Published online Apr 16, 2024. doi: 10.12998/wjcc.v12.i11.1885
Peer-review started: December 30, 2023
First decision: January 17, 2024
Revised: January 31, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: April 16, 2024
Since the inception of fluorine-18 fluorodeoxyglucose (F-18 FDG), positron emission tomography/computed tomography (PET/CT) utilizing F-18 FDG has become widely accepted as a valuable imaging modality in the field of oncology, with global prevalence in clinical practice. Given that a single Torso PET/CT scan encompasses the anatomical region from the skull base to the upper thigh, the detection of incidental abnormal focal hypermetabolism in areas of limited clinical interest is both feasible and not uncommon. Numerous investigations have been undertaken to delineate the distinctive features of these findings, yet the outcomes have proven inconclusive. The incongruent results of these studies present a challenge for physicians, leaving them uncertain about the appropriate course of action. This article provides a succinct overview of the characteristics of fluoro
Core Tip: Unexpected incidental focal fluorine-18 fluorodeoxyglucose uptake on positron emission tomography/computed tomography is not an uncommon finding. The nature of this uptake has been the subject of various studies, with outcomes varying depending on the organ in which it manifests. A noteworthy finding from these investigations reveals that over one-third of such uptakes were determined to be malignant. This observation underscores the importance of conducting further examinations in cases where incidental uptake is identified, as it could potentially serve as a crucial indicator for malignancy.
- Citation: Lee H, Hwang KH. Unexpected focal fluorodeoxyglucose uptake in main organs; pass through or pass by? World J Clin Cases 2024; 12(11): 1885-1899
- URL: https://www.wjgnet.com/2307-8960/full/v12/i11/1885.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i11.1885
The role of fluorine-18 fluorodeoxyglucose (F-18 FDG) is pivotal in establishing positron emission tomography/computed tomography (PET/CT) as a preeminent imaging modality within the realm of oncology. This radiopharmaceutical is readily available and routinely employed worldwide on a daily basis. Unlike conventional radiological images such as those obtained through computed tomography (CT) or magnetic resonance imaging, nuclear medicine images offer a functional perspective, enabling the assessment of molecular-level changes. Given that biochemical alterations precede observable physical changes such as alterations in size[1,2], PET/CT assumes a crucial role in the early detection of disease states. Presently, this imaging technique serves various purposes, including diagnosis, treatment planning, post-treatment evaluation, and follow-up.
Torso PET/CT scans encompass a range extending from the skull base to the upper thigh, with the possibility of conducting whole-body PET/CT scans contingent upon the capabilities of the scanner. The extensive scan range introduces the potential inclusion of regions with diminished clinical interest, leading to the observation of increased fluorodeoxyglucose (FDG) uptake (hypermetabolism) at unexpected sites. Such incidental uptake can pose a challenge for physicians in their interpretation. Studies have been undertaken to investigate incidental hypermetabolic regions, yielding diverse outcomes. This article delves into the distinctive characteristics of F-18 FDG, elucidates imaging findings, and explores the clinical significance of incidental hypermetabolism in several organs where unexpected uptake is relatively commonplace.
The initial synthesis of F-18 FDG was accomplished by Pacák et al[3] in 1968, followed by the successful preparation of F-18 FDG by Ido et al[4] in 1978, thereby facilitating its utilization as a radiopharmaceutical for positron emission tomography (PET) imaging[3-6]. FDG has found extensive applications in diverse fields such as oncology, neurology, and cardiology. Structurally, FDG closely resembles glucose, with the key distinction being the substitution of the hydroxyl group on the 2-carbon of a glucose molecule with a fluorine-18 radionuclide[7,8]. This glucose analogue is actively transported into cells through glucose transporters, primarily GLUT1 and GLUT3, mirroring the cellular uptake of glucose[7-9]. However, owing to its structural dissimilarity, FDG cannot complete the glucose metabolic pathway and becomes trapped within cells[10]. Despite this metabolic divergence, the initial stages of FDG uptake closely parallel those of glucose, enabling FDG to assess and depict cellular glucose metabolism due to their shared metabolic behavior.
Living cells rely on glucose as a primary energy source. Notably, cancer cells exhibit a heightened uptake of glucose, a phenomenon well-described by the Warburg effect[11]. In terms of energy production, cancer cells predominantly favor glycolysis over oxidative phosphorylation, despite its lower efficiency in adenosine triphosphate yields when compared to the latter. The preference for glycolysis, despite its lower efficiency, is attributed to its faster rate, effectively meeting the energy demands of cancer cells[12-15]. This accelerated glycolytic activity contributes to the increased uptake of both glucose and FDG in cancer cells, visualized through PET[16]. However, it is essential to note that FDG, while commonly used as a marker, lacks specificity for cancer cells. Organs with naturally high glucose metabolism, such as the brain or liver, exhibit elevated FDG uptake. Moreover, benign conditions characterized by increased glycolysis also result in the accumulation of FDG in cells[17-21]. Consequently, FDG cannot be considered a selective agent for distinguishing between malignant and benign cells.
The assessment of accumulated FDG in PET images involves both visual interpretation and quantitative analysis. One widely used semi-quantitative index is the standardized uptake value (SUV), a representative dimensionless ratio indicating the relative concentration of FDG in a region of interest[22]. The calculation of SUV is outlined as follows:

The popular application of SUV is evident in the differentiation between malignant and benign lesions. A cutoff value is investigated for specific cancers; subsequently, this determined cutoff serves as a reference value. Furthermore, SUV finds frequent use in the evaluation of treatment efficacy, involving a comparison of values obtained from pre- and post-therapy images. SUV can be quantified in various ways. Noteworthy methods include the determination of the highest SUV for a single pixel (maximum SUV or SUVmax), the average SUV for a freely drawn region (mean SUV or SUVmean), and the average SUV for a small fixed-sized region centered on a highly uptake area (peak SUV or SUVpeak). While SUVmax remains consistent regardless of the evaluator, it is susceptible to noise interference[23,24]. Conversely, SUVmean is prone to alterations based on the delineated area[25,26]. SUVpeak encompasses a relatively larger volume, making it less influenced by noise; however, its application becomes challenging for small or tiny lesions that do not attain a certain size[27-29]. In addition to these parameters, various SUV-derived metrics such as SUV corrected for lean body mass, metabolic tumor volume (MTV), and total lesion glycolysis (TLG) are employed. Acknowledging the imperfections inherent in each parameter, it becomes apparent that no single parameter can entirely substitute others.
The elevated glycolytic activity observed in cancer cells contributes to an increased uptake of FDG, resulting in pronounced visualization on PET imaging[30-32]. This uptake can be quantified, with SUV serving as a widely used metric in clinical settings. While SUV has inherent limitations, a generally accepted threshold is an SUV of 2.5 or higher, indicating a potential malignancy. It is crucial to acknowledge that non-specific FDG uptake can lead to elevated SUV values in normal physiological or benign inflammatory/infectious conditions[33-37].
The degree of FDG uptake varies depending on several factors. In solid tumors, low cellularity or reduced glucose metabolism may result in diminished FDG uptake, as observed in well-differentiated thyroid cancers, low-grade lung adenocarcinomas, low-grade lymphomas, well-differentiated neuroendocrine tumors, renal cell carcinoma, clear cell carcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, low-grade hepatocellular carcinoma, pancreatic cancer, prostate cancer, and so on[38-45]. However, these tumors may exhibit heightened FDG uptake during progression, marked by dedifferentiation or transformation[46-51]. Factors such as the cell cycle phase, oxygen levels, and the surrounding environment, particularly acidity, are implicated in influencing FDG uptake[52-57]. The level of FDG uptake also varies based on cancer cell type and degree of differentiation. Complementary to FDG, other PET radiopharmaceuticals structurally derived from amino acids or choline can be employed for a more comprehensive assessment of cancer avidity[58-61].
The transport of both glucose and FDG across cell membranes is facilitated by common plasma membrane proteins. Consequently, blood glucose concentration plays a pivotal role in influencing FDG transport dynamics. Numerous studies have elucidated that elevated blood sugar levels adversely affect image quality, attributing this phenomenon to the competitive inhibition of FDG transport by blood glucose across cell membranes[62-65]. Recognizing the significance of this relationship, guidelines outlined by the Society of Nuclear Medicine and Molecular Imaging and the European Association of Nuclear Medicine recommend conducting F-18 FDG PET/CT scans under stringent blood glucose control, specifically targeting concentrations lower than 11 mmol/L (approximately 200 mg/dL)[16,66]. Although a subset of recent literature suggests a limited impact of blood glucose levels on imaging outcomes[67-73], it is noteworthy that adherence to the established guidelines remains prevalent in the field.
The non-specific uptake mechanism of FDG renders it susceptible to uptake in both malignant and non-malignant cells, with a particular affinity for those exhibiting elevated glycolysis. Non-malignant cells, akin to their malignant counterparts, may avidly seek out glucose. Notably, active infectious or inflammatory lesions, as well as benign polyps, can manifest high FDG uptake, thereby mimicking the metabolic patterns observed in malignant lesions[74-78]. Consequently, relying solely on heightened FDG uptake poses challenges in accurately differentiating between malignant and non-malignant lesions. FDG, in this context, remains impartial, lacking discriminatory specificity.
The human body harbors several tissues and organs exhibiting noteworthy physiological FDG uptake. The brain, characterized by its elevated glucose metabolism, typically manifests robust FDG uptake, constituting approximately 6% of the administered dose[36]. The liver, too, engages in active facilitated glucose transport, resulting in a discernible FDG uptake. Hepatic FDG uptake generally surpasses that of the blood pool, and owing to the relatively stable metabolic activity of the liver, it frequently serves as a reference for FDG uptake[36,79]. Physiological uptake of FDG in the gas
Studies noted the usefulness of SUV in differentiation between primary and metastatic lesions[88-90], however, challenges persist when confronted with intense FDG uptake.
When elevated FDG metabolism is evident in imaging, the observed uptake manifests as either focal or diffuse[91-94]. In certain organs, diffuse uptake is more likely to be benign or physiological when compared to focal uptake[34,36,95-100]. Conversely, focal uptake holds greater clinical significance; it necessitates careful consideration, as it may indicate the presence of a malignant lesion[101-104]. The pattern of uptake becomes crucial in evaluating the potential pathology.
F-18 FDG PET/CT is a widely performed imaging modality encompassing various anatomical regions, not rarely revealing incidental focal FDG uptake outside the primary area of interest. However, it is imperative to acknowledge that non-FDG-avid or diminutive malignant lesions may be inherently excluded due to the inherent limitations of F-18 FDG PET/CT. The degree of FDG uptake serves as a crucial parameter, with higher uptake correlating with an elevated likelihood of malignancy or advanced disease[105-107]. Consequently, these incidentally observed uptakes should not be casually disregarded. The subsequent discussion delves into the clinical implications of incidentally observed focal F-18 FDG uptake within several organs that are frequently affected[108].
Approximately 85% of thyroid cancer is composed of well-differentiated cancer including papillary and follicular carcinomas[109,110]. They are relatively indolent than other subtypes such as poorly differentiated carcinoma or Hürthle cell cancer. F-18 FDG PET/CT is not routinely engaged in diagnosis and follow-up evaluation of well-differentiated cancer, unless the situation with elevated serum thyroglobulin (generally > 10 ng/mL) and negative radioiodine-131 whole body scan[111]. The prevalence of incidental FDG uptake, including both diffuse and focal, is up to 8%[112], and that of focal uptake is around 2%-4%[113-116]. Diffuse FDG uptake has more chance to be benign diseases such as thyroiditis than cancer[96,117], while approximately up to 60% of focal uptake proved to be malignant[112,116,118-120]. Noticeably, benign Hürthle cell adenoma show focal high FDG uptake as well mimicking a malignant lesion[121-123].
In healthy men with a mean age 55.5 ± 13 (min 28, max 75), the SUV of thyroid ranges from a minimum of 1.2 to a maximum of 2.2 with a mean of 1.5 ± 0.2. In healthy women with a mean age 49 ± 17 (min 18, max 73), it is from 1.1 to 2.4 with a mean of 1.5 ± 0.3[124]. Although studies show some differences in findings, most focal thyroid uptake had SUV greater than 3, and it was able to differentiate malignant from benign lesions in many instances. Other PET parameters such as MTV or TLG are under discussion for differentiation.
Of those malignant lesions, well-differentiated cancers accounted for a large part[102]. These FDG-avid well-differentiated cancers are possibly related to the dedifferentiation or mutation. The upregulation of glucose transporters is one of the possible mechanisms of increased FDG avidity[125,126]. BRAF V600E mutation is also the possible cause of elevated expression of glucose transporters and glycolysis, resulting in high FDG uptake[127-130]. Therefore, incidentally visualized focal thyroid uptake on FDG PET possibly has more aggressive features than non-visualized occult lesions. Also, the fact that more than half (from one-third to two-thirds) of incidental focal uptake turned out to be malignant suggests that further evaluation should be weighted to identify the nature of the uptake.
The incidence of breast cancer in the year 2020 is the overwhelming number one in women, and the mortality rate is the highest among the types of malignant tumors[131,132]. Breast cancer is classified into many subtypes. The two main histological subtypes are invasive and preinvasive[133-135]. Invasive breast cancer is about three times more common than preinvasive one. Ductal carcinoma no special type of invasive breast cancer (also known as invasive ductal carcinoma) makes up close to 80% of all breast cancers. Invasive lobular carcinoma is the other subtype of invasive breast cancer. Preinvasive breast cancer includes ductal carcinoma in situ (DCIS) and lobular carcinoma in situ (LCIS). DCIS accounts for about 80% of preinvasive breast cancer and may develop into invasive breast cancer, whereas LCIS rarely exhibits invasive features. Many molecular subtypes area known including triple-negative, human epidermal growth factor receptor 2 positive, luminal B, luminal A.
The prevalence and incidence of breast cancer is obviously much higher in woman than in man[136-138]. The prevalence of incidental focal breast FDG uptake in woman is varying in some extent, several studies documented it around 1.0%[103,139-143] and the highest was about 23% in a study[144]. Possibly over 50% of the focal uptake proved to be malignant and common histologic type was invasive ductal carcinoma[103,145-148].
As expected, homogeneously diffuse and low breast FDG uptake appears to be a normal finding, and SUVmax is less than 2.5. A study reported that in the age group of 50.9 ± 9.70 (range 32-77), the average SUVmax of normal dense breasts was 1.243, while that of normal nondense breasts was 0.997. Similar results have been reported in other studies[149-151]. Due to low SUV in normal breast, focal uptake can be observed without great difficulty. However, DCIS was reported to have an average SUVmax between 2.0 and 2.4[152,153], which may not be visually distinguishable on images. Moreover, the SUV may be affected by physiological states (density change) such as pregnancy, breastfeeding, menstrual cycle[154,155], and age. Enlarged breast during pregnancy may show SUV similar to liver which has the value from 2 to 5[124]. Suckling of the lactating breast may be associated with increase in expression of glucose transporters resulting in high FDG uptake[156]. SUV decreases as age increases (with diminished breast density).
Breast cancers with lobular feature or small size limit the role of FDG PET/CT in evaluation[157-160]. F-18 FDG PET/CT is not indicated for the routine use except when standard studies bring equivocal results or in advanced disease[161]. In evaluation of breast, axillary lymph node uptake may be a challenge too. Focal FDG uptake in the breast and/or axilla may be observed in the situations such as infection/inflammation[37], primary/metastatic disease[162-164], benign neoplasm (fibroadenoma, intraductal papilloma, ductal epithelial hyperplasia, sclerosing adenosis, and so on)[165], gynecomastia[166], and artefacts[167]. These should be included in differential diagnosis. The most common benign breast tumor, fibroadenoma, often shows low FDG uptake, but it may have high uptake mimicking a malignant lesion[168,169]. Asymmetrical or nodular appearance of gynecomastia also can mimic malignancy. SUV failed in differentiation between malignant and benign lesions in a study[139]. Nevertheless, incidental focal breast FDG uptake has up to more than 50% of malignancy, therefore, a thorough appropriate evaluation is needed.
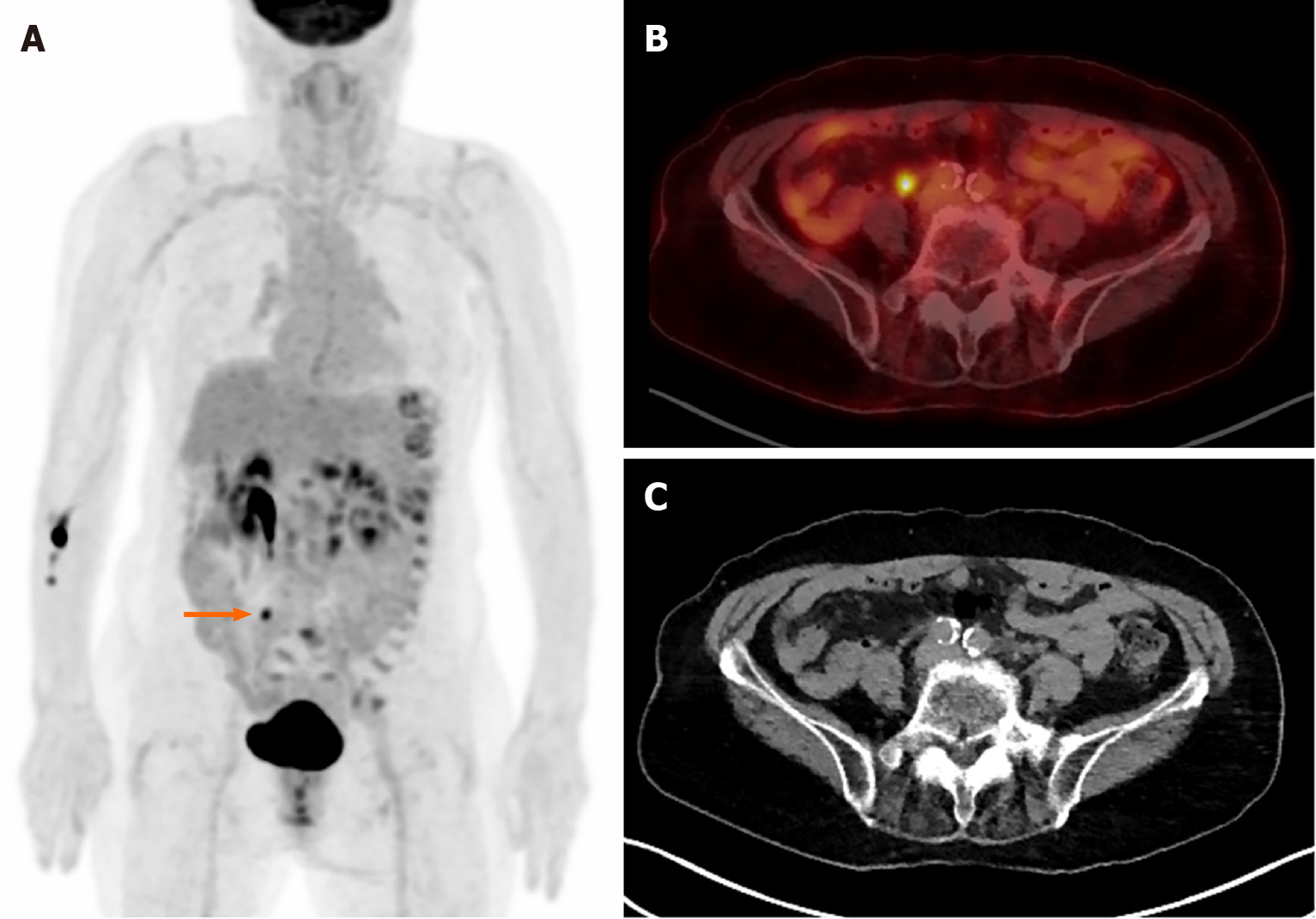
Figure 1 shows incidental right breast uptake in a 32-year-old woman who was diagnosed with endometrial cancer. Increased uptake in the right pelvic cavity indicates primary cancer lesion, and another focal moderate uptake is observed in her right breast unexpectedly (SUVmax 3.29). The uptake turned out to be a coincident malignant lesion which was confirmed as DCIS histopathologically.
Incidental colorectal FDG uptake is up to around 5%[170-172]. Focal uptake has more chance to be malignant[173]. Diffuse and segmental uptake may be due to inflammation, physiological uptake, or FDG excretion[174,175]; and considered to have a low risk of malignancy. The prevalence of focal uptake is up to around 16%[176]. The malignant and premalignant lesions were up to 68% of focal uptake[101,176,177]. Premalignant lesions are not yet malignant; however, they have more chance to develop into malignant lesions. Adenomas (tubular adenomas, villous adenomas, tubulovillous adenomas) are the most frequent premalignant lesions and others include chronic inflammatory bowel diseases, hereditary syndromes (familial adenomatous polyposis, Peutz-Jeghers syndrome, and juvenile polyposis). Colorectal adenomatous polyps are known to develop in up to 40% of people over the age of 60[178].
Different genetic mechanisms are suggested for cancer development by the location (distal or proximal)[179-181]. Proximal colon cancer (up to splenic flexure) accounted for 41%; distal colon cancer 22%; and rectal cancer 28% in the United States from 2009 to 2013[182]. Other paper observed that the most frequent site (42%) of malignant and premalignant lesions was the ascending colon[173], while another reported it as the rectum (60.0%), followed by the sigmoid colon (17.4%)[183]. The distribution of colorectal cancer appears to vary by country, region, race, and age[184-186]. It also varies with sex. Women are more likely to experience proximal colon cancer compared with men[187,188]. Because specific colorectal regions are more likely to involve malignancy than others, consideration of patient characteristics is recommended in reading FDG PET/CT.
Often, mixed single/multiple focal and diffuse uptake is observed together in colorectal region. In this case, diffuse uptake may not preclude further evaluation including colonoscopy and histopathological confirmation. The role of SUV to differentiate malignant/premalignant from benign lesions is still in debate[170,189-193]. Again, more than 50% of malignancy is observed in the incidental focal FDG colorectal uptake.
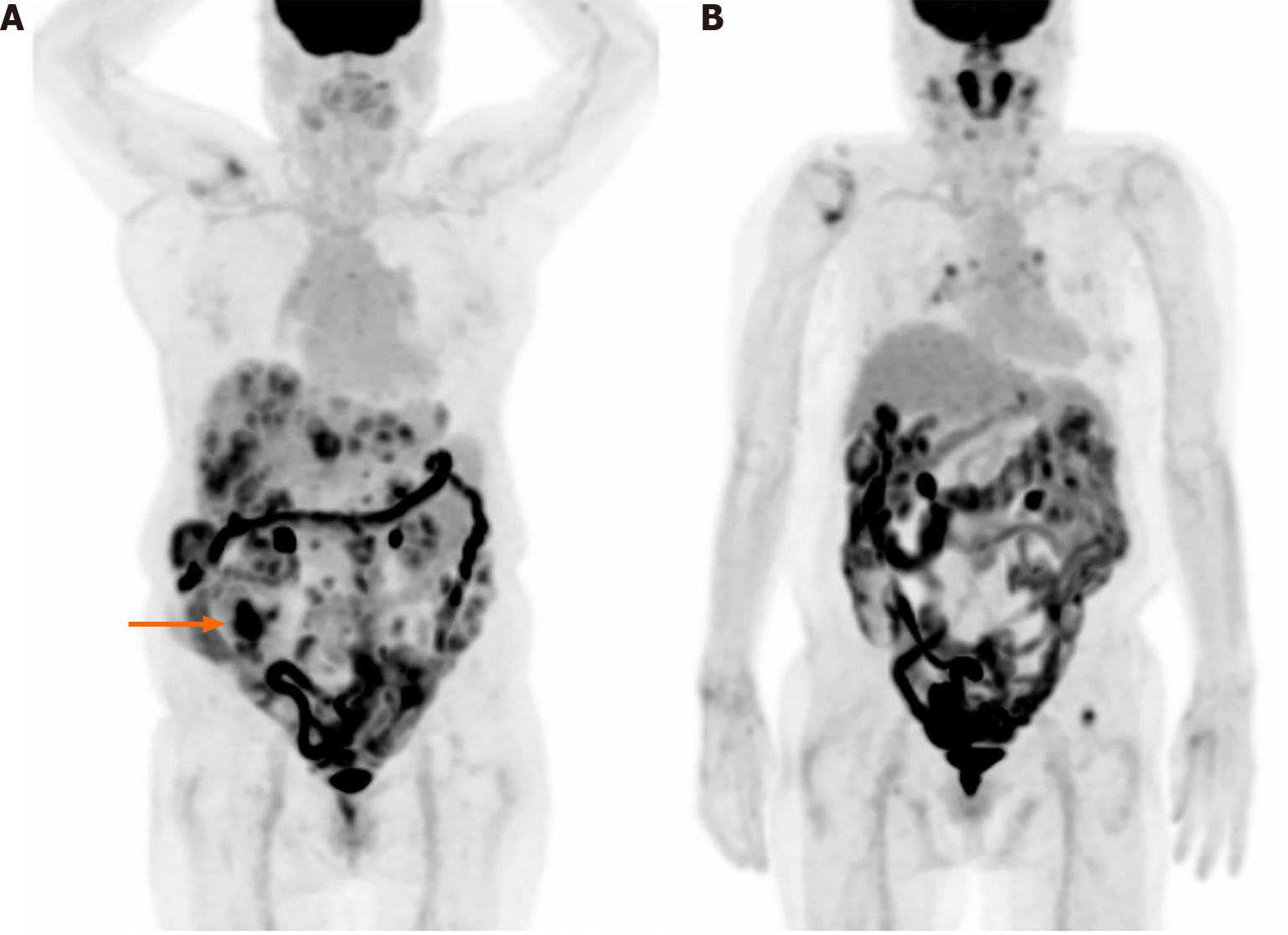
Figure 2 shows F-18 FDG PET/CT images of a 79-year-old woman who was diagnosed with pancreatic body cancer. Suspicious focal uptake is observed in the right lower abdomen. With an aid of CT, it was revealed as urine radioactivity at the right ureter. Another interesting thing is the multiple foci of hypermetabolism along the descending and sigmoid colon. Colonoscopy was conducted and it found no significant abnormal lesion. The uptake is probably due to normal physiological uptake.
Figure 3 shows a maximum intensity projection image of a 76-year-old woman diagnosed with cecal cancer and multiple metastases/seeding nodules. The primary cecal cancer is observed in the right lower abdomen and diffuse intestinal uptake is also shown. The diffuse uptake may be physiological; however, the possibility of hidden pathological lesions, which may be obscured by the intense intestinal uptake, cannot be ruled out.
With the recent advent of United States Food and Drug Administration-approved prostate-specific membrane antigen-targeted PET imaging radiopharmaceuticals, PET/CT has become actively used in the evaluation of prostate cancer. In terms of FDG, prostate is one of the organs that shows low FDG uptake even if it is cancerous. In a group of men with a mean age of 63.6 years (range 22-97), SUVmean and SUVmax in normal prostate were reported as 1.3 ± 0.4 (range 0.1-2.7) and 1.6 ± 0.4 (range 1.1-3.7), respectively[194]. The degree of uptake may overlap among prostate cancer, benign prostate hyperplasia, and normal prostate. SUV showed questionable or suboptimal performance in differentiation between malignant and benign lesions. As a result, F-18 FDG PET/CT is not routinely recommended and performed in detection or initial staging of primary prostate cancer[195,196]. However, incidentally observed focal uptake may have clinical implications[100,104,197,198], particularly in the peripheral zone[199,200]. The prevalence of uptake is up to around 2%[200,201] and malignant rate is up to over 60%[197,201-203]. Multifocal uptake is not a significant differential diagnostic criterion between malignant and benign lesions[204]. The location of focal uptake may be the key in differentiation, that is, focal uptake in the peripheral zone is possibly related with malignancy[204,205]. Although with low FDG uptake in both malignant and benign prostate diseases, focal and peripheral uptake should be noted and lead to further evaluation.
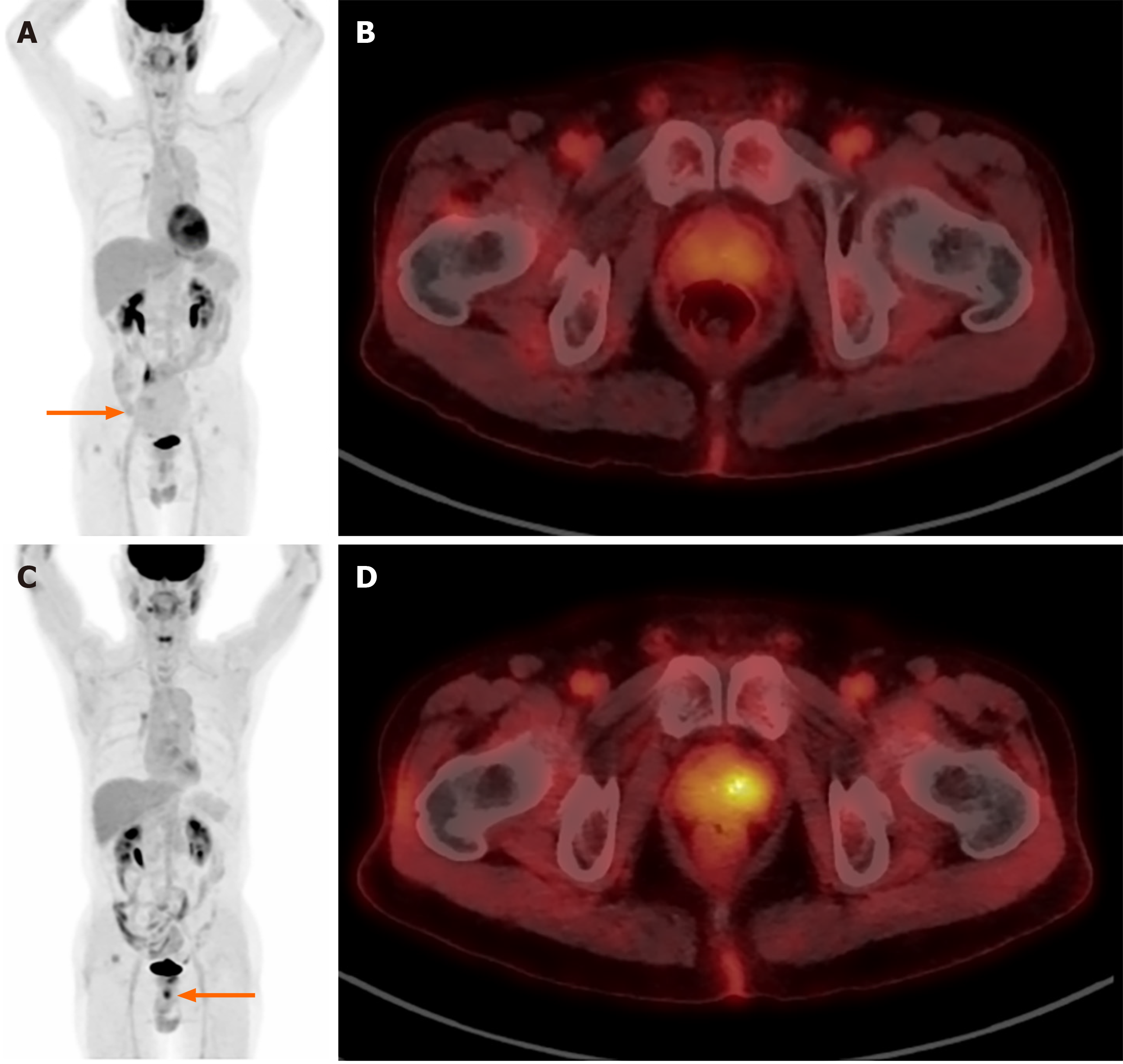
Figure 4 shows F-18 FDG PET/CT images of a 74-year-old man who was diagnosed with small bowel gastrointestinal stromal tumor. Initial maximum intensity projection image Figure 4A shows a large pelvic mass with low FDG uptake (arrow). Also, nearly symmetrical low prostate FDG uptake is observed (Figure 4B). F-18 FDG PET/CT conducted in five years for a suspicious recurrent pelvic mass noticed on CT show an incidental focal FDG uptake (SUVmax 5.6) in the left side of prostate (Figure 4C and D). The uptake was proved as an adenocarcinoma of prostate histopathologically.
This article comprehensively addresses the characteristics of FDG, discusses imaging findings, and outlines the clinical implications of incidental focal FDG uptake across various organs. Existing literature consistently reports that a significant proportion, ranging from approximately one-third to over a half, of incidental focal FDG uptake is indicative of yet unrecognized malignancy. Considering the malignancy rate associated with incidental focal uptake in diverse organs, it is imperative for healthcare professionals not to disregard such unexpected findings. By doing so, they can make more informed and confident clinical decisions, prompting a proactive approach towards further comprehensive evaluation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Okasha H, Egypt S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
| 1. | Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol. 2011;38:55-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Wong CY, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29:815-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Pacák J, Točík Z, Černý M. Synthesis of 2-deoxy-2-fluoro-D-glucose. J Chem Soc D: Chem Commun. 196977-196977. [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Ido T, Wan C-N, Casella V, Fowler JS, Wolf AP, Reivich M, Kuhl DE. Labeled 2-deoxy-D-glucose analogs. 18F-labeled 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2-fluoro-D-mannose and 14C-2-deoxy-2-fluoro-D-glucose. J Labelled Compounds Radiopharm. 1978;14:175-183. [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Guerrero TM, Hoffman EJ, Dahlbom M, Cutler PD, Hawkins RA, Phelps ME. Characterization of a Whole-Body Imaging Technique for Pet. Ieee T Nucl Sci. 1990;37:676-680. [DOI] [Cited in This Article: ] |
| 6. | Cherry SR. Fundamentals of positron emission tomography and applications in preclinical drug development. J Clin Pharmacol. 2001;41:482-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Yu S. Review of F-FDG Synthesis and Quality Control. Biomed Imaging Interv J. 2006;2:e57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O'shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785-2808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Smith TA. Facilitative glucose transporter expression in human cancer tissue. Br J Biomed Sci. 1999;56:285-292. [PubMed] [Cited in This Article: ] |
| 10. | Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19:1154-1161. [PubMed] [Cited in This Article: ] |
| 11. | Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12:1131-1137. [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 165] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Rajendran JG, Mankoff DA, O'Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Coleman CN, Mitchell JB, Camphausen K. Tumor hypoxia: chicken, egg, or a piece of the farm? J Clin Oncol. 2002;20:610-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Annibaldi A, Widmann C. Glucose metabolism in cancer cells. Curr Opin Clin Nutr Metab Care. 2010;13:466-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Smith TA. FDG uptake, tumour characteristics and response to therapy: a review. Nucl Med Commun. 1998;19:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ; European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2053] [Cited by in F6Publishing: 1924] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 17. | Vangu MDT, Momodu JI. F-18 FDG PET/CT Imaging in Normal Variants, Pitfalls and Artifacts in the Abdomen and Pelvis. Front Nucl Med. 2022;1. [DOI] [Cited in This Article: ] |
| 18. | Li Y, Behr S. Acute Findings on FDG PET/CT: Key Imaging Features and How to Differentiate Them from Malignancy. Curr Radiol Rep. 2020;8:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Buyukdereli G, Kara E, Guler M, Kanat N. Evaluation of Visible Physiological F-18 FDG Uptake Patterns in Spinal Cord on PET/CT. Neurosurg Q. 2015;25:403-406. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Purohit BS, Ailianou A, Dulguerov N, Becker CD, Ratib O, Becker M. FDG-PET/CT pitfalls in oncological head and neck imaging. Insights Imaging. 2014;5:585-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Kostakoglu L, Hardoff R, Mirtcheva R, Goldsmith SJ. PET-CT fusion imaging in differentiating physiologic from pathologic FDG uptake. Radiographics. 2004;24:1411-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 470] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519-1527. [PubMed] [Cited in This Article: ] |
| 24. | Nakamoto Y, Zasadny KR, Minn H, Wahl RL. Reproducibility of common semi-quantitative parameters for evaluating lung cancer glucose metabolism with positron emission tomography using 2-deoxy-2-[18F]fluoro-D-glucose. Mol Imaging Biol. 2002;4:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Krak NC, Boellaard R, Hoekstra OS, Twisk JW, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32:294-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med. 2012;53:4-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Oh HH, Lee SE, Choi IS, Choi WJ, Yoon DS, Min HS, Ra YM, Moon JI, Kang YH. The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol. 2011;104:530-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, van Lanschot J, Sloof G, Boellaard R. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2007;34:392-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 584] [Cited by in F6Publishing: 532] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 31. | Czernin J. Clinical applications of FDG-PET in oncology. Acta Med Austriaca. 2002;29:162-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Pinilla I, Rodríguez-Vigil B, Gómez-León N. Integrated FDG PET/CT: Utility and Applications in Clinical Oncology. Clin Med Oncol. 2008;2:181-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Cook GJ, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Eur J Nucl Med. 1999;26:1363-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19:61-77; quiz 150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 624] [Cited by in F6Publishing: 546] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 35. | Cook GJ, Fogelman I, Maisey MN. Normal physiological and benign pathological variants of 18-fluoro-2-deoxyglucose positron-emission tomography scanning: potential for error in interpretation. Semin Nucl Med. 1996;26:308-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 291] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Ahmad Sarji S. Physiological uptake in FDG PET simulating disease. Biomed Imaging Interv J. 2006;2:e59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol. 2012;198:W304-W314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Dondi F, Albano D, Giubbini R, Bertagna F. 18F-FDG PET and PET/CT for the evaluation of gastric signet ring cell carcinoma: a systematic review. Nucl Med Commun. 2021;42:1293-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Jadvar H. Is There Use for FDG-PET in Prostate Cancer? Semin Nucl Med. 2016;46:502-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. AJR Am J Roentgenol. 2011;197:W260-W265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000;174:1005-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Tanizaki Y, Kobayashi A, Shiro M, Ota N, Takano R, Mabuchi Y, Yagi S, Minami S, Terada M, Ino K. Diagnostic value of preoperative SUVmax on FDG-PET/CT for the detection of ovarian cancer. Int J Gynecol Cancer. 2014;24:454-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Kang DE, White RL Jr, Zuger JH, Sasser HC, Teigland CM. Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J Urol. 2004;171:1806-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Higashi K, Ueda Y, Seki H, Yuasa K, Oguchi M, Noguchi T, Taniguchi M, Tonami H, Okimura T, Yamamoto I. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med. 1998;39:1016-1020. [PubMed] [Cited in This Article: ] |
| 45. | Aquino SL, Halpern EF, Kuester LB, Fischman AJ. FDG-PET and CT features of non-small cell lung cancer based on tumor type. Int J Mol Med. 2007;19:495-499. [PubMed] [Cited in This Article: ] |
| 46. | Bahri H, Laurence L, Edeline J, Leghzali H, Devillers A, Raoul JL, Cuggia M, Mesbah H, Clement B, Boucher E, Garin E. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med. 2014;55:1786-1790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, Dickson J, Caplin M, Ell PJ. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447-2455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 48. | Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 49. | Busaidy NL, Cabanillas ME. Differentiated thyroid cancer: management of patients with radioiodine nonresponsive disease. J Thyroid Res. 2012;2012:618985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Hong CM, Ahn BC, Jeong SY, Lee SW, Lee J. Distant metastatic lesions in patients with differentiated thyroid carcinoma. Clinical implications of radioiodine and FDG uptake. Nuklearmedizin. 2013;52:121-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Bannas P, Derlin T, Groth M, Apostolova I, Adam G, Mester J, Klutmann S. Can (18)F-FDG-PET/CT be generally recommended in patients with differentiated thyroid carcinoma and elevated thyroglobulin levels but negative I-131 whole body scan? Ann Nucl Med. 2012;26:77-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Sung Y, Tetrault MA, Takahashi K, Ouyang J, Pratx G, Fakhri GE, Normandin MD. Dependence of fluorodeoxyglucose (FDG) uptake on cell cycle and dry mass: a single-cell study using a multi-modal radiography platform. Sci Rep. 2020;10:4280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Roppongi M, Izumisawa M, Terasaki K, Muraki Y, Shozushima M. (18)F-FDG and (11)C-choline uptake in proliferating tumor cells is dependent on the cell cycle in vitro. Ann Nucl Med. 2019;33:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Türkcan S, Kiru L, Naczynski DJ, Sasportas LS, Pratx G. Lactic Acid Accumulation in the Tumor Microenvironment Suppresses (18)F-FDG Uptake. Cancer Res. 2019;79:410-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, Aime S. In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res. 2016;76:6463-6470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 56. | Peppicelli S, Toti A, Giannoni E, Bianchini F, Margheri F, Del Rosso M, Calorini L. Metformin is also effective on lactic acidosis-exposed melanoma cells switched to oxidative phosphorylation. Cell Cycle. 2016;15:1908-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Corbet C, Draoui N, Polet F, Pinto A, Drozak X, Riant O, Feron O. The SIRT1/HIF2α axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. 2014;74:5507-5519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Verger A, Imbert L, Zaragori T. Dynamic amino-acid PET in neuro-oncology: a prognostic tool becomes essential. Eur J Nucl Med Mol Imaging. 2021;48:4129-4132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Najjar AM, Johnson JM, Schellingerhout D. The Emerging Role of Amino Acid PET in Neuro-Oncology. Bioengineering (Basel). 2018;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Goldstein J, Even-Sapir E, Ben-Haim S, Saad A, Spieler B, Davidson T, Berger R, Weiss I, Appel S, Lawrence YR, Symon Z. Does Choline PET/CT Change the Management of Prostate Cancer Patients With Biochemical Failure? Am J Clin Oncol. 2017;40:256-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Urso L, Rocca GC, Borgia F, Lancia F, Malorgio A, Gagliano M, Zanetto M, Uccelli L, Cittanti C, Ippolito C, Evangelista L, Bartolomei M. The Role of [(18)F]F-Choline PET/CT in the Initial Management and Outcome Prediction of Prostate Cancer: A Real-World Experience from a Multidisciplinary Approach. Biomedicines. 2022;10. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 62. | Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology. 1992;183:643-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med. 1993;34:1-6. [PubMed] [Cited in This Article: ] |
| 64. | Minn H, Leskinen-Kallio S, Lindholm P, Bergman J, Ruotsalainen U, Teräs M, Haaparanta M. [18F]fluorodeoxyglucose uptake in tumors: kinetic vs. steady-state methods with reference to plasma insulin. J Comput Assist Tomogr. 1993;17:115-123. [PubMed] [Cited in This Article: ] |
| 65. | Langen KJ, Braun U, Rota Kops E, Herzog H, Kuwert T, Nebeling B, Feinendegen LE. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med. 1993;34:355-359. [PubMed] [Cited in This Article: ] |
| 66. | Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, Stabin MG, Zubal G, Kachelriess M, Cronin V, Holbrook S. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885-895. [PubMed] [Cited in This Article: ] |
| 67. | Hara T, Higashi T, Nakamoto Y, Suga T, Saga T, Ishimori T, Ishizu K, Kawashima H, Kawase S, Matsumoto K, Togashi K. Significance of chronic marked hyperglycemia on FDG-PET: is it really problematic for clinical oncologic imaging? Ann Nucl Med. 2009;23:657-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Rabkin Z, Israel O, Keidar Z. Do hyperglycemia and diabetes affect the incidence of false-negative 18F-FDG PET/CT studies in patients evaluated for infection or inflammation and cancer? A Comparative analysis. J Nucl Med. 2010;51:1015-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 69. | Lindholm H, Brolin F, Jonsson C, Jacobsson H. The relation between the blood glucose level and the FDG uptake of tissues at normal PET examinations. EJNMMI Res. 2013;3:50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Sprinz C, Zanon M, Altmayer S, Watte G, Irion K, Marchiori E, Hochhegger B. Effects of blood glucose level on 18F fluorodeoxyglucose (18F-FDG) uptake for PET/CT in normal organs: an analysis on 5623 patients. Sci Rep. 2018;8:2126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Sprinz C, Altmayer S, Zanon M, Watte G, Irion K, Marchiori E, Hochhegger B. Effects of blood glucose level on 18F-FDG uptake for PET/CT in normal organs: A systematic review. PLoS One. 2018;13:e0193140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Eskian M, Alavi A, Khorasanizadeh M, Viglianti BL, Jacobsson H, Barwick TD, Meysamie A, Yi SK, Iwano S, Bybel B, Caobelli F, Lococo F, Gea J, Sancho-Muñoz A, Schildt J, Tatcı E, Lapa C, Keramida G, Peters M, Boktor RR, John J, Pitman AG, Mazurek T, Rezaei N. Effect of blood glucose level on standardized uptake value (SUV) in (18)F- FDG PET-scan: a systematic review and meta-analysis of 20,807 individual SUV measurements. Eur J Nucl Med Mol Imaging. 2019;46:224-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Cengiz A. The Relation Between the Blood Glucose Level and the FDG Uptake of Tissues at Normal or Near-Normal PET/CT Imaging. Akd Med J. 2019;5:365-369. [DOI] [Cited in This Article: ] |
| 74. | Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972-1980. [PubMed] [Cited in This Article: ] |
| 75. | Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551-1555. [PubMed] [Cited in This Article: ] |
| 76. | Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 77. | Safaie E, Matthews R, Bergamaschi R. PET scan findings can be false positive. Tech Coloproctol. 2015;19:329-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation--current and emerging clinical applications. Clin Radiol. 2015;70:787-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 79. | Binns DS, Pirzkall A, Yu W, Callahan J, Mileshkin L, Conti P, Scott AM, Macfarlane D, Fine BM, Hicks RJ; OSI3926g Study Team. Compliance with PET acquisition protocols for therapeutic monitoring of erlotinib therapy in an international trial for patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38:642-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, Mantzarides M, Foehrenbach H, Pecking AP, Alberini JL. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging. 2008;35:95-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 81. | Oh JR, Song HC, Chong A, Ha JM, Jeong SY, Min JJ, Bom HS. Impact of medication discontinuation on increased intestinal FDG accumulation in diabetic patients treated with metformin. AJR Am J Roentgenol. 2010;195:1404-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Ozülker T, Ozülker F, Mert M, Ozpaçaci T. Clearance of the high intestinal (18)F-FDG uptake associated with metformin after stopping the drug. Eur J Nucl Med Mol Imaging. 2010;37:1011-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Bybel B, Greenberg ID, Paterson J, Ducharme J, Leslie WD. Increased F-18 FDG intestinal uptake in diabetic patients on metformin: a matched case-control analysis. Clin Nucl Med. 2011;36:452-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Steenkamp DW, McDonnell ME, Meibom S. Metformin may be associated with false-negative cancer detection in the gastrointestinal tract on PET/CT. Endocr Pract. 2014;20:1079-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 85. | Bahler L, Stroek K, Hoekstra JB, Verberne HJ, Holleman F. Metformin-related colonic glucose uptake; potential role for increasing glucose disposal?--A retrospective analysis of (18)F-FDG uptake in the colon on PET-CT. Diabetes Res Clin Pract. 2016;114:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Moran JK, Lee HB, Blaufox MD. Optimization of urinary FDG excretion during PET imaging. J Nucl Med. 1999;40:1352-1357. [PubMed] [Cited in This Article: ] |
| 87. | Garbarino S, Caviglia G, Brignone M, Massollo M, Sambuceti G, Piana M. Estimate of FDG excretion by means of compartmental analysis and ant colony optimization of nuclear medicine data. Comput Math Methods Med. 2013;2013:793142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Kirienko M, Cozzi L, Rossi A, Voulaz E, Antunovic L, Fogliata A, Chiti A, Sollini M. Ability of FDG PET and CT radiomics features to differentiate between primary and metastatic lung lesions. Eur J Nucl Med Mol Imaging. 2018;45:1649-1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 89. | Kosaka N, Tsuchida T, Tsuji K, Shimizu K, Kimura H. Standardized uptake value differences between primary and metastatic lesions in ¹⁸F-FDG PET/CT of patients with lung cancer. Acta Radiol. 2015;56:1329-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | Dijkman BG, Schuurbiers OC, Vriens D, Looijen-Salamon M, Bussink J, Timmer-Bonte JN, Snoeren MM, Oyen WJ, van der Heijden HF, de Geus-Oei LF. The role of (18)F-FDG PET in the differentiation between lung metastases and synchronous second primary lung tumours. Eur J Nucl Med Mol Imaging. 2010;37:2037-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Thuillier P, Crouzeix G, Descourt R, Salaun PY, Abgral R. Progression of Focal to Diffuse Thyroid Uptake Detected by 18F-FDG PET/CT: Malignant Metastatic Disease or Benign Thyroiditis? Clin Nucl Med. 2018;43:e310-e311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Dong A, Zhao T, Wang Y, Zuo C. Focal and diffuse FDG uptake patterns of endotracheal lymphoma. Clin Nucl Med. 2014;39:731-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 93. | Kurata S, Ishibashi M, Hiromatsu Y, Kaida H, Miyake I, Uchida M, Hayabuchi N. Diffuse and diffuse-plus-focal uptake in the thyroid gland identified by using FDG-PET: prevalence of thyroid cancer and Hashimoto's thyroiditis. Ann Nucl Med. 2007;21:325-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Chen W, Li G, Parsons M, Zhuang H, Alavi A. Clinical Significance of Incidental Focal Versus Diffuse Thyroid Uptake on FDG-PET Imaging. PET Clin. 2007;2:321-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 95. | Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, Horiuchi M. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology. 1998;207:775-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, Peller PJ, Bahn RS, Lowe VJ. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48:896-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. Radiographics. 2007;27:145-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 98. | Tatlidil R, Jadvar H, Bading JR, Conti PS. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology. 2002;224:783-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 99. | Kresnik E, Mikosch P, Gallowitsch HJ, Heinisch M, Lind P. F-18 fluorodeoxyglucose positron emission tomography in the diagnosis of inflammatory bowel disease. Clin Nucl Med. 2001;26:867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Sahin E, Elboga U, Kalender E, Basıbuyuk M, Demir HD, Celen YZ. Clinical significance of incidental FDG uptake in the prostate gland detected by PET/CT. Int J Clin Exp Med. 2015;8:10577-10585. [PubMed] [Cited in This Article: ] |
| 101. | Gökden Y, Özülker F, Özülker T. Prevalence and Clinical Significance of Incidental Focal (18)F-FDG Uptake in Colon on PET/CT Imaging. Mol Imaging Radionucl Ther. 2022;31:96-103. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 102. | de Leijer JF, Metman MJH, van der Hoorn A, Brouwers AH, Kruijff S, van Hemel BM, Links TP, Westerlaan HE. Focal Thyroid Incidentalomas on (18)F-FDG PET/CT: A Systematic Review and Meta-Analysis on Prevalence, Risk of Malignancy and Inconclusive Fine Needle Aspiration. Front Endocrinol (Lausanne). 2021;12:723394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Aarstad EM, Nordhaug P, Naghavi-Behzad M, Larsen LB, Gerke O, Hildebrandt MG. Prevalence of focal incidental breast uptake on FDG-PET/CT and risk of malignancy: a systematic review and meta-analysis. Eur J Hybrid Imaging. 2019;3:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Brown AM, Lindenberg ML, Sankineni S, Shih JH, Johnson LM, Pruthy S, Kurdziel KA, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B. Does focal incidental 18F-FDG PET/CT uptake in the prostate have significance? Abdom Imaging. 2015;40:3222-3229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Cheng NM, Hsieh CE, Liao CT, Ng SH, Wang HM, Fang YD, Chou WC, Lin CY, Yen TC. Prognostic Value of Tumor Heterogeneity and SUVmax of Pretreatment 18F-FDG PET/CT for Salivary Gland Carcinoma With High-Risk Histology. Clin Nucl Med. 2019;44:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Chin AL, Kumar KA, Guo HH, Maxim PG, Wakelee H, Neal JW, Diehn M, Loo BW Jr, Gensheimer MF. Prognostic Value of Pretreatment FDG-PET Parameters in High-dose Image-guided Radiotherapy for Oligometastatic Non-Small-cell Lung Cancer. Clin Lung Cancer. 2018;19:e581-e588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Kwon SY, Choi EK, Kong EJ, Chong A, Ha JM, Chun KA, Cho IH, Bom HS, Min JJ, Kim J, Song HC, O JH, Kim SH. Prognostic value of preoperative 18F-FDG PET/CT in papillary thyroid cancer patients with a high metastatic lymph node ratio: a multicenter retrospective cohort study. Nucl Med Commun. 2017;38:402-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 108. | O'Sullivan JW, Muntinga T, Grigg S, Ioannidis JPA. Prevalence and outcomes of incidental imaging findings: umbrella review. BMJ. 2018;361:k2387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 109. | Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Goldner W, Haymart M, Hoh C, Hunt JP, Iagaru A, Kandeel F, Kopp P, Lamonica DM, McIver B, Raeburn CD, Ridge JA, Ringel MD, Scheri RP, Shah JP, Sippel R, Smallridge RC, Sturgeon C, Wang TN, Wirth LJ, Wong RJ, Johnson-Chilla A, Hoffmann KG, Gurski LA. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw. 2018;16:1429-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 110. | Shah JP. Thyroid carcinoma: epidemiology, histology, and diagnosis. Clin Adv Hematol Oncol. 2015;13:3-6. [PubMed] [Cited in This Article: ] |
| 111. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7978] [Cited by in F6Publishing: 8252] [Article Influence: 1031.5] [Reference Citation Analysis (1)] |
| 112. | Akbas A, Dagmura H, Gül S, Daşiran F, Daldal E, Okan I. MANAGEMENT PRINCIPLES OF INCIDENTAL THYROID 18F-FDG UPTAKE IDENTIFIED ON 18F-FDG PET/CT IMAGING. Acta Endocrinol (Buchar). 2022;18:253-257. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 113. | Nielsen MH, Nygaard B, Søndergaard SB, Bennedbæk FN. [Diagnostic strategy of incidental findings of focal thyroid FDG uptake identified on PET/CT]. Ugeskr Laeger. 2011;173:1948-1952. [PubMed] [Cited in This Article: ] |
| 114. | Bertagna F, Treglia G, Piccardo A, Giubbini R. Diagnostic and clinical significance of F-18-FDG-PET/CT thyroid incidentalomas. J Clin Endocrinol Metab. 2012;97:3866-3875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 115. | Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, Baek CH, Chung JH, Lee KH, Kim BT. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609-615. [PubMed] [Cited in This Article: ] |
| 116. | Chen W, Parsons M, Torigian DA, Zhuang H, Alavi A. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl Med Commun. 2009;30:240-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 117. | Liu Y. Clinical significance of thyroid uptake on F18-fluorodeoxyglucose positron emission tomography. Ann Nucl Med. 2009;23:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 118. | Kwak JY, Kim EK, Yun M, Cho A, Kim MJ, Son EJ, Oh KK. Thyroid incidentalomas identified by 18F-FDG PET: sonographic correlation. AJR Am J Roentgenol. 2008;191:598-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Adas M, Adas G, Koc B, Ozulker F. Incidental thyroid lesions on FDG-PET/CT: a prevalence study and proposition of management. Minerva Endocrinol. 2015;40:169-175. [PubMed] [Cited in This Article: ] |
| 120. | Erdoğan M, Korkmaz H, Torus B, Avcı M, Boylubay ŞM, Çiriş M, Yıldız M, Şengül SS. The Role of Metabolic Volumetric Parameters in Predicting Malignancy in Incidental Thyroid Nodules Detected in (18)F-FDG PET/CT Scans. Mol Imaging Radionucl Ther. 2021;30:86-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Pathak KA, Klonisch T, Nason RW, Leslie WD. FDG-PET characteristics of Hürthle cell and follicular adenomas. Ann Nucl Med. 2016;30:506-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Yu R, Auerbach MS. FDG-Avid Hürthle Cell Thyroid Adenoma. Clin Nucl Med. 2019;44:752-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 123. | Rohren EM. Intense FDG uptake in a benign Hurthle cell adenoma. Clin Nucl Med. 2004;29:664-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Zincirkeser S, Sahin E, Halac M, Sager S. Standardized uptake values of normal organs on 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging. J Int Med Res. 2007;35:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 125. | Grabellus F, Nagarajah J, Bockisch A, Schmid KW, Sheu SY. Glucose transporter 1 expression, tumor proliferation, and iodine/glucose uptake in thyroid cancer with emphasis on poorly differentiated thyroid carcinoma. Clin Nucl Med. 2012;37:121-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 126. | Schönberger J, Rüschoff J, Grimm D, Marienhagen J, Rümmele P, Meyringer R, Kossmehl P, Hofstaedter F, Eilles C. Glucose transporter 1 gene expression is related to thyroid neoplasms with an unfavorable prognosis: an immunohistochemical study. Thyroid. 2002;12:747-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Suh HY, Choi H, Paeng JC, Cheon GJ, Chung JK, Kang KW. Comprehensive gene expression analysis for exploring the association between glucose metabolism and differentiation of thyroid cancer. BMC Cancer. 2019;19:1260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 128. | Meyer HJ, Wienke A, Surov A. Associations between GLUT expression and SUV values derived from FDG-PET in different tumors-A systematic review and meta analysis. PLoS One. 2019;14:e0217781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 129. | Chang JW, Park KW, Heo JH, Jung SN, Liu L, Kim SM, Kwon IS, Koo BS. Relationship Between (18)F-fluorodeoxyglucose Accumulation and the BRAF (V600E) Mutation in Papillary Thyroid Cancer. World J Surg. 2018;42:114-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Yoon M, Jung SJ, Kim TH, Ha TK, Urm SH, Park JS, Lee SM, Bae SK. Relationships between transporter expression and the status of BRAF V600E mutation and F-18 FDG uptake in papillary thyroid carcinomas. Endocr Res. 2016;41:64-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Estimated age-standardized incidence and mortality rates (World) in 2020, World, females, all ages (excl. NMSC). Available from: https://gco.iarc.fr/today/online-analysis-multi-bars?v=2020&mode=cancer&mode_population=countries&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=0&include_nmsc_other=1&type_multiple=%257B%2522inc%2522%253Atrue%252C%2522mort%2522%253Atrue%252C%2522prev%2522%253Afalse%257D&orientation=horizontal&type_sort=0&type_nb_items=%257B%2522top%2522%253Atrue%252C%2522bottom%2522%253Afalse%257D. [Cited in This Article: ] |
| 132. | Chhikara BS, Parang K. Global Cancer Statistics 2022: the trends projection analysis. Chem Biol Lette. 2023;10:451 Available from: https://pubs.thesciencein.org/journal/index.php/cbl/article/view/451. [Cited in This Article: ] |
| 133. | Cserni G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica. 2020;112:25-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 134. | Yang WT, Bu H. [Updates in the 5(th) edition of WHO classification of tumours of the breast]. Zhonghua Bing Li Xue Za Zhi. 2020;49:400-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 135. | Lebeau A. [Updated WHO classification of tumors of the breast]. Pathologe. 2021;42:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Kang SY, Lee SB, Kim YS, Kim Z, Kim HY, Kim HJ, Park S, Bae SY, Yoon K, Lee SK, Jung KW, Han J, Youn HJ; Korean Breast Cancer Society. Breast Cancer Statistics in Korea, 2018. J Breast Cancer. 2021;24:123-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 137. | Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72:524-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 508] [Article Influence: 254.0] [Reference Citation Analysis (0)] |
| 138. | Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 600] [Cited by in F6Publishing: 484] [Article Influence: 242.0] [Reference Citation Analysis (0)] |
| 139. | Shin KM, Kim HJ, Jung SJ, Lim HS, Lee SW, Cho SH, Jang YJ, Lee HJ, Kim GC, Jung JH, Park JY. Incidental Breast Lesions Identified by (18)F-FDG PET/CT: Which Clinical Variables Differentiate between Benign and Malignant Breast Lesions? J Breast Cancer. 2015;18:73-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 140. | Kim MY, Cho N, Chang JM, Yun BL, Bae MS, Kang KW, Moon WK. Mammography and ultrasonography evaluation of unexpected focal 18F-FDG uptakes in breast on PET/CT. Acta Radiol. 2012;53:249-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Minamimoto R, Senda M, Jinnouchi S, Terauchi T, Yoshida T, Inoue T. Detection of breast cancer in an FDG-PET cancer screening program: results of a nationwide Japanese survey. Clin Breast Cancer. 2015;15:e139-e146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 142. | Dunne RM, O'Mahony D, Wilson G, McDermott R, O'Keeffe SA. The role of the breast radiologist in evaluation of breast incidentalomas detected on 18-fludeoxyglucose positron emission tomography/CT. Br J Radiol. 2013;86:20130034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 143. | Lim S, Lee EH, Park JM, Chang YW, Kim HH, Jeong SH. Role of combined BI-RADS assessment using mammography and sonography for evaluation of incidental hypermetabolic lesions in the breast on 18F-FDG PET-CT. Acta Radiol. 2013;54:1117-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 144. | Benveniste AP, Marom EM, Benveniste MF, Mawlawi O, Fox PS, Yang W. Incidental primary breast cancer detected on PET-CT. Breast Cancer Res Treat. 2015;151:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 145. | Beatty JS, Williams HT, Gucwa AL, Hughes MP, Vasudeva VS, Aldridge BA, Fields DM, David GS, Lind DS, Kruse EJ, McLoughlin JM. The predictive value of incidental PET/CT findings suspicious for breast cancer in women with non-breast malignancies. Am J Surg. 2009;198:495-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 146. | Litmanovich D, Gourevich K, Israel O, Gallimidi Z. Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: incidence and clinical significance. Eur J Nucl Med Mol Imaging. 2009;36:1558-1564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 147. | Korn RL, Yost AM, May CC, Kovalsky ER, Orth KM, Layton TA, Drumm D. Unexpected focal hypermetabolic activity in the breast: significance in patients undergoing 18F-FDG PET/CT. AJR Am J Roentgenol. 2006;187:81-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Chae EY, Cha JH, Kim HH, Shin HJ, Kim HJ, Oh HY, Koh YH, Moon DH. Analysis of incidental focal hypermetabolic uptake in the breast as detected by 18F-FDG PET/CT: clinical significance and differential diagnosis. Acta Radiol. 2012;53:530-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 149. | Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Cucchiara AJ, Yu JQ, Alavi A. The effect of age, menopausal state, and breast density on (18)F-FDG uptake in normal glandular breast tissue. J Nucl Med. 2010;51:347-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 150. | Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Standardized uptake values of normal breast tissue with 2-deoxy-2-[F-18]fluoro-D: -glucose positron emission tomography: variations with age, breast density, and menopausal status. Mol Imaging Biol. 2006;8:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Vranjesevic D, Schiepers C, Silverman DH, Quon A, Villalpando J, Dahlbom M, Phelps ME, Czernin J. Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med. 2003;44:1238-1242. [PubMed] [Cited in This Article: ] |
| 152. | Yoon HJ, Kim Y, Kim BS. Intratumoral metabolic heterogeneity predicts invasive components in breast ductal carcinoma in situ. Eur Radiol. 2015;25:3648-3658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 153. | Mavi A, Urhan M, Yu JQ, Zhuang H, Houseni M, Cermik TF, Thiruvenkatasamy D, Czerniecki B, Schnall M, Alavi A. Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med. 2006;47:1440-1446. [PubMed] [Cited in This Article: ] |
| 154. | Park HH, Shin JY, Lee JY, Jin GH, Kim HS, Lyu KY, Lee TS. Discussion on the alteration of 18F-FDG uptake by the breast according to the menstrual cycle in PET imaging. Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:2469-2472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 155. | Lin CY, Ding HJ, Liu CS, Chen YK, Lin CC, Kao CH. Correlation between the intensity of breast FDG uptake and menstrual cycle. Acad Radiol. 2007;14:940-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 156. | Camps M, Vilaro S, Testar X, Palacín M, Zorzano A. High and polarized expression of GLUT1 glucose transporters in epithelial cells from mammary gland: acute down-regulation of GLUT1 carriers by weaning. Endocrinology. 1994;134:924-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 157. | Kitajima K, Miyoshi Y. Present and future role of FDG-PET/CT imaging in the management of breast cancer. Jpn J Radiol. 2016;34:167-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 158. | Berg WA. Nuclear Breast Imaging: Clinical Results and Future Directions. J Nucl Med. 2016;57 Suppl 1:46S-52S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 159. | Groheux D, Espié M, Giacchetti S, Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 160. | Avril N, Adler LP. F-18 fluorodeoxyglucose-positron emission tomography imaging for primary breast cancer and loco-regional staging. Radiol Clin North Am. 2007;45:645-657, vi. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 161. | Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Hurvitz SA, Isakoff SJ, Jankowitz RC, Javid SH, Krishnamurthy J, Leitch M, Lyons J, Mortimer J, Patel SA, Pierce LJ, Rosenberger LH, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Wisinski KB, Young JS, Burns J, Kumar R. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:691-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 287] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 162. | Bedi DG, Krishnamurthy R, Krishnamurthy S, Edeiken BS, Le-Petross H, Fornage BD, Bassett RL Jr, Hunt KK. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol. 2008;191:646-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 163. | Ravdel L, Robinson WA, Lewis K, Gonzalez R. Metastatic melanoma in the breast: a report of 27 cases. J Surg Oncol. 2006;94:101-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 164. | Loffeld A, Marsden JR. Management of melanoma metastasis to the breast: case series and review of the literature. Br J Dermatol. 2005;152:1206-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 165. | Benveniste AP, Yang W, Benveniste MF, Mawlawi OR, Marom EM. Benign breast lesions detected by positron emission tomography-computed tomography. Eur J Radiol. 2014;83:919-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 166. | Dong A, Wang Y, Lu J, Zuo C. Spectrum of the Breast Lesions With Increased 18F-FDG Uptake on PET/CT. Clin Nucl Med. 2016;41:543-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 167. | Surov A, Fiedler E, Holzhausen HJ, Ruschke K, Schmoll HJ, Spielmann RP. Metastases to the breast from non-mammary malignancies: primary tumors, prevalence, clinical signs, and radiological features. Acad Radiol. 2011;18:565-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 168. | Makis W, Ciarallo A, Hickeson M, Derbekyan V. Rapidly growing complex fibroadenoma with surrounding ductal hyperplasia mimics breast malignancy on serial F-18 FDG PET/CT imaging. Clin Nucl Med. 2011;36:576-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 169. | Yamaguchi R, Futamata Y, Yoshimura F, Murakami N, Koufuji K, Kutami R, Kojima K, Ohki S, Kurata S, Kaida H, Ishibashi M, Yano H. Mastopathic-type fibroadenoma and ductal adenoma of the breast with false-positive fluorodeoxyglucose positron emission tomography. Jpn J Radiol. 2009;27:280-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 170. | van Hoeij FB, Keijsers RG, Loffeld BC, Dun G, Stadhouders PH, Weusten BL. Incidental colonic focal FDG uptake on PET/CT: can the maximum standardized uptake value (SUVmax) guide us in the timing of colonoscopy? Eur J Nucl Med Mol Imaging. 2015;42:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 171. | Keyzer C, Dhaene B, Blocklet D, De Maertelaer V, Goldman S, Gevenois PA. Colonoscopic Findings in Patients With Incidental Colonic Focal FDG Uptake. AJR Am J Roentgenol. 2015;204:W586-W591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 172. | Hui YH, Kung BT, Yong TA. Incidental Focal Colonic Uptake of 18F-fluorodeoxyglucose on Positron Emission Tomography/Computed Tomography: Its Incidence and Clinical Significance. Hong Kong J Radiol. 2020;23:275-280. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 173. | Shmidt E, Nehra V, Lowe V, Oxentenko AS. Clinical significance of incidental [18 F]FDG uptake in the gastrointestinal tract on PET/CT imaging: a retrospective cohort study. BMC Gastroenterol. 2016;16:125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 174. | Şimşek FS, İspiroğlu M, Taşdemir B, Köroğlu R, Ünal K, Özercan IH, Entok E, Kuşlu D, Karabulut K. What approach should we take for the incidental finding of increased 18F-FDG uptake foci in the colon on PET/CT? Nucl Med Commun. 2015;36:1195-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 175. | Salazar Andía G, Prieto Soriano A, Ortega Candil A, Cabrera Martín MN, González Roiz C, Ortiz Zapata JJ, Cardona Arboniés J, Lapeña Gutiérrez L, Carreras Delgado JL. Clinical relevance of incidental finding of focal uptakes in the colon during 18F-FDG PET/CT studies in oncology patients without known colorectal carcinoma and evaluation of the impact on management. Rev Esp Med Nucl Imagen Mol. 2012;31:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 176. | Treglia G, Taralli S, Salsano M, Muoio B, Sadeghi R, Giovanella L. Prevalence and malignancy risk of focal colorectal incidental uptake detected by (18)F-FDG-PET or PET/CT: a meta-analysis. Radiol Oncol. 2014;48:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 177. | Kousgaard SJ, Gade M, Petersen LJ, Thorlacius-Ussing O. Incidental detection of colorectal lesions on (18) F-FDG-PET/CT is associated with high proportion of malignancy: A study in 549 patients. Endosc Int Open. 2020;8:E1725-E1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 178. | Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551-2557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 179. | Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 526] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 180. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8087] [Cited by in F6Publishing: 7736] [Article Influence: 227.5] [Reference Citation Analysis (1)] |
| 181. | Beart RW, Melton LJ 3rd, Maruta M, Dockerty MB, Frydenberg HB, O'Fallon WM. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983;26:393-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 182. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2526] [Cited by in F6Publishing: 2757] [Article Influence: 393.9] [Reference Citation Analysis (2)] |
| 183. | Golfam F, Golfam P, Neghabi Z. Frequency of all types of colorectal tumors in the patients referred to selected hospitals in tehran. Iran Red Crescent Med J. 2013;15:473-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 184. | Sjöström O, Silander G, Syk I, Henriksson R, Melin B, Hellquist BN. Disparities in colorectal cancer between Northern and SouthernSweden - a report from the new RISK North database. Acta Oncol. 2018;57:1622-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 185. | Qing SH, Rao KY, Jiang HY, Wexner SD. Racial differences in the anatomical distribution of colorectal cancer: a study of differences between American and Chinese patients. World J Gastroenterol. 2003;9:721-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 186. | Thomas CR Jr, Jarosz R, Evans N. Racial differences in the anatomical distribution of colon cancer. Arch Surg. 1992;127:1241-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 187. | Schmuck R, Gerken M, Teegen EM, Krebs I, Klinkhammer-Schalke M, Aigner F, Pratschke J, Rau B, Benz S. Gender comparison of clinical, histopathological, therapeutic and outcome factors in 185,967 colon cancer patients. Langenbecks Arch Surg. 2020;405:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 188. | Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167-5175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 274] [Cited by in F6Publishing: 268] [Article Influence: 29.8] [Reference Citation Analysis (4)] |
| 189. | Servente L, Gigirey V, García Fontes M, Alonso O. Incidental focal colonic uptake in studies (18)F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2018;37:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 190. | Soltau SR, Hess S, Nguyen T, Gerke O, Petersen H, Alavi A, Høilund-Carlsen PF. Clinical significance of incidental focal bowel uptake on (18)F-FDG PET/CT as related to colorectal cancer. Hell J Nucl Med. 2016;19:245-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 191. | Purandare NC, Gawade SK, Puranik AD, Agrawal A, Shah S, Rangarajan V. Etiology and significance of incidentally detected focal colonic uptake on FDG PET/CT. Indian J Radiol Imaging. 2012;22:260-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 192. | Kei PL, Vikram R, Yeung HW, Stroehlein JR, Macapinlac HA. Incidental finding of focal FDG uptake in the bowel during PET/CT: CT features and correlation with histopathologic results. AJR Am J Roentgenol. 2010;194:W401-W406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 193. | Weston BR, Iyer RB, Qiao W, Lee JH, Bresalier RS, Ross WA. Ability of integrated positron emission and computed tomography to detect significant colonic pathology: the experience of a tertiary cancer center. Cancer. 2010;116:1454-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 194. | Jadvar H, Ye W, Groshen S, Conti PS. [F-18]-fluorodeoxyglucose PET-CT of the normal prostate gland. Ann Nucl Med. 2008;22:787-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 195. | Jadvar H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging. 2013;40 Suppl 1:S5-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 196. | Minamimoto R, Senda M, Jinnouchi S, Terauchi T, Yoshida T, Murano T, Fukuda H, Iinuma T, Uno K, Nishizawa S, Tsukamoto E, Iwata H, Inoue T, Oguchi K, Nakashima R. The current status of an FDG-PET cancer screening program in Japan, based on a 4-year (2006-2009) nationwide survey. Ann Nucl Med. 2013;27:46-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 197. | Hwang I, Chong A, Jung SI, Hwang EC, Kim SO, Kang TW, Kwon DD, Park K, Ryu SB. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med. 2013;27:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 198. | Bertagna F, Piccardo A, Dib B, Bertoli M, Fracassi F, Bosio G, Giubbini R, Biasiotto G, Giovanella L, Treglia G. Multicentre study of 18F-FDG-PET/CT prostate incidental uptake. Jpn J Radiol. 2015;33:538-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 199. | McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 357] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 200. | Yang Z, Hu S, Cheng J, Xu J, Shi W, Zhu B, Zhang Y, Yao Z, Pan H. Prevalence and risk of cancer of incidental uptake in prostate identified by fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Clin Imaging. 2014;38:470-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 201. | Bertagna F, Sadeghi R, Giovanella L, Treglia G. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland. Systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin. 2014;53:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 202. | Kang PM, Seo WI, Lee SS, Bae SK, Kwak HS, Min K, Kim W, Kang DI. Incidental abnormal FDG uptake in the prostate on 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography scans. Asian Pac J Cancer Prev. 2014;15:8699-8703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Seino H, Ono S, Miura H, Morohashi S, Wu Y, Tsushima F, Takai Y, Kijima H. Incidental prostate ¹⁸F-FDG uptake without calcification indicates the possibility of prostate cancer. Oncol Rep. 2014;31:1517-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 204. | Cho SK, Choi JY, Yoo J, Cheon M, Lee JY, Hyun SH, Lee EJ, Lee KH, Kim BT. Incidental Focal (18)F-FDG Uptake in the Prostate: Clinical Significance and Differential Diagnostic Criteria. Nucl Med Mol Imaging. 2011;45:192-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 205. | Han EJ, H O J, Choi WH, Yoo IR, Chung SK. Significance of incidental focal uptake in prostate on 18-fluoro-2-deoxyglucose positron emission tomography CT images. Br J Radiol. 2010;83:915-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |