Published online Dec 16, 2014. doi: 10.12998/wjcc.v2.i12.912
Revised: October 2, 2014
Accepted: October 23, 2014
Published online: December 16, 2014
Processing time: 142 Days and 19.3 Hours
Relapsing polychondritis (RP) is a rare autoimmune disease with chronic inflammatory/destructive lesions of the cartilaginous tissues. In one third of the cases it is associated with other autoimmune disorders, mostly with anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV). We report three cases of RP with p-ANCA positive AAV. In the first patient RP developed 1.5 years after the onset of AAV. In the others the signs of RP were present before the onset of severe crescent glomerulonephritis. Patients responded well on steroid and cyclophosphamide. In dialysis dependent cases plasmapheresis was also used successfully. During the 2 and 1.5 years of follow up, they were symptom-free, and had stable glomerular filtration rate. The first patient died after four years of follow-up due to the complications of sudden unset pancytopenia, which raises the possibility of associated hemophagocytic syndrome. In the setting of RP or AAV physicians should always be aware of the possibility of sudden or insidious appearance of the other disease.
Core tip: Relapsing polychondritis (RP) is a rare disease usually diagnosed late when serious symptoms occur. Appearance of renal symptoms significantly increases the possibility of associated associated vasculitis (AAV). We present three cases of RP in whom AAV occurred at different times during the illness. AAV caused rapidly progressive glomerulonephritis (RPGN) in the second and third patient. Aggressive immunosuppression resulted in remission of both RP and AAV. In the RPGN cases dialysis could be discontinued.
- Citation: File I, Trinn C, Mátyus Z, Ujhelyi L, Balla J, Mátyus J. Relapsing polychondritis with p-ANCA associated vasculitis: Which triggers the other? World J Clin Cases 2014; 2(12): 912-917
- URL: https://www.wjgnet.com/2307-8960/full/v2/i12/912.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i12.912
Relapsing polychondritis (RP) is a rare disease characterized by recurrent inflammatory flares of cartilaginous structures of ear, nose, joints, larynx and tracheobronchial tree[1-4]. The aetiology of RP is not clearly defined, but the pathogenesis should involve an autoimmune response to cartilage[5]. About one third of RP cases can be associated with other multi-system diseases, of which primary systemic vasculitides are the most common. anti-neutrophil cytoplasmic antibody (ANCA) may be present in up to 25% of patients with RP[6]. Some of these patients show a classical clinical picture of one of the ANCA associated vasculitides (AAV) and polychondritis is usually thought to be a secondary phenomenon[7-9]. However many RP patients with ANCA positivity do not have any, or only limited, vasculitic symptoms[6] and the occurrence of RP may precede AAV[10-12]. It is possible that the development of ANCA could be provoked by RP, as it was seen by us in rheumatoid arthritis patients[13].
Whatever is the sequence of disease manifestations, the occurrence of renal symptoms significantly raise the possibility of (underlying or secondary) AAV, the need for more aggressive treatment, and indicates worse prognosis[14-17]. We present three cases of RP in whom AAV occurred at different times during the illness. Two patients developed rapidly progressive glomerulonephritis (RPGN). The aggressive treatment resulted in dialysis independence in both cases.
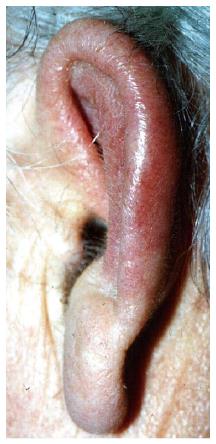
In March 1998 microscopic polyangiitis was diagnosed in a 58 years old male, based on four weeks’ history of fever, anaemia, purpura, arthralgia, episcleritis, axonal neuropathia, and p-ANCA positivity of 38 U/mL (normal < 3 U/mL). Glomerular haematuria, granular casts and mild proteinuria were also present. The serum creatinine was normal, therefore a kidney biopsy was not performed. Renal angiography did not find any aneurysms. Skin biopsy verified small vessel vasculitis. Per os treatment with 1 mg/kg steroid and 2 mg/kg cyclophosphamide resulted in quick resolution of symptoms and p-ANCA negativity, but 2 mo later severe leucopenia and herpes infection of the skin developed, therefore cyclophosphamide was withdrawn. The patient was well on a low dose steroid, but after tapering the dose to 4 mg/d in November 1999 episcleritis reoccurred. Painful swelling and redness of both auricles with sparing of the ear lobe had also developed (Figure 1). Auricular polychondritis spontaneously diminished, but in the next months it relapsed twice. Less severe inflammation of the nose bridge was also present. Based on these clinical symptoms the diagnosis of relapsing polychondritis was established. ANCA remained negative and no other signs of systemic vasculitis reoccurred. Increased steroid dose and azathioprine resulted in remission of polychondritis, therefore six month later azathioprine was withdrawn and only 4-8 mg of methylprednisolone was applied. In June of 2002 fever, weakness and purpura reoccurred. Severe thrombocytopenia (24 G/L), leucopenia (1,2 G/L) and anaemia (Hb 78 g/L) were also present. Bone marrow biopsy showed hyperregenerative cell lines but also a delay in cell maturation thus leading to pancytopenia. Occasionally macrophages containing red blood cell fragments within their cytoplasm were also present. No primary haematological disease was seen and ANCA was negative. Pulse steroid treatment was given resulting in quick improvement of pancytopenia. In August 2002 pancytopenia suddenly reoccurred and the patient died within 24 h after admission into another institution. No autopsy was performed.
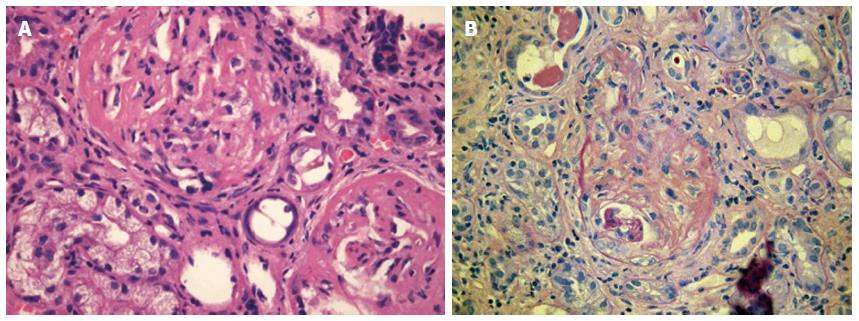
A 63-year old woman was admitted to our Department in July 2012 with two months’ history of 6 kg weight loss, fatigue, subfebrility, elevated C-reactive protein and normocytic anaemia. She had renal failure as well and needed urgent haemodialysis (serum creatinine 1040 μmol/L). Urinalysis disclosed proteinuria and glomerular haematuria, ultrasound showed normal size kidneys. Rapidly progressive glomerulonephritis was suspected. The renal biopsy demonstrated pauci-immune necrotizing glomerulonephritis with fibrocellular crescents being present in 70% of glomeruli (Figure 2). She had elevated anti-MPO titer: 21 U/mL (normal < 5 U/mL). The diagnosis of ANCA associated systemic vasculitis was established. Typical signs of auricular chondritis were also present, her ears were tender and had cauliflower appearance. She complained of dizziness, hearing loss, and compromised smell. Her bilateral mixed hearing loss was diagnosed 8 years earlier. In the recent years she had migrating transient polyarthralgia, recurrent nasal obstruction and red eyes, but medical consultation was not sought except due to hypertension in 2010. These signs and symptoms led to the diagnosis of relapsing polychondritis. Pulse steroid of 3 × 1 g was given and five sessions of plasmapheresis were performed. Treatment resulted in immediate resolution of the inflammatory symptoms. Maintenance immunosuppression was continued in a dose of 0.5 mg/kg per day prednisolone and 1.5 mg/kg per day cyclophosphamide per os. Renal function improved, in February 2013 dialysis could be discontinued, cyclophosphamide was withdrawn. Prednisolone was stopped in June 2014 (estimate GFR 20 mL/min per 1.73 m2, proteinuria 0.5 g/L). During the 2 years of follow-up, no relapse of vasculitis or polychondritis was observed. Anti-MPO level remained negative, her hearing and the shape of her ears returned to normal.
A 56-year-old woman - with a ten year history of hypertension - was referred to our Department in October 2012 due to RPGN requiring dialysis. ANCA associated glomerulonephritis was established based on > 100 U/mL anti-MPO (normal < 5 U/mL) and pauci-immune glomerulonephritis (fibrocellular crescents in 10 of 32 of glomeruli) seen in the kidney biopsy. In recent years she repeatedly experienced hoarseness, sore throat, laryngotracheal pain, swollen, tender, painful ears and low grade fever. Symptoms sometimes disappeared spontaneously, sometimes she was treated with antibiotics and analgesics. She also had migrating arthralgia. Based on these signs preceding polychondritis was also diagnosed. Before the start of her complaints she punctured her finger while vaccinating rabbits against myxomatosis. At that time (September 2011) laboratory tests revealed normal renal function without proteinuria and haematuria. In September 2012 her throat and ear complaints reoccurred accompanied by fever, fatigue, weight loss, macroscopic haematuria and oliguria. Considering this acute episode she was started on the following therapy: pulse prednisolone (4 × 0.5 g), plasmapheresis (5 session), and 1.5 mg/kg oral cyclophosphamide. The treatment resulted in resolution of the inflammatory symptoms, renal function improved, and dialysis could be discontinued. Anti-MPO level decreased to 16 U/mL. The patient was discharged in good condition with 0.8 mg/kg per day methylprednisolone and 1.5 mg/kg per day cyclophosphamide. After three weeks she needed admission to our intensive care unit due to high fever, repeated convulsions, agitation and unconsciousness. ANCA titer was normal, renal function has not deteriorated, and her ears and throat did not show signs of inflammation. Therefore cerebral symptoms were suspected not caused by a vasculitic episode but rather by an immunosuppression-related cerebral infection. Herpes encephalitis was diagnosed by liquor herpes simplex virus polymerase chain reaction positivity. Intravenous acyclovir and immunoglobulin was administered, the steroid dose increased and cyclophosphamide discontinued. This therapy resulted in a slow but full recovery regarding cerebral symptoms. The steroid was gradually tapered and stopped after 1 year. There was no relapse of polychondritis or vasculitis during the 18 mo follow-up, eGFR stabilized about 20 mL/min per 1.73 m2, there was no proteinuria, and ANCA tests were negative.
The most important laboratory findings are presented in the Table 1.
| Case 1, male 58 yr03, 1998 | Case 2 female 56 yr7, 2012 | Case 3, female 63 yr10, 2012 | |
| Proteinuria (g/d) | 0.2 | 1.03 | 4.7 |
| Haematuria (vvt/hpf) | 15 | 516 | 107 |
| Hb (g/L) | 95 | 63 | 69 |
| CRP (mg/L) | 209 | 101 | 103 |
| UN (mmol/L) | 6.3 | 41 | 28 |
| Creatinine (μmol/L) | 105 | 1040 | 534 |
| GFR (mL/min per 1.73 m2) | > 60 | 3 | 7 |
| Anti-MPO ab (U/mL) | 38 (normal < 3) | 21.8 (normal < 5) | > 100 (normal < 5) |
We present three cases of ANCA associated vasculitis, who also merit the diagnostic criteria for RP. They are not exceptional cases, because vasculitis can be seen in 14% of RP patients[1], ANCA positivity in up to 25% of RP patients[6]. The annual incidence of AAV and RP (which is about 10-30/million and 3.5/million population respectively) makes it unlikely that these cases represent simple coincidence.
About one third of RP cases can be associated with other autoimmune diseases, of which vasculitis is the most common[1-4]. All types of vasculitis were already reported with RP, including microscopic polyangiitis[10,14], polyangiitis with granulomatosis[18], eosinophil granulomatosis with polyangiitis[19], among them most frequently AAV. In our three cases microscopic polyangiitis was diagnosed based on the general and renal signs of systemic vasculitis, the absence of eosinophilia, allergic rhinitis/asthma or sinusitis/otitis. The p-ANCA, anti-MPO positivity is also characteristic for MPA.
There are no specific clinically applicable tests to confirm the diagnosis of RP. Antibodies against type II collagen and matrilin-1 (cartilage matrix protein prominent in tracheal, auricular, and nasal cartilages) can be detected in sera of patients with RP, however their sensitivity and specificity is very low[5]. Therefore the diagnosis of RP is based on clinical signs[20]. Currently the diagnosis of RP requires the presence of a proven inflammation in at least 2 of 3 of the auricular, nasal, or laryngotracheal cartilages, alternatively, a proven inflammation in one of the above cartilages and two other signs including ocular inflammation, hearing loss, vestibular dysfunction, or seronegative arthritis[21].
In our cases the auricular chondritis was the diagnosis-raising sign, but there were other signs in every case to meet the diagnostic criteria of RP. In the first patient recurrent polychondritis developed 1.5 years after the onset of typical vasculitis (neuropathy, purpura, haematuria) when the steroid dose was tapered off. This supports the concept, that RP is a secondary phenomenon of underlying AAV. However, at that time vasculitis was not active, furthermore ANCA was negative. This observation is counter to the findings outlined in a recent case report[9].
In Case 3 the ear and throat symptoms preceded the vasculitis by one year. Her symptoms started after an accidental needle-puncture while vaccinating rabbits against myxomatosis. It is possible that the attenuated Myxoma virus was the trigger activating the immune system by molecular mimicry. In Case 2 AAV and auricular chondritis occurred at the same time, but her hearing loss preceded them by eight years. From that time she had recurrent auricular, nasal and ophthalmological symptoms, which raises the possibility of RP. The diagnosis of RP is difficult in the early stage because the incidence of each symptom is less than 50% at the onset[1]. The diagnosis is usually delayed by 3 years[22] but the delay can be as long as 10 years[23]. Extremely precise case history and clinical evaluation is needed. In this case the biopsy of an involved cartilage could help. Biopsies, however, often show only nonspecific granulation tissue, so the pathognomonic findings for RP may be not be easy to obtain[24].
In spite of the fact that glomeruli do not contain type II collagen, renal involvement was reported in 29/129 cases in the Mayo Clinic study[17]. Haematuria was the most frequent abnormality occurring in 26% of 337 patients[1]. It was observed in all our cases, indicating a proliferative glomerulonephritis. Rapid decline in glomerular filtration rate (GFR) was seen in two cases raising the suspicion of pauci-immune crescentic glomerulonephritis, which was verified by a kidney biopsy. This type of glomerular lesion is diagnostic for AAV-s, even if ANCA is not present. In the early phase of kidney damage only focal segmental glomerular necrosis can be present. These lesions were the most frequently observed pathological finding in RP and rose the suspition of vasculitis even decades earlier when ANCA was not yet available[14-17]. Less frequently other types of glomerulonephritides, such as IgA nephropathy[25], membranous nephropathy[26] had also been reported. We think that these lesions could not to be linked to RP. When renal signs appear it is very important to differentiate renal vasculitis from other causes: e.g., membranous nephropathy could be caused by non-steroid anti-inflammatory drugs, used for the treatment of arthralgia in RP.
Renal vasculitis indicates a worse prognosis and the need for more aggressive immunosuppressive treatment. Due to the poor response of AAV to steroids alone, first-line regimes used in patients with RP/AAV overlap should include additional cyclophosphamide or other immunosuppression. Our patients responded well on steroid and cyclophosphamide treatment. In dialysis-dependent cases we combined it with plasmapheresis. This regime resulted in dialysis independence in spite of the advanced histological picture. The patients became symptom-free both regarding RP and AAV. They have severely decreased GFR, which could have been prevented had they been referred to us earlier. The first patient died after four years of follow-up due to the complications of sudden unset pancytopenia. No primary haematological disease was seen on bone marrow biopsy. Therefore it was thought to be a result of a flare of the underlying autoimmune disease, which was supported by the fact that pulse steroid treatment had been effective. The presence of hemophagocytosis and the recurrence of pancytopenia with sudden respiratory failure raise the possibility of hemophagocytic syndrome. Its association with adult onset autoimmune disease has recently gained attention[27,28]. The association of RP and AAV can lead to critical conditions and treatment needs to be initiated promptly and undertaken by an experienced team. RP patients need a regular and prolonged follow up for renal symptoms and ANCA-s as well.
In any case of RP or AAV physicians should be aware of sudden or insidious appearance of the other disease.
A 58-year-old male diagnosed with microscopic polyangiitis experienced painful swelling and redness of both auricles, a 63-year-old woman had renal failure, tender and cauliflower-like ears, a 56-year-old woman -with a history of relapsing polychondritis-presented with rapidly progressive glomerulonephritis.
Swelling and redness of both ears, arthralgia, red eyes, hearing loss, tracheobronchial pain pointed to relapsing polychondritis, while purpura, general (fever, fatigue, weight loss), and renal (haematuria, oliguria) symptoms to vasculitis.
Microscopic polyangiitis, polyangiitis with granulomatosis, eosinophil granulomatosis with polyangiitis, other vasculitides, systemic lupus erythematodes, other causes of rapidly progressive glomerulonephritis (RPGN) can be considered.
High C-reactive protein, anaemia, p-anti-neutrophil cytoplasmic antibody/anti-MPO positivity, haematuria, proteinuria, elevated serum creatinine, decreased glomerular filtration rate (Table).
Chest X-ray was unremarkable, abdominal ultrasound showed normal size kidneys.
Skin biopsy of the first patient showed small vessel vasculitis, renal biopsy of the other two patients was consistent with pauci-immune crescentic glomerulonephritis.
Immunosuppressive treatment with steroid, cyclophosphamide, azathioprine; in the RPGN cases plasmapheresis was the specific medication.
There are only scattered case reports about the association of relapsing polychondritis (RP) and associated vasculitides (AAV).
Pauci-immune glomerulonephritis is diagnosed based on extensive extracapillary proliferation (leading to crescent formation) and necrotic lesions in the capillary tuft with negative immunofluorescent and electron microscopic finding.
In any case of RP or AAV physicians should be aware of sudden or insidious appearance of the other disease.
It is properly writen article on case series of relapsing polychondritis and vasculitis.
P- Reviewer: Espinoza LR, Kusztal M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Kent PD, Michet CJ, Luthra HS. Relapsing polychondritis. Curr Opin Rheumatol. 2004;16:56-61. [PubMed] [Cited in This Article: ] |
| 2. | Gergely P, Poór G. Relapsing polychondritis. Best Pract Res Clin Rheumatol. 2004;18:723-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Lahmer T, Treiber M, von Werder A, Foerger F, Knopf A, Heemann U, Thuermel K. Relapsing polychondritis: An autoimmune disease with many faces. Autoimmun Rev. 2010;9:540-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Cantarini L, Vitale A, Brizi MG, Caso F, Frediani B, Punzi L, Galeazzi M, Rigante D. Diagnosis and classification of relapsing polychondritis. J Autoimmun. 2014;48-49:53-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Arnaud L, Mathian A, Haroche J, Gorochov G, Amoura Z. Pathogenesis of relapsing polychondritis: a 2013 update. Autoimmun Rev. 2014;13:90-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Papo T, Piette JC, Le Thi Huong Du, Godeau P, Meyer O, Kahn MF, Bourgeois P. Antineutrophil cytoplasmic antibodies in polychondritis. Ann Rheum Dis. 1993;52:384-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Handrock K, Gross WL. Relapsing polychondritis as a secondary phenomenon of primary systemic vasculitis. Ann Rheum Dis. 1993;52:895-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Schina M, Karsaliakos P, Apostolou T, Mousoulis G. Relapsing polychondritis as a secondary phenomenon of primary systemic vasculitis. Clin Nephrol. 2008;70:446-449. [PubMed] [Cited in This Article: ] |
| 9. | Mattiassich G, Egger M, Semlitsch G, Rainer F. Occurrence of relapsing polychondritis with a rising cANCA titre in a cANCA-positive systemic and cerebral vasculitis patient. BMJ Case Rep. 2013;2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Weber F, Kowald E, Schmuth M, Sepp N. Microscopic polyangiitis in a patient with relapsing polychondritis. Rheumatology (Oxford). 2001;40:233-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Masterson R, Sheerin N, Abbs I, Goldsmith D. Late allograft loss due to recurrence of p-ANCA-associated systemic vasculitis in a patient with relapsing polychondritis. Nephrol Dial Transplant. 2001;16:1705-1707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Barzegar C, Vrtovsnik F, Devars JF, Mignon F, Pradalier A. Vasculitis with mesangial IgA deposits complicating relapsing polychondritis. Clin Exp Rheumatol. 2002;20:89-91. [PubMed] [Cited in This Article: ] |
| 13. | Szilasi M, Mátyus J, File I, Szücs G, Rákóczi E, Pfliegler G, Szabó Z, Végh E, Szekanecz Z. Association of ANCA-associated vasculitis-rheumatoid arthritis overlap syndrome in four patients: rituximab may be the right choice? Autoimmunity. 2012;45:304-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Neild GH, Cameron JS, Lessof MH, Ogg CS, Turner DR. Relapsing polychondritis with crescentic glomerulonephritis. Br Med J. 1978;1:743-745. [PubMed] [Cited in This Article: ] |
| 15. | Ruhlen JL, Huston KA, Wood WG. Relapsing polychondritis with glomerulonephritis. Improvement with prednisone and cyclophosphamide. JAMA. 1981;245:847-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Botey A, Navasa M, del Olmo A, Montoliu J, Ferrer O, Cardesa A, Darnell A, Revert L. Relapsing polychondritis with segmental necrotizing glomerulonephritis. Am J Nephrol. 1984;4:375-378. [PubMed] [Cited in This Article: ] |
| 17. | Chang-Miller A, Okamura M, Torres VE, Michet CJ, Wagoner RD, Donadio JV, Offord KP, Holley KE. Renal involvement in relapsing polychondritis. Medicine (Baltimore). 1987;66:202-217. [PubMed] [Cited in This Article: ] |
| 18. | Small P, Black M, Davidman M, de Champlain ML, Kapusta MA, Kreisman H. Wegener’s granulomatosis and relapsing polychondritis: a case report. J Rheumatol. 1980;7:915-918. [PubMed] [Cited in This Article: ] |
| 19. | Conn DL, Dickson ER, Carpenter HA. The association of Churg-Strauss vasculitis with temporal artery involvement, primary biliary cirrhosis, and polychondritis in a single patient. J Rheumatol. 1982;9:744-748. [PubMed] [Cited in This Article: ] |
| 20. | McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore). 1976;55:193-215. [PubMed] [Cited in This Article: ] |
| 21. | Michet CJ, McKenna CH, Luthra HS, O’Fallon WM. Relapsing polychondritis. Survival and predictive role of early disease manifestations. Ann Intern Med. 1986;104:74-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 404] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 22. | Trentham DE, Le CH. Relapsing polychondritis. Ann Intern Med. 1998;129:114-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 257] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Paroli MP, Priori R, Spinucci G, Abicca I, Valesini G. Uveitis with retinal occlusive vasculitis and sensorineural hypoacusia as first symptoms of relapsing polychondritis. Clin Exp Rheumatol. 2012;30:S101-S103. [PubMed] [Cited in This Article: ] |
| 24. | Tsuda T, Nakajima A, Baba S, Tanohara K, Masuda I, Yamada T, Takagi K, Yamakawa T, Kamatani N, Hara M. A case of relapsing polychondritis with bilateral sensorineural hearing loss and perforation of the nasal septum at the onset. Mod Rheumatol. 2007;17:148-152. [PubMed] [Cited in This Article: ] |
| 25. | Dalal BI, Wallace AC, Slinger RP. IgA nephropathy in relapsing polychondritis. Pathology. 1988;20:85-89. [PubMed] [Cited in This Article: ] |
| 26. | Lee J, Lee EK. A case of membranous nephropathy associated with relapsing polychondritis. Kidney Res Clin Pract. 2012;31:253–256. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Tabata R, Tabata C, Terada M, Nagai T. Hemophagocytic syndrome in elderly patients with underlying autoimmune diseases. Clin Rheumatol. 2009;28:461-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheumatol. 2014;66:2297-2307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |