Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8350
Peer-review started: August 3, 2023
First decision: October 24, 2023
Revised: November 14, 2023
Accepted: December 5, 2023
Article in press: December 5, 2023
Published online: December 16, 2023
Pulmonary arterial hypertension (PAH) in pregnancy is one of the major obstetric complications and is considered a contraindication to pregnancy as it is classified as a class IV risk in the revised risk classification of pregnancy by the World Health Organisation. Pregnancy, with its adaptive and expectant mechanical and hormonal changes, negatively affects the cardiopulmonary circulation in pregnant women. Do patients with repaired simple congenital heart disease (CHD) develop other pulmonary and cardiac complications during pregnancy? Can pregnant women with sudden pulmonary hypertension be treated and managed in time? In this paper, we present a case of a 39-year-old woman who underwent cesarean section at 33 wk' gestation and developed PAH secondary to repaired simple CHD. Our research began by a PubMed search for "pulmonary hypertension" and "pregnancy" and "CHD" case reports. Three cases were selected to review PAH in pregnancy after correction of CHD defects. These studies were reviewed, coupled with our own clinical experience.
Herein, a case involving a woman who underwent atrial septal defect repair at the age of 34, became pregnant five years later, and had a sudden onset of PAH and right heart failure secondary to symptoms of acute peripheral edema in the third trimester of her pregnancy. As a result, the patient underwent a cesarean section and gave birth to healthy twins. Within three days after cesarean delivery, her cardiac function deteriorated as the pulmonary artery pressure increased. Effec
This case served as a reminder to obstetricians of the importance of pregnancy after repair of CHD. It is crucial for patients with CHD to receive early correction. It suggests doctors should not ignore edema of twin pregnancy. Also, it provides a reference for the further standardization of antenatal, in
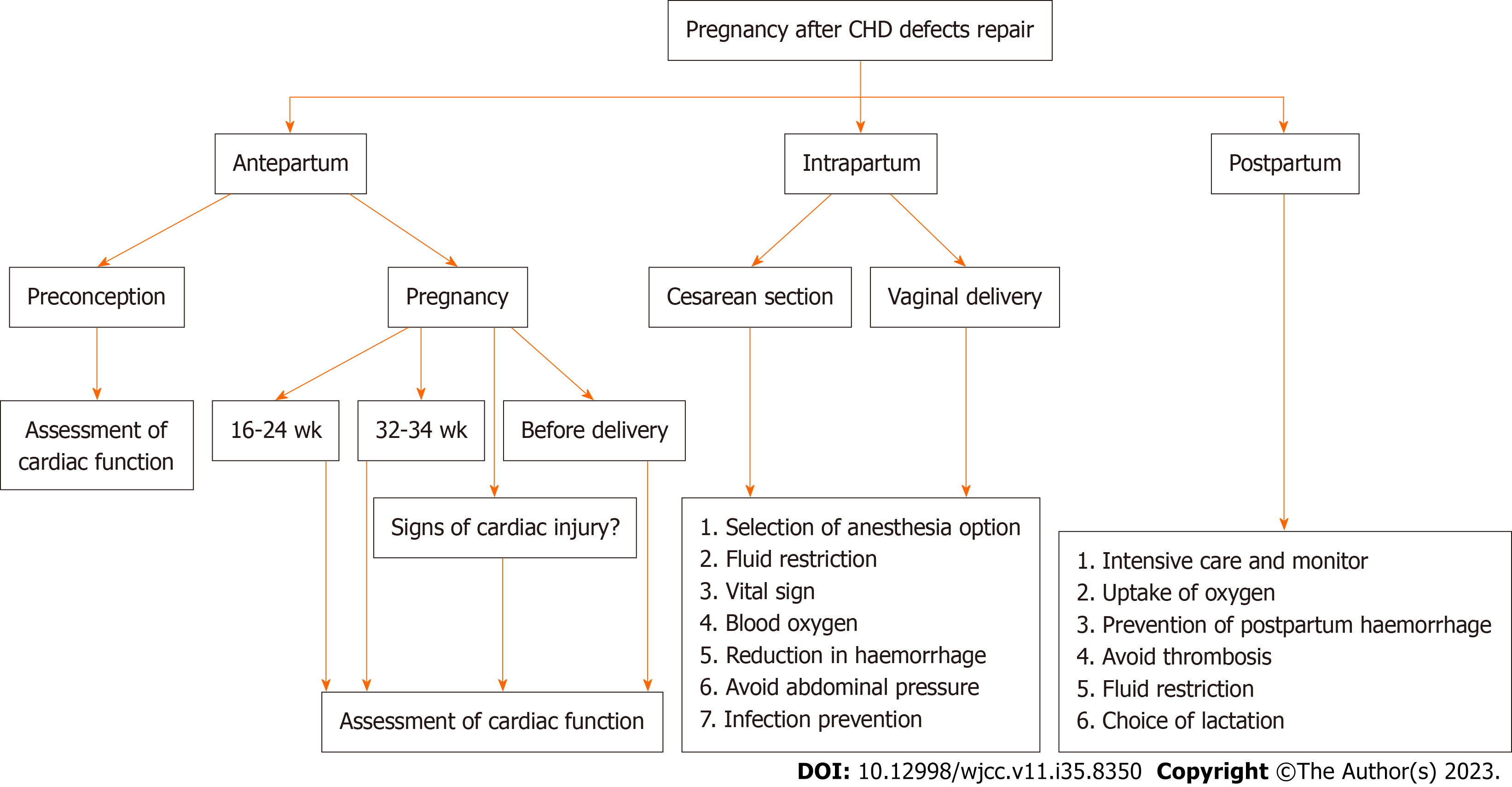
Core Tip: Long-term hemodynamic abnormalities caused by the late repair of congenital heart disease (CHD) defects are another essential factor in the deterioration of the heart function of pregnant women. This emphasizes the importance of early correction for patients with CHD, which is often overlooked by obstetricians. In this case, the timing of the deterioration in cardiac function coincided with the peak of blood volume and cardiac output during pregnancy, that is, 32-34 wk of pregnancy and within 3 d after delivery. These time periods are vital for cardiac function assessment.
- Citation: Tong CX, Meng T. Twin pregnancy with sudden heart failure and pulmonary hypertension after atrial septal defect repair: A case report. World J Clin Cases 2023; 11(35): 8350-8356
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8350.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8350
Successfully repaired simple congenital heart disease (CHD) lesions have been classified as World Health Organisation (mWHO) pregnancy risk class I. Pregnancy after defects correction has a 2%-5% risk of maternal cardiac events and only requires one or two cardiology evaluations during pregnancy[1]. However, there is a possibility of developing pulmonary hypertension after heart defect correction, which may be present immediately or several years after surgery. Studies have shown that pulmonary arterial hypertension (PAH) after defects correction is related to delayed correction age, and the prognosis of these patients is poor, possibly related to the impaired ability of the right vein to adapt to increased afterload after birth[2].
In the event of PAH being diagnosed, pregnancy is not recommended for this type of woman[3]. During pregnancy, maternal hormonal and blood flow changes are associated with an increased risk of pulmonary hypertension. Symptoms of pulmonary hypertension are non-specific, with initial symptoms including shortness of breath, fatigue and weakness. Progressive right ventricular dysfunction can cause abdominal distention and peripheral edema[3]. These symptoms are confused with the normal physiological manifestations of the pregnant women in the third trimester, especially during twin pregnancies, making it difficult for pregnant women and obstetricians to link these symptoms to worsening heart function, which can lead to untreated deterioration and rapid progression. The maternal mortality rate of PAH is approximately 9 to 28%[1], with acute postpartum heart failure being the leading cause of death[4,5]. Pregnant women with PAH are most at greatest risk the first week after delivery, which accounts for approximately 61% of maternal deaths[6]. At present, there is no definitive management of hospital stay and termination of pregnancy for pregnant women with repaired CHD or with PAH. If a pregnant woman has pulmonary hypertension onset outside the hospital, resuscitation of the mother and fetus will be delayed.
There are few reports about pregnancy with PAH after CHD defects correction, and a review of previously reported cases of PAH in pregnancy after correction of heart defects was undertaken. This study outlines the clinical characteristics, perinatal management, and outcomes of pregnant women after CHD repair. It also provides a time-point basis for further standardizing the management of pregnancy in patients after correction of CHD defects.
A 39-year-old, 32-wk pregnant woman presented to the obstetric clinic with complaint worsened peripheral edema for one week.
After eight months of pregnancy, her edema became unbearable in the past one week and affected her sleep and rest.
The patient was 34 years old when she was diagnosed with an atrial septal defect (ASD) on physical examination and the defect was successfully repaired the same year. This pregnant woman had conceived with twins through in vitro fertilization eight months earlier. Cardiac function was not systematically monitored during this pregnancy, but this pregnant woman complained of no chest tightness, shortness of breath, and other abnormal manifestations during pregnancy.
The patient denied any family history of cardiovascular disease.
On physical examination, the vital signs were as follows: Body temperature, 36.7 ℃; blood pressure, 156/83 mmHg; heart rate, 90 beats per min; respiratory rate, 18 breaths per min, blood oxygen, 98%. The blood pressure dropped to 128/76 mmHg after 20 min of rest. No notable abnormalities were heard on cardiopulmonary auscultation. The patient was severely oedematous with orange peel abdominal skin.
Brain natriuretic peptide (BNP) increased up to 162 pg/mL, and other laboratory indicators, including complete blood count, urinalysis, coagulation, liver and kidney function, myocardial enzymes, troponin, were all normal. 24-h urine output is 1000 mL.
Echocardiography several days prior to admission showed a normal cardiac structure. On the day of admission, the electrocardiogram was normal. The deep vein ultrasound of both lower limbs was normal, and the fetal ultrasound indicated that the intrauterine development of both fetuses was normal. There was no pulmonary hypertension after closure of ASD. Echocardiography conducted a few days prior to admission showed normal pulmonary artery pressures.
Four days after admission, the patient complained of more severe oedema, which severely interfered with her rest, and presented with chest tightness, shortness of breath. The cardiac function was re-evaluated. The echocardiography report: mild pulmonary hypertension (pulmonary artery systolic pressure: 42 mmHg), mild tricuspid regurgitation, and larger left heart.
We diagnosed her as 33 wk of twin pregnancy, mild pulmonary hypertension, heart failure, mWHO IV and New York Heart Association (NYHA) class III-IV.
Our multidisciplinary consultations team planned cesarean delivery under epidural anesthesia, paid attention to fluid management during the perioperative period to ensure stable hemodynamics, prevent heart failure, strictly prevent postpartum hemorrhage, ensure oxygenation after the operation, prevent infection, and prevent thrombosis. The whole surgical procedure went smoothly and the intraoperative and postoperative vital signs remained stable. The patient had no apparent symptoms of chest tightness, shortness of breath, and dyspnea. She felt that the peripheral edema was relieved. After the operation, she was given routine oxygen inhalation, infection prevention, myocardial nutrient treatment, and strict fluid management. However, on the 2nd d after the cesarean section, the mother developed wor
The patient was transferred to the local hospital on the 8th d after the cesarean section for further treatment of pulmonary hypertension. The patient received targeted PAH treatment with a phosphodiesterase type 5 inhibitor (sildenafil). The current maternal prognosis is good.
We set out to write in April 2022 with the aim of determining the clinical manifestations of PAH after CHD defects correction in pregnant women. We then performed a search on PubMed applying “(“PAH” [Mesh] AND “CHD” [Mesh]) AND “pregnant” [Mesh]” and identified three cases of PAH after correction of CHD defects in pregnancy, as outlined in Table 1[7,8].
| Ref. | Age at pregnancy (yr) | Age (defects repair) | Congenital heart defects | The gestational age of events, wk | The gestational age of termination | Complications | Symptoms | Maternal outcomes |
| Ross-Ascuitto et al[8] | 19 | 4 | VSD | Three days within delivery | 38 wk | PAH | No obvious symptoms | Uneventfully discharge |
| Jackson et al[7] | 32 | 4 | VSD | 20 wk | 20 wk | PAH; Eisenmenger syndrome | Shortness of breath | Being evaluated for a combined heart-lung transplant |
| Jackson et al[7] | 23 | 3 | VSD | Three days within delivery | 26 wk | PAH; Heart failure | Dyspnea; hypoxemia | Died on postoperative day 5 |
Patients with the ASD generally have a better prognosis and treatment effect if surgically repair is performed at a younger age. If the repair is performed at an older age, changes in the ventricular structure and the increase in pulmonary artery pressure can affect the outcome. At present, there is still clinical controversy regarding the repair effect of an ASD in adults and even the elderly[9,10]. The pregnant woman, in this case, did not undergo atrial defect repair until the age of 34. The correction time was late, and the heart was in a chronic state of left-to-right shunt abnormality and as a result, long-term changes in ventricular structure and pulmonary artery pressure occurred, which influenced the prognosis. This article reminds of the importance of early repair of the ASD. Even after the defect repair operation, delayed repair of the ASD can still affect the hemodynamics of this pregnancy. We set out to write in April 2022 with the aim of determining the clinical manifestations of PAH after CHD defects correction in pregnant women. We then performed a search on PubMed applying “(“PAH” [Mesh] AND “CHD” [Mesh]) AND “pregnant” [Mesh]”. Three cases were identified regarding pregnancies with PAH after CHD defects correction, as outlined in Table 1[7,8]. And a retrospective study shows that compared with pregnancy after ASD repair, pregnant women who have not undergone defect repair have a higher probability of miscarriage, premature delivery, and heart-related symptoms during pregnancy[11]. These all emphasize the importance of early repair of simple lesions of CHD. The time node of hemodynamic changes during pregnancy is crucial. The cardiac output of a pregnant woman peaks at 32-34 wk of gestation and lasts until three days after delivery. In this case, the patient began to have severe peripheral edema at 32 wk of pregnancy and was unable to stand in clinostatic position. Then the pregnant woman was diagnosed as pulmonary hypertension and right heart failure at 33 wk of gestation. After the termination of pregnancy, the patient's cardiac function experienced a short-term relief. However, the patient suddenly developed symptoms of severe cardiac insufficiency with high pulmonary hypertension, unstable vital signs, elevated blood pressure and a large heart the day after the procedure. The reasons for this are as follows. First, the fluid movement caused by labor increases the preload, which increases the cardiac output by about 10%-15%, with a corresponding increase in the load of the right ventricle and pulmonary vascular bed. After delivery, uterine contraction leads to another 300-500 mL of autotransfusion from the uterus to the mother. Second, when the obstruction caused by the uterine compression of the inferior vena cava is lifted, the subaortic vena cava pressure decreases abruptly and venous blood flow increases. Moreover, cardiac output does not increase until 48 h after delivery. Third, there will be an increase in pulmonary vascular resistance due to uterine contraction, rapid abdominal decom
Pregnancy with a successfully repaired ASD is considered class I in the 2018 mWHO classification. ASD that has not been repaired surgically is considered class II, and pulmonary hypertension is thought to be class IV[1]. The 2011 European Society of Cardiology (ESC) guidelines concluded that repaired ASD usually does not increase maternal-fetal risk[12]. However, the 2018 ESC guidelines relatively rigorously abolished the view that repaired ASD usually does not increase the risk of maternal-fetal risk, which is consistent with the occurrence of pulmonary hypertension and right heart failure in the third trimester of pregnancy in this case[13]. The American College of Obstetricians and Gynecologists (ACOG) puts the probability of a maternal cardiac event during pregnancy in patients with repaired ASDs at 2%-5%. Physicians need to evaluate the heart function of pregnant women 1-2 times during pregnancy[1]. In addition to the peak period of blood return volume, the main complaint of the patient must be addressed. In order to diagnose pulmonary hypertension and exclude other cardiac conditions, echocardiography is the most useful imaging modality[14]. In this case, there was no abnormality on the echocardiography 6 d prior to admission and the pregnant woman did not show symptoms of cardiac insufficiency during pregnancy. If it were not for the patient's complaint, we would not have noticed the deterioration in her heart function. Due to the complex hemodynamic and physiological changes during pregnancy, peripheral edema often occurs in the third trimester, especially for pregnant women with high body mass index (BMI). The symptoms of peripheral edema in pregnant women with twins can be easily overlooked as the BMI is usually greater in twin pregnancies. Therefore, whether it is peripheral edema caused by cardiac insufficiency is difficult for doctors to assess. As a result, physicians often ignore edema, and the mother and fetus miss the best rescue time. This article suggests that even for normal pregnant women with a high BMI, when acute edema worsens, with or without chest tightness and shortness of breath, the potential changes in cardiac function should be considered.
The ACOG believes that the mortality rate of pregnant women with heart disease who are older than 40 years old is 30 times higher than that of pregnant women younger than 20 years old. Although twins and multiple pregnancies are not mentioned, pregnant women with twins and multiple pregnancies usually increase in BMI, and overweight is also an essential underlying in increasing maternal cardiogenic death. In this case, twins, high BMI, and advanced age are all high-risk factors for maternal-fetal risk during pregnancy. This article highlights the influence of high BMI and advanced age on the heart function of pregnant women and the importance of multi-disciplinary pregnancy management[1].
Our multidisciplinary consultations team planned cesarean delivery under combined spinal epidural anesthesia as general anesthesia suppresses cardiac contractility, while positive pressure ventilation increases pulmonary vascular resistance and exacerbates pulmonary hypertension. Combined spinal epidural anesthesia can therefore provide better sensory block, avoid vascular dilation, and do not increase the risk of hypotension. Also, it is a single administration after puncture, which lasts for a short time and is easy to cause hypotension. Lumbar anesthesia is a devastating blow to right heart failure. Therefore, it is recommended that intraspinal anesthesia be used in patients with PAH undergoing caesarean delivery if there are no contraindications to intraspinal puncture, according to the latest statement. General anaesthesia can be considered for patients with abnormal coagulation function and poor cardiopulmonary function. Continuous epidural anesthesia has good controllability, which can not only produce good anesthesia effects but also reduce hemodynamic fluctuations and effectively suppresses surgical stress reaction. At the same time, it can block the dilation of blood vessels in the area, reduce cardiac blood volume, and to some extent reduce pulmonary hypertension and right heart burden. Additionally, the increased blood flow in the blocked area is beneficial to tissue oxygenation.
Upon reviewing different literatures, we identified the specific risks in pregnancy related to different hemodynamic conditions produced by heart defects[15-17]. There was no difference in obstetric complications between pregnant women with unrepaired and repaired ASD. However, cardiac complications were comparable between women with unrepaired and repaired ASDs. Women with unrepaired ASD seem to be more susceptible to arrhythmias as compared to women with repaired ASD[15]. Cardiac complications during pregnancy and NYHA deterioration have been associated with modified WHO class III/IV, prior medication use, and higher body mass index[16].
Pregnant women with PAH should be treated by a multidisciplinary team, including obstetricians, anesthesiologists, intensivists, pulmonary hypertension specialists and cardiologists. The optimal duration of extracorporeal therapy for combined pulmonary hypertension in pregnancy is between 13-28 wk of gestation. In this case, the patient was diagnosed with pulmonary hypertension in the third trimester. A caesarean section is recommended to terminate the pregnancy prior to cardiac surgery. Intraoperative anesthesia management, blood pressure management, uterine contraction management, bleeding management, and perioperative volume management all have important impact on the patient's cardiac function. Effective treatment in the ICU after the operation also includes improved oxygenation, diuresis, anti-heart insufficiency, and controlling fluid intake. The heart function of the parturient is significantly improved, and the BNP decreases by ≥ 30% after treatment. After effective management, a satisfactory outcome was achieved and the patient was transferred back to the local hospital for follow-up specialist management.
Early repair of the ASD is vital for the prognosis of patients with CHD. Clinicians and emergency rescue teams should pay more attention to the main complaints of high-risk pregnant women and assessment of cardiac function in pregnant women with high BMI and advanced age. They should be more attentive to the changes of hemodynamics of these patients in three time periods during pregnancy. Once the heart function deteriorates, effective peripartum cardiac management and pregnancy termination are vital for maternal and fetal rescue. All of the above has awakened obstetricians' attention to pregnancies with repaired simple lesions of CHD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rotondo JC, Italy; Teragawa H, Japan S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | American College of Obstetricians and Gynecologists' Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet Gynecol. 2019;133:e320-e356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 200] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 2. | Manes A, Palazzini M, Leci E, Bacchi Reggiani ML, Branzi A, Galiè N. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: a comparison between clinical subgroups. Eur Heart J. 2014;35:716-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 3. | Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3636] [Cited by in F6Publishing: 3916] [Article Influence: 435.1] [Reference Citation Analysis (0)] |
| 4. | Jaïs X, Olsson KM, Barbera JA, Blanco I, Torbicki A, Peacock A, Vizza CD, Macdonald P, Humbert M, Hoeper MM. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J. 2012;40:881-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Sliwa K, van Hagen IM, Budts W, Swan L, Sinagra G, Caruana M, Blanco MV, Wagenaar LJ, Johnson MR, Webb G, Hall R, Roos-Hesselink JW; ROPAC investigators. Pulmonary hypertension and pregnancy outcomes: data from the Registry Of Pregnancy and Cardiac Disease (ROPAC) of the European Society of Cardiology. Eur J Heart Fail. 2016;18:1119-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Low TT, Guron N, Ducas R, Yamamura K, Charla P, Granton J, Silversides CK. Pulmonary arterial hypertension in pregnancy-a systematic review of outcomes in the modern era. Pulm Circ. 2021;11:20458940211013671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Jackson GM, Dildy GA, Varner MW, Clark SL. Severe pulmonary hypertension in pregnancy following successful repair of ventricular septal defect in childhood. Obstet Gynecol. 1993;82:680-682. [PubMed] [Cited in This Article: ] |
| 8. | Ross-Ascuitto N, Ascuitto RJ, Darnell J. A pregnant woman with moderate pulmonary hypertension. Pediatr Cardiol. 1995;16:31-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Humenberger M, Rosenhek R, Gabriel H, Rader F, Heger M, Klaar U, Binder T, Probst P, Heinze G, Maurer G, Baumgartner H. Benefit of atrial septal defect closure in adults: impact of age. Eur Heart J. 2011;32:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Liava'a M, Kalfa D. Surgical closure of atrial septal defects. J Thorac Dis. 2018;10:S2931-S2939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Actis Dato GM, Rinaudo P, Revelli A, Actis Dato A, Punta G, Centofanti P, Cavaglià M, Barbato L, Massobrio M. Atrial septal defect and pregnancy: a retrospective analysis of obstetrical outcome before and after surgical correction. Minerva Cardioangiol. 1998;46:63-68. [PubMed] [Cited in This Article: ] |
| 12. | European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM), Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L; ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147-3197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 972] [Cited by in F6Publishing: 935] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 13. | Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA; ESC Scientific Document Group. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165-3241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 811] [Cited by in F6Publishing: 1071] [Article Influence: 178.5] [Reference Citation Analysis (0)] |
| 14. | Schiller NB. Pulmonary artery pressure estimation by Doppler and two-dimensional echocardiography. Cardiol Clin. 1990;8:277-287. [PubMed] [Cited in This Article: ] |
| 15. | van Hagen IM, Roos-Hesselink JW. Pregnancy in congenital heart disease: risk prediction and counselling. Heart. 2020;106:1853-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Ntiloudi D, Zegkos T, Bazmpani MA, Parcharidou D, Panagiotidis T, Hadjimiltiades S, Karvounis H, Giannakoulas G. Pregnancy outcome in women with congenital heart disease: A single-center experience. Hellenic J Cardiol. 2018;59:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Steiner JM, Lokken E, Bayley E, Pechan J, Curtin A, Buber J, Albright C. Cardiac and Pregnancy Outcomes of Pregnant Patients With Congenital Heart Disease According to Risk Classification System. Am J Cardiol. 2021;161:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |