Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8357
Peer-review started: August 22, 2023
First decision: November 1, 2023
Revised: November 11, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 16, 2023
Ischemic gastritis is a clinically rare disease with high mortality that infrequently reported in the medical literature and under-recognized clinically and histopathologically. Early diagnosis and treatment can only be achieved through upper gastrointestinal endoscopy after symptoms appear.
A 68-year-old woman with a history of intracranial aneurysm developed dizziness, chest tightness and unconsciousness for 2 d. Computed tomography angiography showed diffuse coronary atherosclerosis, moderate to severe stenosis in the proximal end of the left anterior descending branch, multiple calcified plaques in the proximal end of the circumflex branch and right coronary artery, and mild to moderate stenosis. The patient also developed diffuse atherosclerosis in the splenic and mesenteric arteries, with mild lumen stenosis and atherosclerosis in the abdominal aorta and its branches. Endoscopy showed submucosal congestion and damage of the entire gastric mucosa, of which the fundus and body of the stomach were most seriously affected. The mucosa was swollen, with a deep purple color, surface erosion and dark red oozing blood. Pathological examination showed bleeding and necrosis of the gastric mucosa, with residual contours of the gastric glands, consistent with ischemic gastritis.
Ischemic gastritis is a rare disease that may be difficult to diagnose as its symptoms may be similar to those of other gastrointestinal diseases. Diagnosis is usually based on endoscopic and pathological examinations, which show insufficient blood supply to the gastric mucosa leading to mucosal damage and necrosis.
Core Tip: Ischemic gastritis is a rare disease characterized by insufficient blood supply to the gastric mucosa, leading to mucosal damage and necrosis. It can occur in patients with various underlying diseases, such as cardiovascular disease, arterial thrombosis or embolism, vasculitis or hypotension. The clinical manifestations of ischemic gastritis range from mild gastrointestinal symptoms to severe bleeding or perforation, and diagnosis is usually based on endoscopic and pathological examination. However, ischemic gastritis may be difficult to diagnose as it may be similar to other gastrointestinal diseases and may require a high degree of suspicion.
- Citation: Wei RY, Zhu JH, Li X, Wu JY, Liu JW. Diffuse arterial atherosclerosis presenting with acute ischemic gastritis: A case report. World J Clin Cases 2023; 11(35): 8357-8363
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8357.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8357
Ischemic gastritis is a rare event that occurs in the vascular-rich stomach which can be seen in patients with sepsis, severe atherosclerosis, vasculitis, acute gastric dilatation, gastric volvulus, or due to idiopathic causes[1]. Gastric ischemia is a serious condition with estimated mortality rates of an estimated 30% thirty-day mortality and reports of up to a 40% delayed 1-year mortality rate, in part due to delays in diagnosis leading to development of severe complications[2]. It is a rare phenomenon with limited cases reported in the literature, typically caused by either local or diffused vascular insufficiency[3]. Older people and patients with vascular risk (such as renal failure and diabetes) have an increased risk of ischemic gastritis[4]. These risks, combined with hemodynamic disruption, lead to high mortality rates[2]. The characteristic symptoms of this disease include abdominal pain and gastrointestinal bleeding[5]. Computed tomography (CT) shows wall thickening, wall emphysema and fluid retention, but rarely vascular obstruction[6,7]. Upper gastrointestinal endoscopy shows multiple ulcers and ischemic changes[2,8]. We here report a 68-year-old female patient with a history of intracranial aneurysm who developed rare diffuse atherosclerosis and ischemic gastritis. The baseline clinical and laboratory data, medical history, endoscopic and CT results, treatment and outcome, clinical characteristics, prognosis and early detection in this patient were described.
A 68-year-old woman with dizziness, chest tightness, and unclear consciousness lasting for 2 d.
When performing the CT scanning, the patient suddenly vomited about 50 mL of bright red blood. As an emergency, she was treated with injection of Pantoprazole to inhibit gastric acid and protect gastric mucosa, and urapidil to control blood pressure. She underwent coronary CT angiography (CTA), which indicated diffuse mixed plaques in the proximal and middle left anterior descending branch with moderate to severe stenosis in the lumen. For further treatment, she was admitted to hospital with a diagnosis of coronary atherosclerosis. She had a history of hypertension for 10 years, with a maximum blood pressure of 240/120 mmHg and irregular antihypertensive treatment.
One year ago, a cerebral aneurysm was discovered. The patient denied a history of infectious diseases such as hepatitis and tuberculosis, diabetes, heart disease, cerebrovascular disease, lung disease, kidney disease, malignant tumor, drug and food allergy, blood transfusion and adverse reactions, trauma and surgery. She had an irregular vaccination history.
Personal and family history was unremarkable.
In the preliminary evaluation, the body temperature was 37.1 °C, pulse rate was 87 beats/min, respiration was 20 breaths/min, and blood pressure was 148/94 mmHg. She had clear consciousness, depression, no yellow staining on the skin and sclera, no swelling of superficial lymph nodes in the neck, supraclavicular region, armpits and groin, etc. Thoracic symmetry, no deformity, no tenderness of the chest wall, clear sound on percussion of both lungs, clear breath sounds in both lungs, no dry or wet rales heard in both lungs, heart rate of 87 beats/min, arrhythmia, no obvious murmurs or additional heart sounds heard in the auscultation area of each valve, flat and soft abdomen, tenderness around the umbilical cord, no rebound pain, no renal or hepatic percussion pain, liver and spleen were not palpable below the costal margin, Murphy’s sign was negative, negative mobility dullness, bowel sounds 3 times/min, and no concave edema in both lower limbs. The neurological examination did not elicit pathological signs.
White blood cells 18.2 × 109/L (normal, 3.5–9.5 × 109/L), red blood cells 5.79 × 1012/L (normal, 4.3–5.8 × 1012/L), hemoglobin 183 g/L (normal, 130–175 g/L), platelets 131 × 109/L (normal 125–300 × 109/L), alanine transaminase 53 U/L (normal, 0–50 U/L), lactate dehydrogenase 265 U/L (normal, 120–246 U/L), glucose 8.44 mmol/L (normal, 4.10–5.90 mmol/L); D-dimer 3.38 mg/L (normal, 0–0.55 mg/L), and fibrin degradation product 8.50 μg/mL (normal, 0–5.00 μg/mL). Blood gas analysis: pH 7.335 (normal, 7.35–7.45), oxygen partial pressure 76.3 mmHg (normal, 83–108 mmHg), total carbon dioxide 20.6 mmol/L (normal, 24–32 mmol/L), remaining alkali -5.4 mmol/L (normal, -3.0 to 3.0mmol/L), anion gap 5.6 mmol/L (normal, 8.0–16.0 mmol/L), sodium (whole blood) 147.0 mmol/L (normal, 135–145 mmol/L), whole blood lactate 2.0 mmol/L (normal, 0.5–1.7 mmol/L), oxygenated hemoglobin 92.0% (normal, 93%-98%), brain natriuretic peptide 19.8 pg/mL (normal, < 100 pg/mL), myoglobin 27.5 ng/mL (normal, < 106 ng/mL), cytokeratin 1.46 ng/mL (normal, < 2.37 ng/mL), and troponin < 0.012 ng/mL (normal, < 0.034 ng/mL).
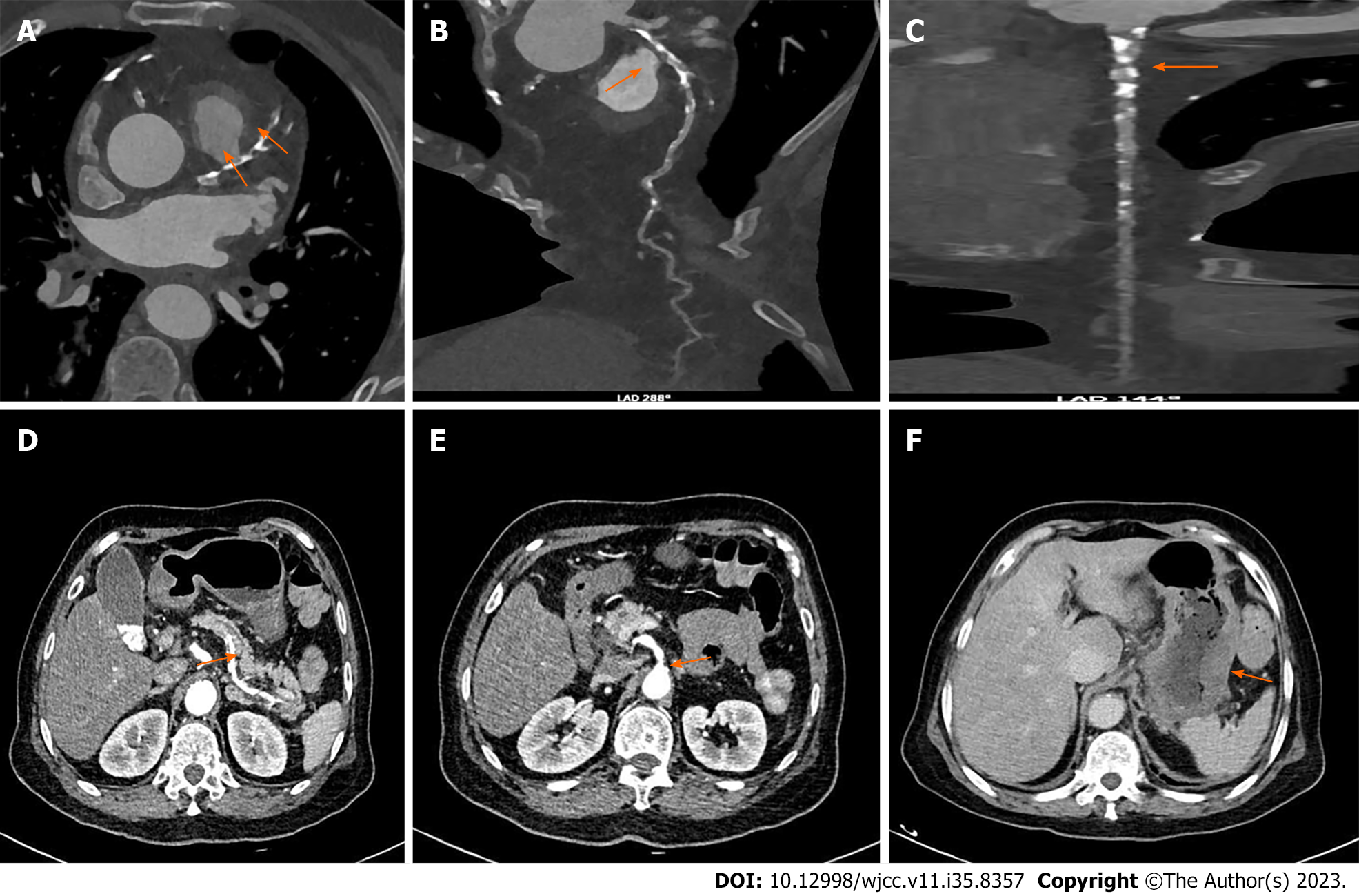
CTA: (1) Coronary atherosclerosis, diffuse mixed plaque with moderate to severe stenosis in the proximal and middle segments of the left anterior descending branch; multiple calcifications and mixed plaques with mild to moderate stenosis in the proximal and middle segments of the circumflex branch; and multiple localized calcified plaques with mild stenosis in the proximal segments of the right coronary artery; and (2) Diffuse mixed plaques in splenic and mesenteric arteries with stenosis of lumen, and atherosclerosis of abdominal aorta and its branches (Figure 1).
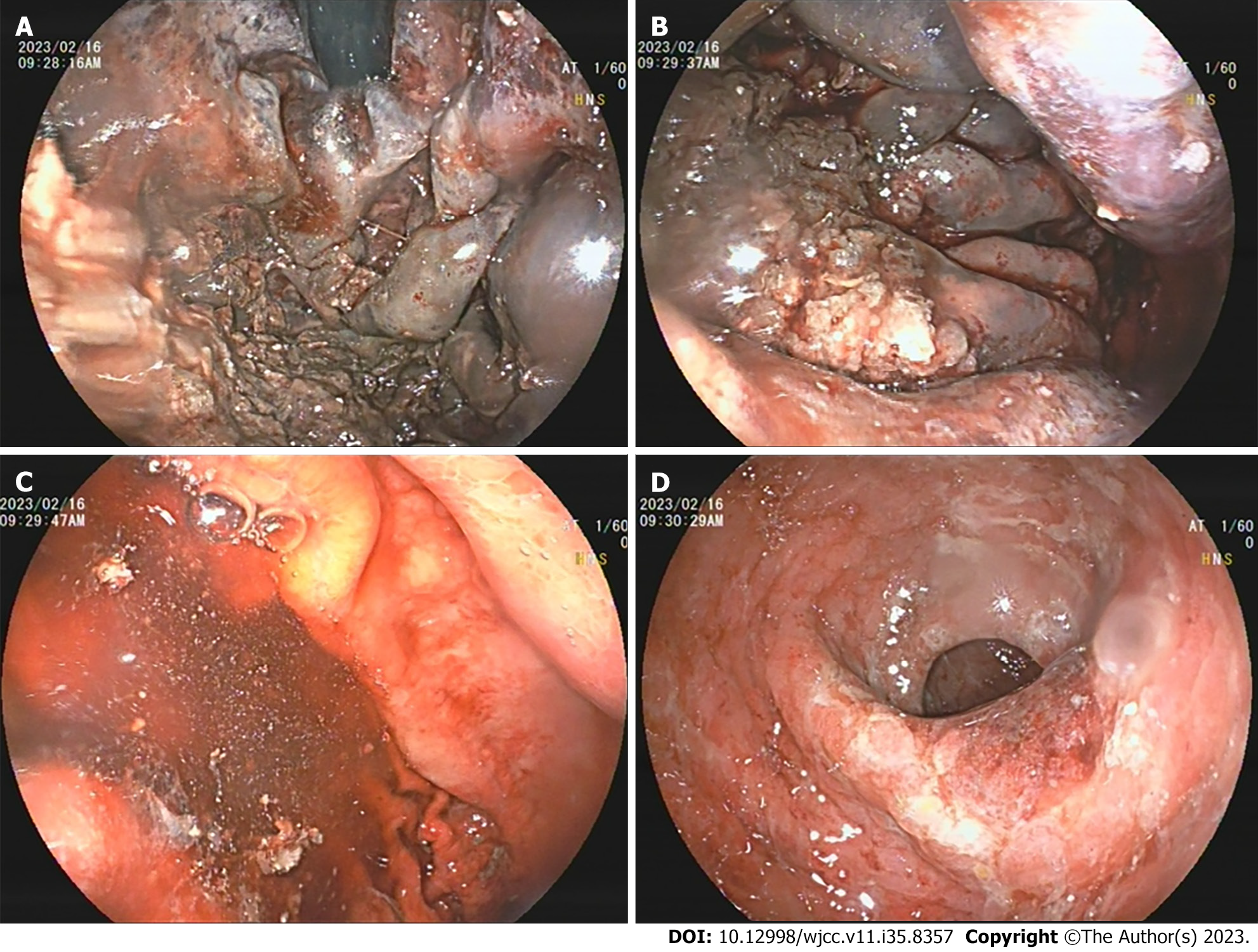
Gastroscopy: Diffuse submucosal congestion throughout the entire gastric mucosa, with the most severe manifestations in the fundus and body of the stomach. The mucosa was swollen and appeared dark purple, with surface erosion accompanied by a small amount of dark red oozing blood (Figure 2).
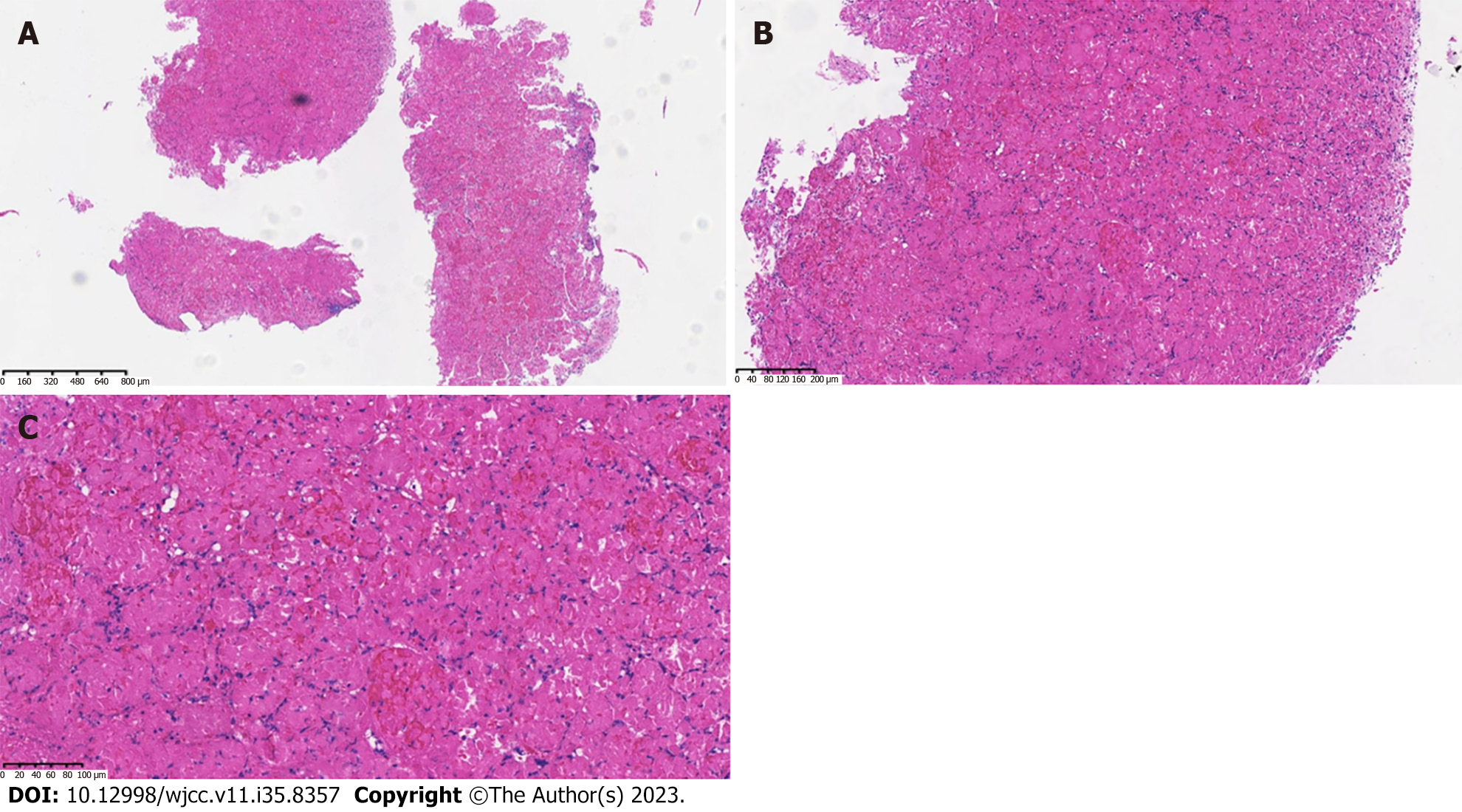
Pathology: Gastric mucosal bleeding, necrosis, and visible residual contour of gastric glands (Figure 3).
Diffuse arterial atherosclerosis presenting with acute ischemic gastritis.
On admission, the patient was given omeprazole injection to prevent acid production and protect the stomach; cefoperazone and sulbactam (Shupushen) to combat infection; atorvastatin calcium tablets to reduce lipids and stabilize plaques; metoprolol sustained-release tablets to improve ventricular remodeling and control ventricular rate; nifedipine controlled-release tablets to reduce blood pressure; and aspirin and clopidogrel tablets to prevent platelet elevation.
After hospitalization, the patient experienced no recurrence of hematemesis, bloody stools, or black stools, confirming the efficacy of the treatment. Subsequently, the patient requested discharge. One month after discharge, the patient’s condition was good, and there was no occurrence of abdominal pain, hematemesis, or black stools.
Ischemic gastritis is a rare disease caused by insufficient blood supply to the stomach, leading to damage and inflammation of gastric mucosa[9,10]. This condition is usually related to postintervention embolization, hypotension, and hemodynamic disorder during celiac disease axis stenosis, vasculitis and idiopathic etiology[11]. Atherosclerosis can affect any artery in the body, including the artery that supplies blood to the gastrointestinal tract[12]. When the blood flow to the stomach decreases, it leads to inflammation and gastric mucosal damage[11]. The clinical manifestations of ischemic gastritis include abdominal pain, nausea and vomiting. In severe cases, it can lead to perforation, peritonitis and sepsis[2]. Its diagnosis requires a combination of a range of radiological and endoscopic findings. Undulations of gastric contours are common imaging manifestations[7,13]. In ischemic gastritis, the formation of gas due to mucosal surface damage or bacterial superinfection caused by bloodborne dissemination leads to the occurrence of intramural gas consistent with emphysema gastritis, with higher mortality[14,15]. Early diagnosis and timely treatment are crucial to prevent disease progression and reduce incidence and mortality. In our case, the patient had a history of intracranial aneurysm, and CT showed atherosclerosis, which may lead to ischemic gastritis. In addition, endoscopic examination showed submucosal congestion, mucosal swelling, dark purple color, surface erosion, and dark red bleeding, which are consistent with diagnosis of ischemic gastritis. Pathological examination confirmed gastric mucosal bleeding and necrosis.
Ischemic gastritis is a rare disease, which has been underreported in the literature except for a few case reports and case series. To our knowledge, there have been no previous studies evaluating its prevalence in the community. From 2009 to 2022, 17 patients with isolated gastric ischemia were identified in a retrospective case series of ischemic gastritis conducted in a single center[16-23], while another large multicenter case series study included 12 patients with ischemic gastritis; most of which was caused by interventional radioembolization, hemodynamic changes in the case of celiac disease axis stenosis, systemic hypotension, vasculitis and other unknown reasons[9]. The 30-day and 1-year mortality rates are 33% and 41%, respectively.
Conservative management is the first-line treatment, which may lead to complete regression of ischemic gastritis, including cases complicated with emphysema gastritis[24,25]. In addition to conservative management, some cases can also be recovered through invasive therapy, including total gastrectomy[3,4,26] or vascular reconstruction. Due to the poor overall condition of most patients, careful examination of surgical indications is necessary. In some cases, although performance of the gastric mucosa has improved, early death still occurs[27,28]. Ischemic gastritis is one of the phenotypes associated with systemic blood flow damage. Impaired systemic blood flow occurs in patients with systemic diseases or comorbidities leading to gastric ischemia. The improvement of endoscopic examination results may not be directly related to prognosis. In contrast, CT was helpful in the diagnosis of our case[29]. Thickening of the gastric wall, emphysema and fluid retention suggest ischemic changes. These were observed on CT scans a few days before the onset of symptoms such as hematemesis, and persisted even after mucosal recovery on endoscopy, which is considered useful for early diagnosis and subsequent follow-up. Since ischemic gastritis is associated with severe systemic arteriosclerosis, improvement in the gastric mucosal surface may not improve the prognosis. In some cases, surgery or endovascular treatment can be life-saving including coronary stent devices which have boosted the prognosis for patients with coronary artery disease[30,31], and CT scans can improve prognosis through early diagnosis and intervention[3,4].
The limitations of this case report include a lack of long-term follow-up data and limited ability to generalize the results to other patient populations. In addition, the analysis of the causes of ischemic gastritis was based on a single case and may not be representative of the larger patient population. Despite these limitations, this case report provides valuable insights for the diagnosis and treatment of ischemic gastritis and emphasizes the need for further research in this field. Clinicians should be alert to signs and symptoms of ischemic gastritis, especially for patients with known risk factors of atherosclerosis, and should consider adopting multidisciplinary nursing methods to optimize patient outcomes. This case reminds us that, in the management of complex diseases (especially those involving multiple organ systems), comprehensive diagnostic assessment and multidisciplinary collaboration are crucial. Further research is needed to better understand the pathophysiology and etiology of ischemic gastritis, and to determine an effective treatment plan to improve patient outcomes.
This rare case of ischemic gastritis highlights the importance of atherosclerosis as a potential root cause of multiple symptoms and conditions, including chest tightness, dizziness and gastrointestinal bleeding. In the diagnosis and treatment of atherosclerotic diseases, the risk factors and medical history of patients should be carefully evaluated, and appropriate intervention should be considered to reduce the risk of cardiovascular adverse events. In addition, this case emphasizes the importance of interdisciplinary cooperation among medical professionals, including cardiologists and gastroenterologists, in the diagnosis and management of complex cases involving multiple organ systems. In order to better understand the pathophysiology and etiology of ischemic gastritis and determine an effective treatment plan, further research is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tudoran C, Romania S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | Quentin V, Dib N, Thouveny F, L'Hoste P, Croue A, Boyer J. Chronic ischemic gastritis: case report of a difficult diagnosis and review of the literature. Endoscopy. 2006;38:529-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Tang SJ, Daram SR, Wu R, Bhaijee F. Pathogenesis, diagnosis, and management of gastric ischemia. Clin Gastroenterol Hepatol. 2014;12:246-52.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Richieri JP, Pol B, Payan MJ. Acute necrotizing ischemic gastritis: clinical, endoscopic and histopathologic aspects. Gastrointest Endosc. 1998;48:210-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Tomishima K, Sato S, Amano N, Murata A, Tsuzura H, Kanemitsu Y, Shimada Y, Iijima K, Genda T, Wada R, Nagahara A. A case of ischemic gastroduodenal disease in a patient who was receiving hemodialysis treatment that was managed by conservative treatment. Clin J Gastroenterol. 2018;11:386-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Bakker RC, Brandjes DP, Snel P, Lawson JA, Lindeman J, Batchelor D. Malabsorption syndrome associated with ulceration of the stomach and small bowel caused by chronic intestinal ischemia in a patient with hyperhomocysteinemia. Mayo Clin Proc. 1997;72:546-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Herman J, Chavalitdhamrong D, Jensen DM, Cortina G, Manuyakorn A, Jutabha R. The significance of gastric and duodenal histological ischemia reported on endoscopic biopsy. Endoscopy. 2011;43:365-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Guniganti P, Bradenham CH, Raptis C, Menias CO, Mellnick VM. CT of Gastric Emergencies. Radiographics. 2015;35:1909-1921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Nishino H, Takano S, Yoshitomi H, Furukawa K, Takayashiki T, Kuboki S, Suzuki D, Sakai N, Kagawa S, Nojima H, Sasaki K, Miyazaki M, Ohtsuka M. Ischemic gastropathy after distal pancreatectomy with en bloc celiac axis resection versus distal pancreatectomy for pancreatic body/tail cancer. Surg Open Sci. 2019;1:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Elwir S, Shaukat A, Mesa H, Colbach C, Dambowy P, Shaw M. Ischemic Gastritis: A Multicenter Case Series of a Rare Clinical Entity and a Review of the Literature. J Clin Gastroenterol. 2016;50:722-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Gray S, Hanna A, Ganti L. Gastric Ischemia Secondary to Abdominal Distension. Cureus. 2021;13:e12793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Becker S, Bonderup OK, Fonslet TO. Ischaemic gastric ulceration with endoscopic healing after revascularization. Eur J Gastroenterol Hepatol. 2006;18:451-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Iqbal U. Gangrenous Gastritis: Unusual Cause Of Upper Gi Bleeding. J Ayub Med Coll Abbottabad. 2019;31:634-635. [PubMed] [Cited in This Article: ] |
| 13. | Tsukasa S, Samejima T, Ohi H, Otsuji M, Saeki K, Tohjinbara H, Aozaki S, Maruta S, Tokushige J, Nishimata H. [X-ray diagnosis of acute gastritis]. Rinsho Hoshasen. 1986;31:1511-1520. [PubMed] [Cited in This Article: ] |
| 14. | Tuero C, Docio G, Artajona A, Arin B, Cires M, Monton S. Acute massive gastric distention with emphysematous gastritis: a case report and literature review. Cir Cir. 2022;90:838-841. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 15. | Watson A, Bul V, Staudacher J, Carroll R, Yazici C. The predictors of mortality and secular changes in management strategies in emphysematous gastritis. Clin Res Hepatol Gastroenterol. 2017;41:e1-e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Ichita C, Sasaki A, Isogai N, Sumida C, Nishino T, Kubota J, Shionoya K, Kimura K. White gastric mucosa during endoscopy as a new endoscopic feature of chronic ischemic gastritis: A case report. DEN Open. 2023;3:e192. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Shionoya K, Sasaki A, Moriya H, Kimura K, Nishino T, Kubota J, Sumida C, Tasaki J, Ichita C, Makazu M, Masuda S, Koizumi K, Kawachi J, Tsukiyama T, Kako M. Clinical features and progress of ischemic gastritis with high fatalities: Seven case reports. World J Clin Cases. 2022;10:8686-8694. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (1)] |
| 18. | Osman M, Mohamed AB, Salim A. Diffuse Arterial Atherosclerosis Presenting With Acute Ischemic Gastritis. Cureus. 2022;14:e29115. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 19. | Ehteshami-Afshar S, Sankey C, Pinto Taylor E. Ischemic Gastritis in a Patient with Chronic Constipation. J Gen Intern Med. 2022;37:966-967. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 20. | Valdés Delgado T, Bellido Muñoz F, Hegueta Delgado P. Ischemic gastritis and severe diabetic ketoacidosis. Rev Esp Enferm Dig. 2021;113:622-623. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 21. | Quiñones Castro R, Vaquero Ayala L, Álvarez Cañas MC. Ischemic gastritis due to oral iron. Rev Esp Enferm Dig. 2019;111:971-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Tariq A, Mehta N, Peroutka K. Follicular B Cell Lymphoma with Accompanying Ischemic Gastritis Completely Resolved by Rituximab. Am J Case Rep. 2017;18:617-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Chambon JP, Bianchini A, Massouille D, Perot C, Lancelevée J, Zerbib P. Ischemic gastritis: a rare but lethal consequence of celiac territory ischemic syndrome. Minerva Chir. 2012;67:421-428. [PubMed] [Cited in This Article: ] |
| 24. | Arezzo A, Famiglietti F, Garabello D, Morino M. Complete resolution of emphysematous gastritis after conservative management. Clin Gastroenterol Hepatol. 2011;9:e30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Nasser H, Ivanics T, Leonard-Murali S, Shakaroun D, Woodward A. Emphysematous gastritis: A case series of three patients managed conservatively. Int J Surg Case Rep. 2019;64:80-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Ishii Y, Sato H, Terai S. Rare case of gastric perforation due to ischemic gastropathy. Gastrointest Endosc. 2020;92:1266-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Højgaard L, Krag E. Chronic ischemic gastritis reversed after revascularization operation. Gastroenterology. 1987;92:226-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | de Widt-Levert LM, Nelis GF, Jörning PJ. Dyspepsia as initial symptom of splanchnic vascular insufficiency. Eur J Gastroenterol Hepatol. 1996;8:815-818. [PubMed] [Cited in This Article: ] |
| 29. | Anucha J, Pinto J, Culpepper-Morgan J, Genao A, Resnick N. Ischemic gastropathy treated with celiac artery revascularization. Cureus. 2019;11:e5949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Tudoran M, Tudoran C, Ciocarlie T, Pop GN, Berceanu-Vaduva MM, Velimirovici DE, Abu-Awwad A, Berceanu-Vaduva DM. Aspects of heart failure in patients with ischemic heart disease after percuta drug-eluting stents vs bare-metal stentsneous coronary revascularization with polymer-coated drug-eluting stents vs bare-metal stents. Mater Plast. 2019;56:37-40. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Khatri M, Kumar S, Mahfooz K, Sugandh F, Dembra D, Mehak F, Rachna Panjwani GA, Islam H, Islam R, Ibn E Ali Jaffari SM, Patel T, Kumar A, Kumar N, Varrassi G. Clinical outcomes of polymer-free versus polymer-coated drug-eluting stents in patients with coronary artery disease: A systematic review and Meta-analysis. Cureus. 2023;15:e38215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |