longer limited to radical disfiguring surgery or the use of high dose radiation therapy with its feared ocular toxicity. The use of drugs that target sonic hedgehog or EGFR pathways for advanced cutaneous carcinomas of the periorbital region should be viewed as palliative but can be associated with long-term and durable response and may be an option for older individuals who would otherwise need radical surgery with significant morbidity. Although specific published data on use of these agents for eyelid or periocular carcinomas are currently limited to few case reports, these relatively new drugs should be further studied for their efficacy in locally advanced BCC or SCC of periocular region. Correlation of response to mutational profile of each tumor would be intriguing and should be further evaluated. These newly available treatments should be considered only in patients with locally advanced unresectable or metastatic disease, multiple tumors, advanced age or multiple comorbidities. Future treatment strategies to decrease the duration of treatment with Hedgehog inhibitors or EGFR inhibitors and the use of these drugs in the neoadjuvant setting before surgery may be interesting to explore as a means of decreasing morbidity associated with periocular surgery.
P- Reviewer: Inan UU S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ