Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8300
Peer-review started: November 3, 2023
First decision: November 16, 2023
Revised: November 27, 2023
Accepted: November 30, 2023
Article in press: November 30, 2023
Published online: December 16, 2023
Immunoglobulin A nephropathy (IgAN) is a common form of chronic glomerulonephritis. Currently, IgAN is one of the main causes of chronic renal failure in China; its prognosis varies greatly between patients, with renal function at the time of diagnosis and prognosis being strongly correlated. Mycophenolate mofetil (MMF) is a drug with a good immunomodulatory effect and is commonly used clinically. However, its effects in IgAN have not yet been clearly demonstrated. Therefore, herein, we retrospectively compared the effectiveness and safety of prednisone alone or combined with MMF for the treatment of primary IgAN with moderate-to-severe renal impairment.
To evaluate the effectiveness and safety of prednisone and MMF in treating IgAN with moderate-to-severe renal dysfunction.
Between January 2011 and December 2020, 200 patients with moderate-to-severe IgAN were included in this study, all of whom were admitted to Wuxi People's Hospital affiliated with Nanjing Medical University. All patients underwent a renal puncture biopsy, which revealed primary IgAN with a glomerular filtration rate (GFR) of 30–60 mL/min. The patients were divided into a glucocorticoid therapy group (GTG) and an immunosuppressive therapy group (ITG) according to the different treatment regimens, with 100 patients in each group. Based on general treatments, such as angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers, patients in the GTG were administered prednisone 0.5–0.8 mg/ (kg·d-1) for 4–8 wk, which was reduced by 5 mg every two weeks until the maintenance(30 mg/d) dose was reached and maintained for 12 mo. In the ITG, MMF was administered at 1.0 g/d for 6–12 mo, followed by a maintenance dosage of 0.5 g/d for 12 mo. Age, sex, blood pressure, 24-h urinary egg white measurement, serum creatinine (Scr), blood uric acid, blood albumin, blood potassium (K), hemoglobin, GFR, alanine aminotransferase, total cholesterol (T-CHO), fasting blood glucose, and body mass index were recorded. The 24-h urinary protein, Scr, and GFR levels were recorded 3, 6, 9, and 12 mo after treatment. Follow-up data were also collected.
No discernible differences existed between the two groups in terms of age, sex, blood pressure, creatinine, 24-h urinary protein level, GFR, or other biochemical indicators at the time of enrollment. Both regimens significantly reduced the 24-h urinary protein quantitation and stabilized renal function. Nine months after treatment, the 24-h urinary protein and Scr of the ITG decreased more significantly than those of the GTG. By the 12th month of treatment, the 24-h urinary protein and Scr in both groups continued to decrease compared to those by the 9th month. In addition, the overall response rate in the ITG was significantly higher than that in the GTG. The occurrence of side effects did not vary significantly between the two regimens; however, endpoint events were significantly more common in the GTG than in the ITG. The follow-up time for the GTG was noticeably lower than that for the ITG.
Prednisone combined with MMF was effective for the treatment of IgAN with moderate-to-severe renal dysfunction.
Core Tip: In this study, the clinical and follow-up data of patients were retrospectively analyzed to explore the effectiveness and safety of prednisone combined with mycophenolate mofetil to guide the selection of clinical treatment of primary immunoglobulin A nephropathy (IgAN) with renal dysfunction. The results showed that mycophenolate ethyl ester combined with prednisone were more effective in treating patients with IgAN than prednisone alone, and could effectively improve renal function.
- Citation: Meng MJ, Hu L, Fan Y, Gao H, Chen HZ, Chen CM, Qi Z, Liu B. Efficacy of prednisone combined with mycophenolate mofetil for immunoglobulin A nephropathy with moderate-to-severe renal dysfunction. World J Clin Cases 2023; 11(35): 8300-8309
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8300.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8300
Primary immunoglobulin A nephropathy (IgAN) refers to primary glomerulonephritis with a pathological manifestation of IgA-dominated immune complex deposition in the mesangial region after the exclusion of secondary factors[1,2]. IgAN is a primary glomerular disease that affects people worldwide; the typical age of disease onset is 20–40 years and it is more common in men. IgAN accounts for 45.26%–58.2% of the primary glomerular diseases in China[3,4]. Furthermore, IgAN is the primary condition most frequently causing end-stage renal disease (ESRD), accounting for 26.69% of cases. The pathogenesis of IgAN is extremely complex; the clinical and pathological manifestations are not the same and the prognosis is very different between patients. IgAN can clinically manifest as isolated microscopic hematuria, gross hematuria, renal involvement, elevated blood pressure, and massive proteinuria[5]. Pathologically, mesangial cell proliferation, focal segmental sclerosis, capillary hyperplasia, and renal tubulointerstitial disease are often observed and sometimes accompanied by crescent formation in IgAN[6]. Therefore, IgAN is currently considered a clinical syndrome that is characterized mainly by changes in renal immunopathology[7]. IgAN often progresses to ESRD, and the renal survival rates after 5 and 10 years of IgAN have been reported to be 85.1% and 77.1%, respectively, in China[8]. Approximately 15%–20% of patients with IgAN progress to ESRD 10 years after onset and 30%–40% progress to ESRD after 20 years[9].
Glucocorticoids have been used to treat IgAN for many years because they reduce inflammation and urinary protein excretion[10]. However, there have been few studies on the application of glucocorticoids in the management of renal dysfunction in patients with IgAN; therefore, the effectiveness of glucocorticoids in this subset of patients is still not supported by sufficient evidence in medical literature. Because the main feature of IgAN is a change in renal immunopathology, immunosuppressants are often used in clinical practice to treat the disease. Mycophenolate mofetil (MMF) is a drug with a good immunomodulatory effect and is commonly used clinically. It mainly inhibits T and B lymphocyte proliferation, interferes with cytotoxic T cell maturation and antibody production in B cells, exerts a strong immunosuppressive effect, and has been clinically utilized for the treatment of IgAN[11-13]. Currently, researchers and clinicians are advocating for hormone-free therapy to treat IgAN with immunosuppressants alone or immunosuppressants combined with low-dose hormones, particularly in patients with mild-to-moderate kidney injury, to reduce urinary protein, protect renal function, and not increase the incidence of adverse reactions. However, the results are inconsistent and there are many controversies. Therefore, in this study, we retrospectively compared the effectiveness and safety of prednisone combined with MMF and prednisone alone for the treatment of primary IgAN with moderate to severe renal impairment.
Between January 2011 and December 2020, 200 patients with moderate-to-severe IgAN participated in this study, all of whom had been admitted to Wuxi People’s Hospital affiliated with Nanjing Medical University. All patients underwent a renal puncture biopsy that revealed primary IgAN with a glomerular filtration rate (GFR) of 30–60 mL/min. The patients were divided into two groups of 100 cases each: the immunosuppressive therapy group (ITG) and the glucocorticoid therapy group (GTG). Based on general therapy, such as angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), patients in the GTG were given 0.5–0.8 mg /(kg·d-1) of prednisone for 4–8 wk, which was reduced by 5 mg every two weeks until the maintenance dosage (30 mg/d) was reached and maintained for 12 mo. In the ITG, based on the treatment regimen of the hormone therapy group, 1.0 g/d of MMF was administered for 6–12 mo; the maintenance dose was 0.5 g/d for 12 mo.
The inclusion criteria were as follows: (1) 18–60 years old; (2) primary IgAN diagnosed by renal biopsy; (3) moderate to severe impairment of renal function at diagnosis: 30 mL/min < GFR < 60 mL/min; (4) 24-h urinary protein quantification > 1.0 g; (5) the quantity of glomeruli obtained through renal biopsy was ≥ 10, and the proportion of glomerulosclerosis revealed by renal pathology was < 80%; tubule atrophy, and renal interstitial fibrosis > 30% and < 80%; and (6) complete clinical, pathological, and follow-up data were available.
The exclusion criteria were as follows: (1) Use of immunosuppressants or cytotoxic drugs; (2) necrotizing capillary vasculitis and crescent nephritis were found by renal biopsy, excluding purpura nephritis, ankylosing spondylitis, systemic lupus erythematosus, hepatitis B virus-associated nephritis, or other secondary IgAN; (3) patients with liver function impairment (aminotransferase increased by > 1.5 times), type 1 and type 2 diabetes mellitus, and patients with tumors; and (4) patients with a history of mental illness or cognitive impairment, and pregnant and lactating women.
Prior information was gathered during renal biopsy. Age, sex, blood pressure, 24-h urinary egg white measurement, serum creatinine (Scr), blood uric acid, blood albumin (ALB), blood potassium (K), hemoglobin (Hb), estimated GFR (eGFR), alanine aminotransferase (ALT), T-CHO, fasting blood glucose (Glu), and body mass index (BMI) were measured. The 24-h urinary protein, Scr, and eGFR levels were recorded 3, 6, 9, and 12 mo after treatment. Follow-up data were collected, including of the duration of follow-up (if an endpoint event occurred then the patient was withdrawn from the follow-up) and of any adverse events, including pneumonia, leukopenia, anemia, gastrointestinal discomfort, decreased transaminase levels, and diabetes. The follow-up duration was 12 mo.
The evaluation criteria were as follows: (1) Obvious effect: After treatment, the urinary protein quantity was < 0.3 g/d and Scr was not > 15% higher than the baseline value before treatment; (2) effective: The urinary protein quantity after treatment was still ≥ 0.3 g/d; however, it was > 50% lower than the baseline value, and the Scr was not > 15% higher than the baseline value before treatment; and (3) ineffective: Blood creatinine increased by > 15% from the baseline value before treatment, else the urine protein quantitation did not meet the above standards.
The endpoint events were defined as follows: (1) Primary endpoint: during treatment follow-up, participants entered ESRD on dialysis or the eGFR decreased by > 50%; (2) safety endpoint: severe infection, liver function impairment, or other serious complications and comorbidities; and (3) patients quit or were lost during follow-up.
Statistical analyses were performed using SPSS 17.0 (IBM corporation, Armonk, NY, United States). Normally distributed measurement data are shown as mean ± standard deviation; non-normally distributed data are shown as the median. Categorical variables are described as rate. Chi-square analysis was performed to analyze the count data whereas the t-test was used to evaluate changes from pre- to post-treatment. The mean renal survival time was calculated using the Kaplan–Meier method. P values of < 0.05 were considered to indicate statistically significant differences.
At the time of enrollment, there was no discernible variation in age, sex, blood pressure, 24-h urinary protein quantification, Scr, ALB, K, Hb, eGFR, ALT, T-CHO, Glu, or BMI between the two groups (P > 0.05), as shown in Table 1.
| Project | GTG | ITG | t value | P value |
| Male:Female | 60:40 | 61:39 | 0.021 | 0.885 |
| Age (yr) | 33.15 ± 9.91 | 34.42 ± 10.04 | -0.901 | 0.369 |
| DBP (mmHg) | 93.05 ± 6.98 | 93.03 ± 7.73 | 0.019 | 0.985 |
| SBP (mmHg) | 140.60 ± 9.94 | 141.40 ± 11.17 | -0.535 | 0.593 |
| K (mmol/L) | 4.88 ± 0.97 | 4.72 ± 0.96 | 1.149 | 0.252 |
| Hb (g/L) | 128.60 ± 14.97 | 129.65 ± 16.29 | -0.472 | 0.638 |
| GFR (mL-1min-1∙(1.73∙m2)-1) | 55.36 ± 3.34 | 54.70 ± 3.27 | 1.405 | 0.162 |
| ALT (U/L) | 36.53 ± 3.20 | 37.12 ± 3.30 | -1.279 | 0.203 |
| T-CHO (mmol/L) | 5.59 ± 0.99 | 5.55 ± 0.99 | 0.321 | 0.748 |
| Glu (mmol/L) | 5.19 ± 0.69 | 5.10 ± 0.66 | 0.883 | 0.378 |
| BMI (kg/m2) | 23.06 ± 2.51 | 23.29 ± 2.55 | -0.717 | 0.474 |
| Alb (g/L) | 22.94 ± 3.20 | 23.11 ± 3.02 | -0.402 | 0.688 |
| 24-h urinary protein quantification (g) | 4.01 ± 1.43 | 3.86 ± 1.37 | 0.76 | 0.448 |
| Scr (μmol/L) | 121.51 ± 14.58 | 122.16 ± 15.29 | -0.311 | 0.756 |
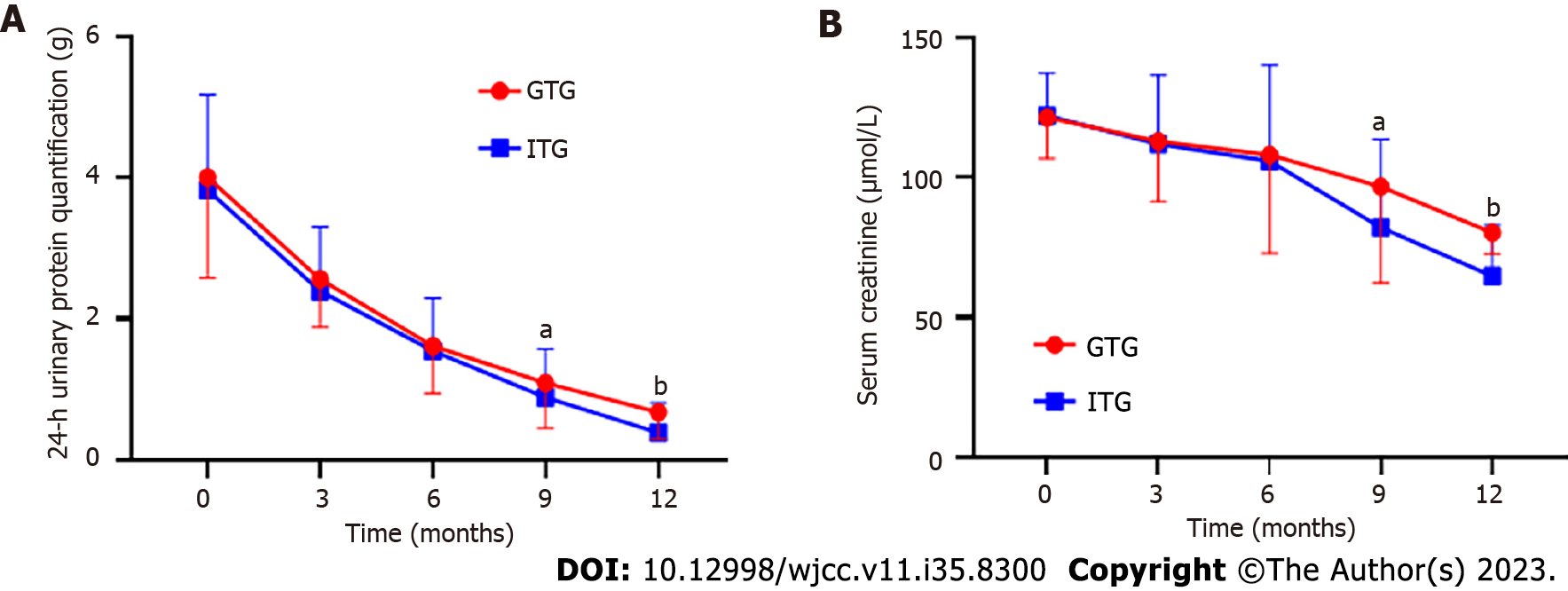
As shown in Table 2, from 3 to 12 mo after treatment, 24-h urinary protein quantitation in both groups began to decrease from baseline values with statistical significance (P < 0.05). However, at 9 mo after treatment, the 24-h urinary protein quantitative level of the ITG decreased more significantly than that of the GTG (P < 0.05). By the 12th month after treatment, the 24-h urinary protein levels in both groups continued to decrease compared with those by the 9th month, as shown in Figure 1A. Similar to the 24-h urinary protein quantitation, Scr in both groups began to decline from baseline 3 to 12 mo after treatment (P < 0.05). However, the Scr level of the ITG decreased more significantly than that of the GTG at 9 mo after treatment (P < 0.05). At 12 mo after treatment, Scr levels in both groups continued to decline compared to that by 9 mo after treatment, as shown in Figure 1B.
| Project | Time | GTG | ITG | t value | P value |
| 24-h urinary protein quantification (g) | Before the treatment | 4.01 ± 1.43 | 3.86 ± 1.37 | 0.597 | 0.551 |
| 3 mo after treatment | 2.57 ± 0.69a | 2.40 ± 0.91a | 1.192 | 0.236 | |
| 6 mo after treatment | 1.62 ± 0.67a | 1.54 ± 0.75a | 0.632 | 0.529 | |
| 9 mo after treatment | 1.09 ± 0.61a | 0.89 ± 0.50a | 1.985 | 0.049 | |
| 12 mo after treatment | 0.68 ± 0.38a | 0.39 ± 0.42a | 4.062 | < 0.001 | |
| Scr (μmol/L) | Before the treatment | 121.51 ± 14.58 | 122.16 ± 15.29 | 0.244 | 0.808 |
| 3 mo after treatment | 113.02 ± 21.63a | 112.00 ± 24.72a | 0.247 | 0.805 | |
| 6 mo after treatment | 108.15 ± 35.2 a | 105.99 ± 34.22a | 0.34 | 0.72 | |
| 9 mo after treatment | 96.81 ± 34.60 a | 82.23 ± 31.41a | 2.454 | 0.016 | |
| 12 mo after treatment | 80.30 ± 7.45 a | 64.76 ± 18.32 a | 11.053 | < 0.001 |
As shown in Table 3, in the GTG, 21 cases showed an obvious curative effect and treatment was effective in 45 cases with a total effective rate of 66%. In the ITG, 15 cases showed an obvious curative effect and treatment was effective in 65 cases with a total effective rate of 80%. The total effective rates of the two groups were statistically significant (P < 0.05).
| Project | Ineffective | Obvious effect | Effective | Total effective rate |
| GTG | 34 | 21 | 45 | 66 |
| ITG | 20 | 15 | 65 | 80 |
| χ2 | 4.972 | |||
| P value | 0.026 |
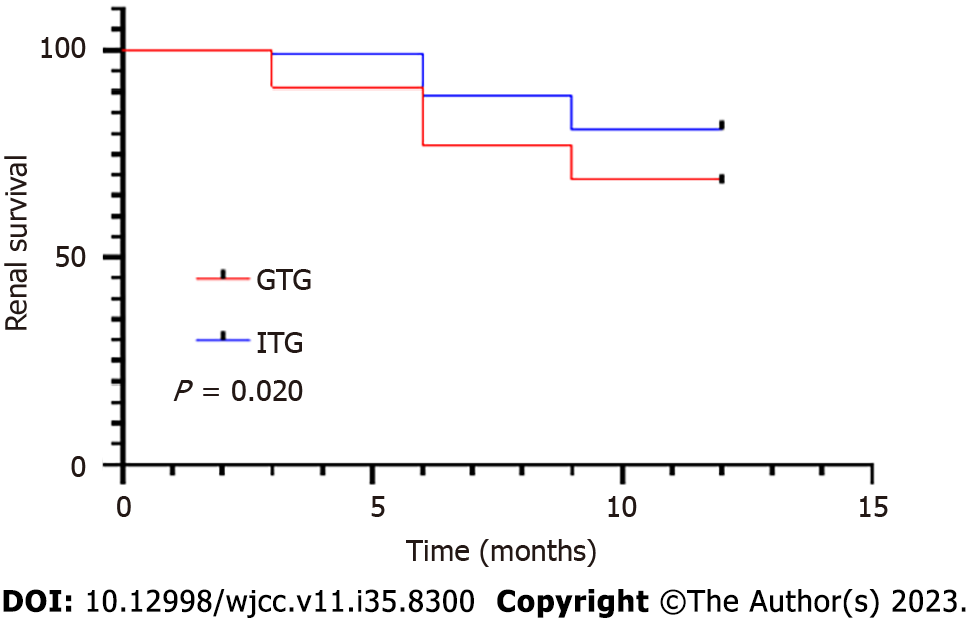
To further evaluate the efficacy of therapy in both groups, the incidence of endpoint events was assessed, and the patients in both groups were followed up with for 12 mo. As shown in Table 4 and Figure 2, in the GTG, renal endpoint events occurred in 14 patients, safety endpoint events occurred in 18 patients, with an incidence of endpoint events was 32%. In the ITG, renal endpoint events occurred in seven patients, safety endpoint events occurred in 12 patients, and the incidence of endpoint events was 19%. There was a statistically significant difference in the incidence of endpoint events between the two groups (P < 0.05). The follow-up time of the GTG and ITG were 10.11 ± 3.09 and 11.07 ± 2.08 mo, respectively. A statistically significant difference was observed between the two groups (P < 0.05).
| Project | GTG | ITG | χ2 | P value |
| Renal endpoint | 14 | 7 | ||
| Serious infection | 4 | 2 | ||
| Serious liver injury | 4 | 2 | ||
| Serious complication | 10 | 8 | ||
| Incidence of endpoint events | 32% | 19% | 4.448 | 0.035 |
| Follow-up time (mo) | 10.11 ± 3.09 | 11.07 ± 2.08 | 2.000 | 0.048 |
From the treatment period to the end of the follow-up period, in the GTG, 18 patients had gastrointestinal reactions, 15 had anemia, 11 had pneumonia, 13 had elevated transaminase levels, 13 had leukopenia, and 11 had diabetes. In the ITG, 22 patients had gastrointestinal reactions, 24 had anemia, 18 had pneumonia, 17 had elevated transaminase, 12 had leukopenia, and 12 had diabetes. The frequency of adverse reactions did not differ significantly between the two groups, as shown in Table 5.
| Project | GTG | ITG | χ2 | P value |
| Gastrointestinal reaction | 18 | 22 | 0.50 | 0.480 |
| Anemia | 15 | 24 | 2.580 | 0.108 |
| Pneumonia | 11 | 18 | 1.976 | 0.160 |
| Transaminitis | 13 | 17 | 0.627 | 0.428 |
| Leukocytopenia | 13 | 12 | 0.046 | 0.831 |
| Diabetes | 11 | 12 | 0.049 | 0.825 |
Primary IgAN is a disease with various clinicopathological manifestations, and its prognosis differs owing to these different manifestations. The Kidney Disease: Improving Global Outcomes (KDIGO) Guidelines for glomerulonephritis considers large proteinuria, hypertension, decreased eGFR, and severe renal pathological changes at initiation and follow-up as potential contributors to the development of renal disease[14]. However, there is currently no unified treatment plan for IgAN, and only IgAN crescent body nephritis with clear indications for cyclophosphamide application is indicated in the guidelines. For patients who have IgAN with renal impairment, particularly those with eGFR values of 30–60 mL/min, no consensus exists on whether further aggressive treatment is required.
The pathogenesis of IgAN has not been clearly defined clinically, and abnormal immune mechanisms and inflammatory injury are believed to be the main pathogenic factors[5,15,16]. In the past, it was clinically considered to be a benign process that did not require intervention; however, in-depth research showed that it was a non-benign process with progressive lesions and even caused end-stage renal failure. However, clinical treatment plans differ; the main treatment is symptomatic treatment[17-19]. Methylprednisolone (MP) is a commonly-used glucocorticoid[20]. By regulating the synthesis and metabolism of proteins, sugars, and fats, MP can exert anti-inflammatory, antiviral, and anti-shock effects, and inhibit the immune response[21,22]. It can also act on the renin-angiotensin system and inhibit kidney lesions by binding to amino acids in the transmembrane region of angiotensin II[23]. Alleviation of renal interstitial fibrosis and glomerulosclerosis can delay the progression of kidney disease, and the treatment effect is accurate. However, studies have shown that MP increases the risk of serious adverse events, and in the early stage, it can increase the level of blood creatinine and affect the prognosis[24,25].
MMF is a new type of immunosuppressant that is used mainly to prevent organ transplantation rejection, not to treat IgAN; however, it was found that MMF has a certain effect on IgAN[26,27]. In this study, we further evaluated the efficacy and safety of glucocorticoids combined with morphol mycophenate in patients with IgAN at risk of progression based on strict blood pressure control using ACEI/ARB and other antihypertensive agents. There were no noteworthy differences between the two groups in terms of age, sex, blood pressure, creatinine, 24-h urinary protein level, eGFR, and other biochemical indicators at the time of enrollment. At baseline, patients in both groups had higher Scr and 24-h urinary protein quantification levels and lower eGFR and albumin levels, suggesting a higher risk of disease progression. A comparison of prednisone alone and prednisone combined with moxylmycophenate revealed that both treatments significantly reduced the 24-h urinary protein levels and stabilized renal function during treatment. The statistical results showed that at 9 mo after treatment, the 24-h urinary protein quantity and Scr of the ITG decreased more significantly than that of the GTG (P < 0.05). By the 12th month after treatment, the 24-h urinary protein and Scr in both groups continued to decrease compared with those by the 9th month. In addition, the overall response rate of the ITG was 80%, which was significantly higher than that of the GTG (69%). These results indicate that the combination of mycophenolate and prednisone in patients with IgAN is more effective than prednisone alone and can effectively improve renal function.
After absorption, MMF selectively acts on hypoxanthine mononucleotide dehydrogenase, blocks guanine nucleotide synthesis, and plays an anti-inflammatory role by inhibiting the synthesis of cell surface adhesion molecules[28]. It can also inhibit an increase in the numbers of fibroblasts, endothelial cells, and vascular smooth muscle cells and reduce the decline in renal function caused by damage to the renal parenchyma[29]. Combined treatment with MP can play a synergistic role, improve the clinical therapeutic effects, and effectively improve kidney function. MMF also has the advantages of low toxicity and fewer side effects, which can effectively prevent and treat glomerulosclerosis[30]. In this study, the frequency of adverse reactions did not significantly differ between the two treatment regimens; however, compared with the incidence of endpoint events in the ITG (19%), that in the GTG was 32%, which is a noticeable increase. The follow-up time of the GTG (10.11 ± 3.09 mo) was significantly lower than that of the ITG (11.07 ± 2.08 mo). These results suggest that the safety of mycophenate combined with prednisone in the treatment of IgAN is attributed to prednisone alone.
There are several limitations of this study; for instance, the sample size was small and the follow-up time of patients was short. We believe that it is necessary to carry out future studies with a large sample and long-term follow-up, and observe the efficacy, recurrence, and adverse reactions of patients according to the degree of renal impairment or according to the kidney pathological grade.
In summary, prednisone combined with mycophenolate ethyl ester for the treatment of IgAN with moderate-to-severe renal function decline has a significant therapeutic effect, can effectively improve renal function, has high security, and can be used as an optimized treatment plan in clinical practice.
Immunoglobulin A nephropathy (IgAN) accompanied with renal dysfunction is a common disease. There is no standard treatment for IgAN with renal dysfunction, and glucocorticoid therapy is usually administered. However, single glucocorticoid is not a complete response of IgAN with renal dysfunction. IgAN is a disease characterized by abnormal immune system, which may be due to glomerular pathological damage caused by the deposition of IgA or its circulating immune complexes in the glomerulus. Therefore, treatment with glucocorticoid and immunosuppressive drugs may be more effective for patients with IgAN with renal dysfunction.
IgAN is among the most prevalent primary glomerular diseases worldwide. Among primary glomerular diseases in China, IgAN accounts for 45.26%–58.2%. Furthermore, as the most prevalent primary cause of ESRD, IgAN accounts for 26.69% of ESRD cases. Glucocorticoids have been used in IgAN for many years because they have advantageous effects on reducing inflammation and urinary protein excretion. The main feature of IgAN is a change in renal immunopathology, which is often treated with immunosuppressants. Therefore, it is important to research the moderate-to-severe IgAN therapy of glucocorticoids combined with immunosuppressants.
To explore the efficacy and security of prednisone combined with mycophenolate mofetil in IgAN therapy with moderate-to-severe renal dysfunction. We hope that, a safer and more effective treatments will be developed.
This study included 200 patients with moderate-to-severe renal dysfunction and IgAN patients. All patients were divided into the glucocorticoid therapy group (GTG) and immunosuppressive therapy group (ITG) according to different treatment regiments, with 100 patients in each group. The baseline data and follow-up data of patients who underwent kidney biopsy were collected. Finally, the above data were compared and analyzed.
The baseline data before treatment were not significantly different between groups. After treatment, serum creatinine and 24-h urinary protein levels in both groups decreased, but the decrease in the ITG differed from that in the GTGs in the 9th month. In addition, the overall response rate in the ITG was significantly higher than that in the GTG. The GTG had more endpoint events than the ITG, but the adverse reactions were similar between the regimens.
The addition of immunosuppressants on the basis of glucocorticoids is a better treatment option for moderate-to-severe renal dysfunction in patients with IgAN.
Future research will involve large-scale sample-controlled studies and long-term follow-up, and will track outcomes, relapse rates, and side effects of patients in relation to their level of renal impairment or pathological grade of their kidneys.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Putterman C, United States S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Lv J, Wong MG, Hladunewich MA, Jha V, Hooi LS, Monaghan H, Zhao M, Barbour S, Jardine MJ, Reich HN, Cattran D, Glassock R, Levin A, Wheeler DC, Woodward M, Billot L, Stepien S, Rogers K, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Zhang H, Perkovic V; TESTING Study Group. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA. 2022;327:1888-1898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 2. | Wheeler DC, Toto RD, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Pecoits-Filho R, Correa-Rotter R, Rossing P, Sjöström CD, Umanath K, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100:215-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 165] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 3. | Xie X, Li J, Liu P, Wang M, Gao L, Wan F, Lv J, Zhang H, Jin J. Chimeric Fusion between Clostridium Ramosum IgA Protease and IgG Fc Provides Long-Lasting Clearance of IgA Deposits in Mouse Models of IgA Nephropathy. J Am Soc Nephrol. 2022;33:918-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Zeng H, Wang L, Li J, Luo S, Han Q, Su F, Wei J, Wei X, Wu J, Li B, Huang J, Tang P, Cao C, Zhou Y, Yang Q. Single-cell RNA-sequencing reveals distinct immune cell subsets and signaling pathways in IgA nephropathy. Cell Biosci. 2021;11:203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Rajasekaran A, Julian BA, Rizk DV. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am J Med Sci. 2021;361:176-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 6. | Zhang H, Barratt J. Is IgA nephropathy the same disease in different parts of the world? Semin Immunopathol. 2021;43:707-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Sallustio F, Serino G, Cox SN, Dalla Gassa A, Curci C, De Palma G, Banelli B, Zaza G, Romani M, Schena FP. Aberrantly methylated DNA regions lead to low activation of CD4+ T-cells in IgA nephropathy. Clin Sci (Lond). 2016;130:733-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Qin A, Pei G, Tang Y, Tan L, Wei X, Zhong Z, Zhou L, Chen C, Qin W. Corticosteroids Improve Renal Survival: A Retrospective Analysis From Chinese Patients With Early-Stage IgA Nephropathy. Front Med (Lausanne). 2020;7:585859. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 9. | Praga M, Caravaca F, Yuste C, Cavero T, Hernández E, Morales E, Mérida E, Moreno JA, Sevillano A, Gutiérrez E. IgA nephropathy: What patients are at risk of progression to end-stage renal disease and how should they be treated? Nefrologia (Engl Ed). 2018;38:347-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Qian G, Zhang X, Xu W, Zou H, Li Y. Efficacy and safety of glucocorticoids for patients with IgA nephropathy: a meta-analysis. Int Urol Nephrol. 2019;51:859-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Hou FF, Xie D, Wang J, Xu X, Yang X, Ai J, Nie S, Liang M, Wang G, Jia N; MAIN Trial Investigators. Effectiveness of Mycophenolate Mofetil Among Patients With Progressive IgA Nephropathy: A Randomized Clinical Trial. JAMA Netw Open. 2023;6:e2254054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 26] [Reference Citation Analysis (0)] |
| 12. | Samsonov D, Zolotnitskaya A, Matloff R, Pereira T, Solomon S. Mycophenolate Mofetil for Severe IgA Vasculitis Nephropathy in Children: An Observational Study. Kidney Med. 2022;4:100534. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Fontana F, Delsante M, Vicari M, Pala C, Alfano G, Giovanella S, Ligabue G, Leonelli M, Manenti L, Rossi GM, Magistroni R, Fiaccadori E, Donati G. Mycophenolate mofetil plus steroids compared to steroids alone in IgA nephropathy: a retrospective study. J Nephrol. 2023;36:297-300. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 14. | Barbour S, Beaulieu M, Gill J, Espino-Hernandez G, Reich HN, Levin A. The need for improved uptake of the KDIGO glomerulonephritis guidelines into clinical practice in Canada: a survey of nephrologists. Clin Kidney J. 2014;7:538-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Selvaskandan H, Barratt J, Cheung CK. Immunological drivers of IgA nephropathy: Exploring the mucosa-kidney link. Int J Immunogenet. 2022;49:8-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Suzuki Y, Monteiro RC, Coppo R, Suzuki H. The Phenotypic Difference of IgA Nephropathy and its Race/Gender-dependent Molecular Mechanisms. Kidney360. 2021;2:1339-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Qing J, Li C, Hu X, Song W, Tirichen H, Yaigoub H, Li Y. Differentiation of T Helper 17 Cells May Mediate the Abnormal Humoral Immunity in IgA Nephropathy and Inflammatory Bowel Disease Based on Shared Genetic Effects. Front Immunol. 2022;13:916934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Zheng Y, Lu P, Deng Y, Wen L, Wang Y, Ma X, Wang Z, Wu L, Hong Q, Duan S, Yin Z, Fu B, Cai G, Chen X, Tang F. Single-Cell Transcriptomics Reveal Immune Mechanisms of the Onset and Progression of IgA Nephropathy. Cell Rep. 2020;33:108525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Liu L, Khan A, Sanchez-Rodriguez E, Zanoni F, Li Y, Steers N, Balderes O, Zhang J, Krithivasan P, LeDesma RA, Fischman C, Hebbring SJ, Harley JB, Moncrieffe H, Kottyan LC, Namjou-Khales B, Walunas TL, Knevel R, Raychaudhuri S, Karlson EW, Denny JC, Stanaway IB, Crosslin D, Rauen T, Floege J, Eitner F, Moldoveanu Z, Reily C, Knoppova B, Hall S, Sheff JT, Julian BA, Wyatt RJ, Suzuki H, Xie J, Chen N, Zhou X, Zhang H, Hammarström L, Viktorin A, Magnusson PKE, Shang N, Hripcsak G, Weng C, Rundek T, Elkind MSV, Oelsner EC, Barr RG, Ionita-Laza I, Novak J, Gharavi AG, Kiryluk K. Genetic regulation of serum IgA levels and susceptibility to common immune, infectious, kidney, and cardio-metabolic traits. Nat Commun. 2022;13:6859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Hofer M, Ranstam J, Atroshi I. Extended Follow-up of Local Steroid Injection for Carpal Tunnel Syndrome: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2130753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Hao X, Han J, Zeng H, Wang H, Li G, Jiang C, Xing Z, Hao Y, Yang F, Hou X. The effect of methylprednisolone prophylaxis on inflammatory monocyte subsets and suppressive regulatory T cells of patients undergoing cardiopulmonary bypass. Perfusion. 2019;34:364-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Zhang N, Lin J, Lin VPH, Milbreta U, Chin JS, Chew EGY, Lian MM, Foo JN, Zhang K, Wu W, Chew SY. A 3D Fiber-Hydrogel Based Non-Viral Gene Delivery Platform Reveals that microRNAs Promote Axon Regeneration and Enhance Functional Recovery Following Spinal Cord Injury. Adv Sci (Weinh). 2021;8:e2100805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | El-Ashmawy NE, Khedr NF, El-Bahrawy HA, Hamada OB. Anti-inflammatory and Antioxidant Effects of Captopril Compared to Methylprednisolone in L-Arginine-Induced Acute Pancreatitis. Dig Dis Sci. 2018;63:1497-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023;8:129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 54] [Reference Citation Analysis (0)] |
| 25. | Chen HL, Peng K, Zeng DM, Yan J, Huang YQ, Jiang PY, Du YF, Ling X, Wu J. High-Salt Diet Aggravates Endothelial-to-Mesenchymal Transition in Glomerular Fibrosis in Dahl Salt-Sensitive Rats. Am J Hypertens. 2023;36:660-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Floege J, Rauen T, Tang SCW. Current treatment of IgA nephropathy. Semin Immunopathol. 2021;43:717-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Li Y, Yang S, Liang M. Efficacy and safety of mycophenolate mofetil treatment in IgA nephropathy: a systematic review. BMC Nephrol. 2014;15:193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Jena A, Mishra S, Deepak P, Kumar-M P, Sharma A, Patel YI, Kennedy NA, Kim AHJ, Sharma V, Sebastian S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun Rev. 2022;21:102927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 29. | Song Y, Hu W, Xiao Y, Li Y, Wang X, He W, Hou J, Liu Y, Liang G, Huang C. Keratinocyte growth factor ameliorates mycophenolate mofetil-induced intestinal barrier disruption in mice. Mol Immunol. 2020;124:61-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Tunnicliffe DJ, Palmer SC, Henderson L, Masson P, Craig JC, Tong A, Singh-Grewal D, Flanc RS, Roberts MA, Webster AC, Strippoli GF. Immunosuppressive treatment for proliferative lupus nephritis. Cochrane Database Syst Rev. 2018;6:CD002922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |