Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1371
Peer-review started: December 31, 2023
First decision: January 16, 2024
Revised: January 19, 2024
Accepted: February 8, 2024
Article in press: February 8, 2024
Published online: March 6, 2024
Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are very serious skin allergies, with an etiology related to infections and medication. Since the coronavirus disease 2019 (COVID-19) pandemic, severe acute respiratory syndrome coronavirus-2 has also been considered to cause SJS/TEN.
We report the case of a woman in her thirties who took acetaminophen after contracting COVID-19. After 3 d of fever relief, she experienced high fever and presented with SJS/TEN symptoms, accompanied by intrahepatic cholestasis. Three days of corticosteroid treatment did not alleviate the skin damage; there
DPMAS therapy is beneficial for abrogating SJS/TEN because plasma adsorption and perfusion techniques reduce the inflammatory mediators (e.g., tumor necrosis factor-alpha and interleukin-10 and-12) speculated to be involved in the patho
Core Tip: A woman in her thirties took acetaminophen after contracting coronavirus disease 2019. After 3 d of fever relief, she experienced high fever, with Stevens–Johnson syndrome and toxic epidermal necrolysis symptoms and intrahepatic cholestasis. Because 3 d of corticosteroid treatment did not alleviate the skin damage, double plasma molecular adsorption system (DPMAS) therapy was initiated, with treatment intervals of 48 h. Her skin symptoms improved gradually and were completely resolved after seven DPMAS treatments.
- Citation: Tan YW, Liu LP, Zhang K. Double plasma molecular adsorption system for Stevens–Johnson syndrome/toxic epidermal necrolysis: A case report. World J Clin Cases 2024; 12(7): 1371-1377
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1371.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1371
Stevens–Johnson syndrome (SJS), first reported by Dr. Albert Stevens and Dr. Frank Chambliss Johnson in 1922[1], is classified as severe erythema multiforme characterized by blisters on the skin, typical or atypical target lesions, and extensive mucosal damage, accompanied by systemic symptoms such as fever and visceral damage. More severe cases are classified as toxic epidermal necrolysis (TEN) or overlapping syndrome (SJS/TEN)[2] and can be caused by drugs, infections, malignant tumors, or idiopathic factors. Since the coronavirus disease 2019 (COVID-19) pandemic, a few cases of SJS caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection or vaccines have been reported[3-5]. Treatments for SJS/TEN include glucocorticoids, intravenous immunoglobulin (IVIG), cyclosporine, N-acetylcysteine, thalidomide, infliximab, etanercept, and plasma exchange. However, these treatments are unsatisfactory and SJS/TEN has a high mortality rate. Herein, we report successful SJS/TEN treatment using corticosteroids combined with a double plasma molecular adsorption system (DPMAS) in a patient who developed the skin condition after contracting COVID-19.
A woman in her thirties developed symptoms of fever (with a maximum body temperature of 39.2 ℃), headache, and sore throat in mid-December 2022.
The patient took 0.5 g of acetaminophen three times daily on the morning prior to the day she was tested for SARS-CoV-2. She was then diagnosed with COVID-19. After 3 d, her body temperature gradually returned to normal and her sore throat improved. One week later, the patient experienced fever (with a maximum temperature of 39.8 ℃) and began to develop red papules and blisters from her head to limbs.
The patient had no history of drug allergies or contact with toxic substances.
The patient had no similar family history or that of other genetic diseases.
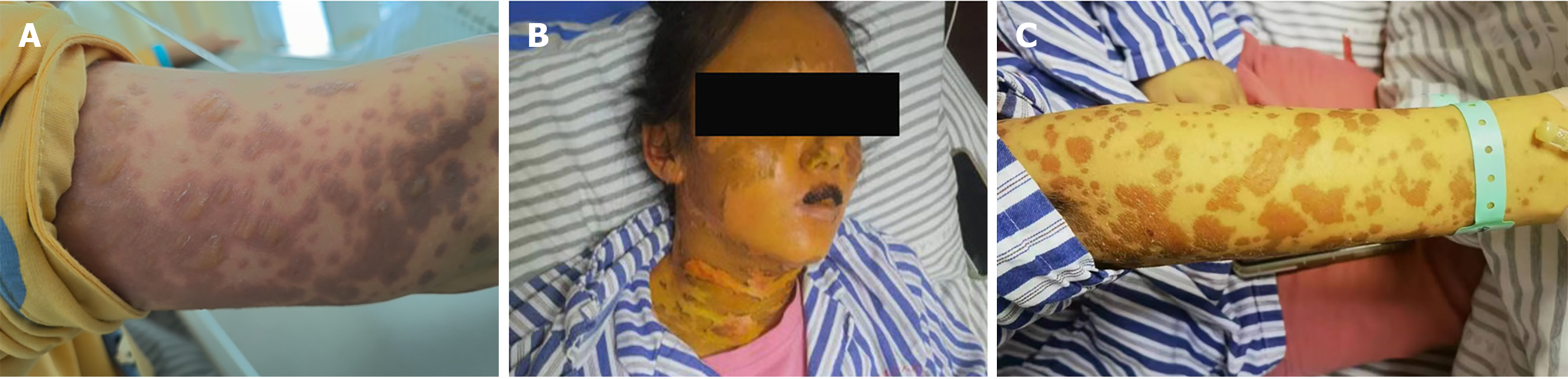
After 3 d, the rash did not resolve. The vesicles fused and spread to the mucous membranes, including those of the eyelids and lips; beginning on the face and torso and spreading centrifugally throughout the body (over 90% of the body surface area) (Figure 1A). The rash was diagnosed as SJS/TEN. The patient simultaneously presented with yellowing skin, light-colored stools, and a serum total bilirubin (TBIL) level of 240 μmol/L with an increase in the liver enzymes alanine aminotransferase and alkaline phosphatase.
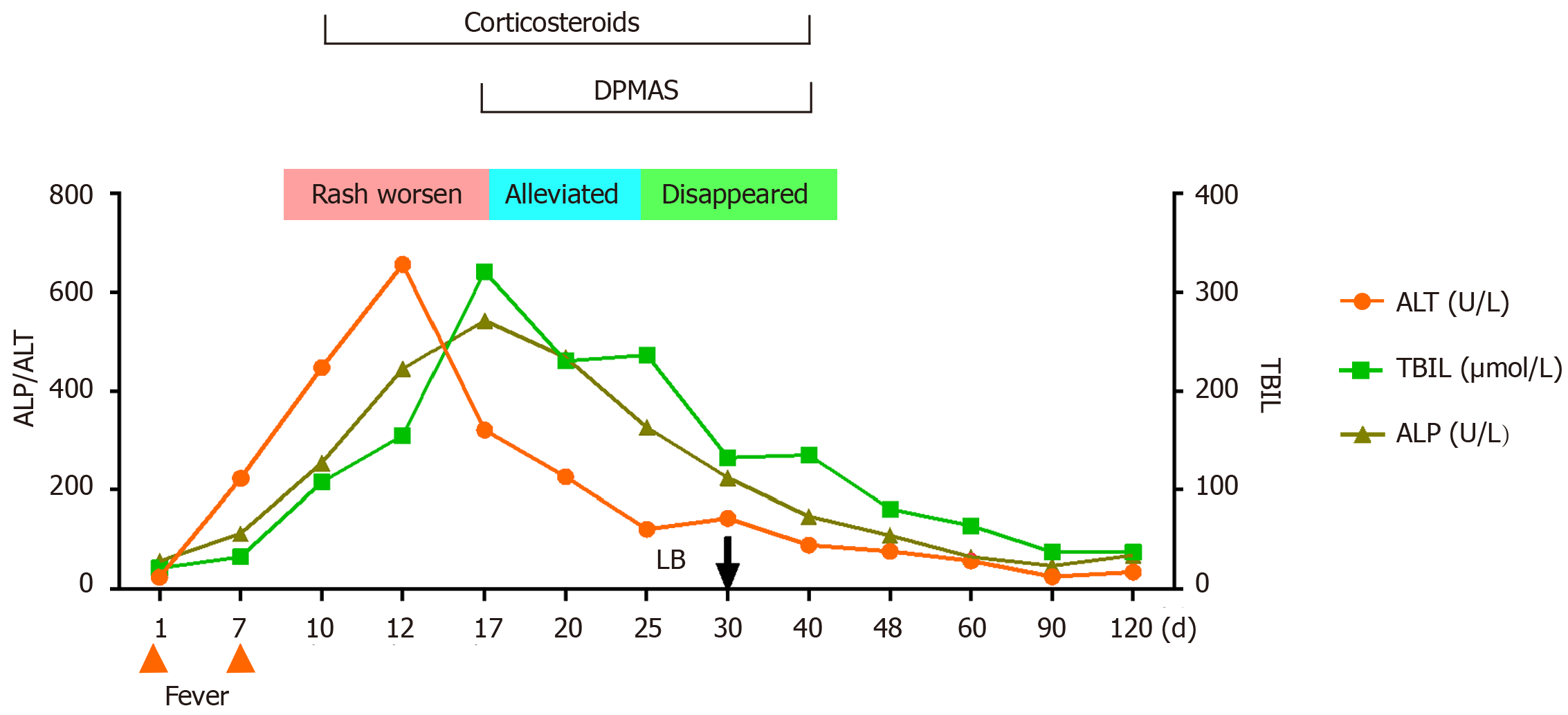
Figure 2 presents a flowchart of the changes during the disease course. Test results for viral hepatitis A to E were all negative, as were those for anti-nuclear, anti-mitochondrial, and anti-liver and kidney microsomal antibodies.
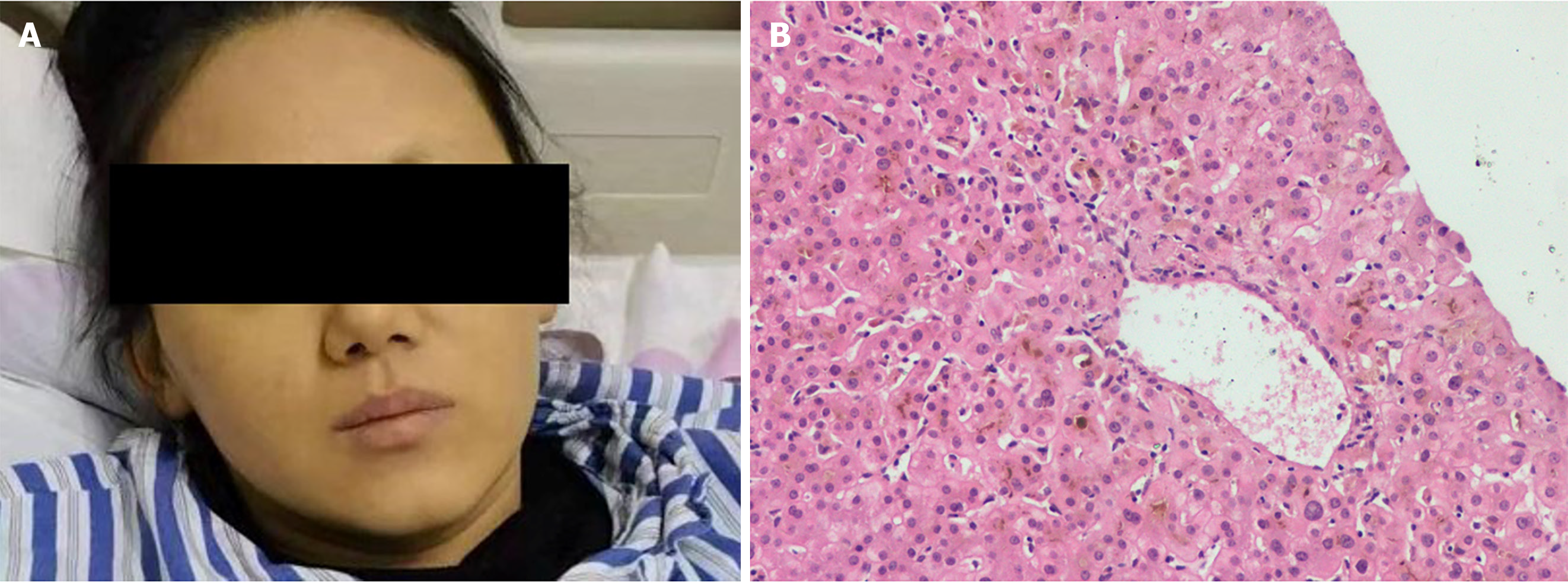
A liver biopsy was performed 1 month later. The histopathology showed a nonspecific inflammatory reaction; cholestasis and mild inflammation of the liver cells; and the absence of liver necrosis, ductopenia, and bile duct inflammation damage (Figure 3B).
The patient was diagnosed with SJS/TEN.
Cyclosporine was discontinued and N-Acetylcysteine and ursodeoxycholic acid were initiated, with DPMAS treatment performed at intervals of 48 h. On day 13 of the disease course and after three courses of DPMAS treatment, the skin rash on her face and arm started falling off (Figure 1B and C). Thus, DPMAS treatment was discontinued and methylprednisolone was maintained. However, 3 d later, new maculopapules appeared on her inner thigh, arms, and face (Figure 4). Moreover, her TBIL level had progressively increased to 321 μmol/L. DPMAS therapy was re-initiated and her rash improved significantly after seven courses of treatment, with no new skin lesions appearing. Subsequently, DPMAS was stopped, and the dosage of intravenous methylprednisolone was reduced to 20 mg/d.
Methylprednisolone and N-acetylcysteine were discontinued and only ursodeoxycholic acid treatment was continued. After 60 d of hospitalization, her facial skin returned to normal, but remained pigmented (Figure 3A). Her TBIL level decreased to normal within 4 months.
SJS is a rare but severe immune-mediated skin disease, with TEN representing a more severe form, involving more than 30% of the total body surface area[6]. In our patient, the infection started on the face and trunk, expanded centrifugally, and rapidly spread throughout her entire body within 1 wk, eventually involving the eyes, lips, and other mucous membranes. This case was categorized as TEN because the patient’s entire body was affected within 1 wk.
Although the pathophysiology of SJS/TEN has not been fully elucidated, it is known to involve type IV hypersensitivity, in which activated cytotoxic T cells release granulysin and cytokines [e.g., tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-15] to induce keratinocyte apoptosis. The etiology of SJS/TEN is often associated with antibiotics, antiepileptics, nonsteroidal anti-inflammatory drugs, and immune checkpoint inhibitors. Rarely, it has been associated with viruses (influenza virus, Epstein–Barr virus, coxsackievirus, cytomegalovirus, parvovirus, human herpes virus 6 and 7) and bacteria (group A Streptococcus, Mycoplasma pneumoniae). Very rarely, malignancies and vaccines (for influenza, tetanus, smallpox, chickenpox, and anthrax) have been implicated.
SARS-CoV-2 is also a causative factor, with reports of 22 SJS/TEN cases related to COVID-19 vaccination and 19 related to COVID-19 infection by August 2022[7]. Of these, 11 patients with symptoms covering over 30% of body surface area met the criteria for TEN, and four died of respiratory distress. The interval between COVID-19 vaccination or infection and rash onset ranged from 1 to 42 d (median, 6 d).
Acetaminophen (also called paracetamol) is known to cause SJS/TEN. According to a review published in June 2021[8], 36 cases of acetaminophen-associated SJS/TEN were reported in 29 studies, including 24 patients with SJS, 10 with TEN, and 2 with SJS/TEN overlap. The interval between receiving the medication and rash appearance varied from the same day to 21 d (median, 3 d). All patients survived. In our patient, acetaminophen was used to treat the fever caused by COVID-19, and it was difficult to distinguish whether SJS/TEN was caused by the viral infection or acetaminophen use. We used the Naranjo Adverse Drug Reaction Probability Scale to score the likelihood of acetaminophen causing TEN[9], and obtained a total score of 3 (Q1, +1; Q2, +2; Q5, -1; Q10, +1), which indicates a likely possibility (Table 1). Her liver pathology was nonspecific and difficult to identify.
| Question | Yes | No | Don’t know | Patient’s score |
| (1) Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | +1 |
| (2) Did the adverse event appear after the suspected drug was administered? | +2 | -1 | 0 | +2 |
| (3) Did the adverse reaction improve when the drug was discontinued, or a specific antagonist was administered? | +1 | 0 | 0 | 0 |
| (4) Did the adverse reaction reappear when the drug was re-administered? | +2 | -1 | 0 | 0 |
| (5) Are there alternative causes (other than the drug) that could on their own have caused the reaction? | -1 | +2 | 0 | -1 |
| (6) Did the reaction reappear when a placebo was given? | -1 | +1 | 0 | 0 |
| (7) Was the drug detected in the blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| (8) Was the reaction more severe when the dose was increased, or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| (9) Did the patient have a similar reaction to the same or similar drug in any previous exposure? | +1 | 0 | 0 | 0 |
| (10) Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | +1 |
Currently, no specific drug has been recognized as a supportive therapy that provides definite benefits for patients with SJS/TEN. The efficacy of systemic corticosteroid use is ambiguous, with early observational studies showing that patients receiving these drugs have significantly higher rates of infection (e.g., Candida-related sepsis) and overall complications, including higher mortality[10,11]. Conversely, patients receiving corticosteroid therapy have a survival advantage over those receiving only supportive care[12].
IVIG is one of the most commonly used and consensus-approved therapies for SJS/TEN. It is typically used as a first-line adjuvant therapy for critically ill patients in tertiary care settings. However, data on IVIG (low-and high-dose regimens) are also ambiguous, with one study showing that it did not confer significant mortality benefit[13].
TNF inhibitors (anti-TNF drugs) are the latest candidates being investigated for SJS/TEN treatment. Moreover, a single infusion of 5 mg/kg infliximab can prevent the shedding of skin cells and induce the rapid re-epithelialization of exfoliated skin[12,14]. The true benefits of anti-TNF drugs in SJS/TEN are difficult to determine, with limited studies in this regard. Therefore, plasma exchange may be beneficial for treating SJS/TEN. However, studies have found no statistically significant improvements in mortality, hospital stay length, or re-epithelialization attributed to this therapeutic method[15-18].
DPMAS technology is a blood purification method that uses different adsorbents to nonselectively bind to and remove endogenous or exogenous toxins from the blood. The process consists of a blood and plasma circuit, a flowchart of which is presented in our previous case report[19]. First, whole blood is collected from the femoral vein and pumped into a plasma separator. Subsequently, a bilirubin adsorbent (BS330) and macroporous neutral resin (HA330-II) are used to adsorb the plasma. Finally, the plasma is fused with the separated blood cells and introduced into the body. The resin in the BS330 adsorption column exhibits specifically absorbed bilirubin and bile acids via electrostatic and lipophilic interactions. The resin in the HA330-II blood perfusion device is a relatively broad-spectrum adsorbent with a large pore structure and surface area. The adsorption of macromolecular toxins (e.g., the inflammatory mediators IL-6 and IL-10) occurs through van der Waals forces and skeletal molecular sieves. The binding energies of the two adsorbents allow them to rapidly remove bilirubin, antibodies, thyroid hormones, inflammatory mediators, and other harmful substances, thereby reducing inflammation and improving the immune response.
The pathogenesis of SJS/TEN is driven by type IV hypersensitivity, which involves reactions in which allergenic substances or drug–peptide complexes are recognized by T cell receptors, leading to downstream CD8+ cytotoxic T cell-and natural killer cell-mediated cytotoxicity and cytokine expression [specifically TNF-α and interferon-gamma (IFN-γ)][20]. In our patient, increased levels of TNF-α and IFN-γ (12.48 and 7.64 pg/mL, respectively) were found, both of which decreased significantly after DPMAS treatment (1.94 and 1.25 pg/mL, respectively). Therefore, we speculate that DPMAS adsorption plays a role similar to that of TNF inhibitors in reducing the levels of these proinflammatory cytokines to suppress SJS/TEN.
In conclusion, a patient who had contracted COVID-19 was diagnosed with SJS/TEN, the cause of which may have been either the viral infection or acetaminophen administration. After corticosteroid treatment failed to achieve significant improvement, the patient was cured via DPMAS treatment.
We acknowledge that dermatologist Dr. He Xiaoyu initially diagnosed and treated this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dasuqi SA, Saudi Arabia S-Editor: Liu H L-Editor: A P-Editor: Li X
| 1. | Stevens AM, Johnson FC. A new eruptive fever associated with stomatitis and ophthalmitis: Report of two cases in children. Am J Dis Child. 1922;24:526-533. [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 365] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 388] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Narang I, Panthagani AP, Lewis M, Chohan B, Ferguson A, Nambi R. COVID-19-induced toxic epidermal necrolysis. Clin Exp Dermatol. 2021;46:927-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Punyaratabandhu P, Chirachanakul P. Cutaneous eruption in COVID-19-infected patients in Thailand: An observational descriptive study. J Dermatol. 2021;48:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Dash S, Sirka CS, Mishra S, Viswan P. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Frantz R, Huang S, Are A, Motaparthi K. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina (Kaunas). 2021;57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 7. | Zou H, Daveluy S. Toxic epidermal necrolysis and Stevens-Johnson syndrome after COVID-19 infection and vaccination. Australas J Dermatol. 2023;64:e1-e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 8. | Milosavljević MN, Pejčić AV, Milosavljević JZ. A review of published cases of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with the use of acetaminophen. Cutan Ocul Toxicol. 2021;40:280-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7061] [Cited by in F6Publishing: 7634] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 10. | Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg. 1986;204:503-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 289] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Kelemen JJ 3rd, Cioffi WG, McManus WF, Mason AD Jr, Pruitt BA Jr. Burn center care for patients with toxic epidermal necrolysis. J Am Coll Surg. 1995;180:273-278. [PubMed] [Cited in This Article: ] |
| 12. | Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, Kardaun S, Sidoroff A, Liss Y, Schumacher M, Roujeau JC; RegiSCAR study group. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133:1197-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 13. | Lee HY, Lim YL, Thirumoorthy T, Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. Br J Dermatol. 2013;169:1304-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Kamanabroo D, Schmitz-Landgraf W, Czarnetzki BM. Plasmapheresis in severe drug-induced toxic epidermal necrolysis. Arch Dermatol. 1985;121:1548-1549. [PubMed] [Cited in This Article: ] |
| 16. | Bamichas G, Natse T, Christidou F, Stangou M, Karagianni A, Koukourikos S, Chaidemenos G, Chrysomallis F, Sombolos K. Plasma exchange in patients with toxic epidermal necrolysis. Ther Apher. 2002;6:225-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Furubacke A, Berlin G, Anderson C, Sjöberg F. Lack of significant treatment effect of plasma exchange in the treatment of drug-induced toxic epidermal necrolysis? Intensive Care Med. 1999;25:1307-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Chaidemenos GC, Chrysomallis F, Sombolos K, Mourellou O, Ioannides D, Papakonstantinou M. Plasmapheresis in toxic epidermal necrolysis. Int J Dermatol. 1997;36:218-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Tan YW, Chen L, Zhou XB. Efficacy of artificial liver support system in severe immune-associated hepatitis caused by camrelizumab: A case report and review of the literature. World J Clin Cases. 2021;9:4415-4422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Schneider JA, Cohen PR. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34:1235-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |