Copyright
©The Author(s) 2023.
World J Clin Cases. Sep 26, 2023; 11(27): 6543-6550
Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6543
Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6543
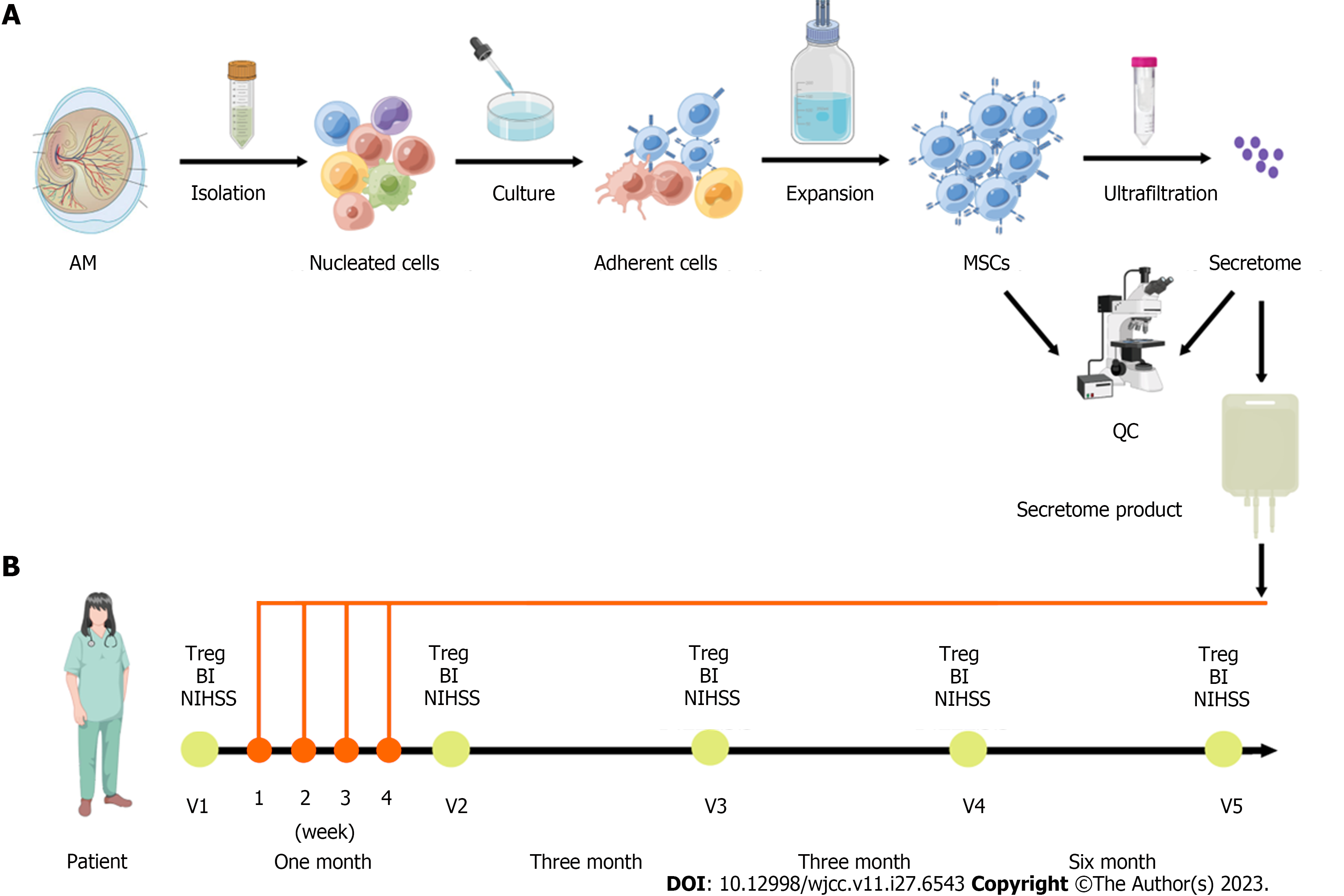
Figure 1 Schematic diagram on clinical-grade manufacturing process of the mesenchymal stromal cell-secretome product and schedule of the treatments of the mesenchymal stromal cell-secretome.
A: Amniotic membrane (AM) mesenchymal stromal cells (MSCs) were isolated, cultured, and expanded, and quality control (QC) evaluated cell characterization, surface markers, differentiation potential, presence of fungi and bacteria, and endotoxin level. The conditioned medium was concentrated by centrifugation using ultrafiltration units with a 3-kDa cutoff. The concentrated media was analyzed for protein concentration and for secreted cytokines content; B: Schematic representation of the follow-up visits (V) before (V1) and after the treatments of the MSC-secretome (V2-V5). The patient was enrolled in March 2021 and received four infusions of 50 mg MSC-secretome once a week for 4 wk. The clinical and laboratory evaluation of the patient was checked at each follow-up visit. BI: Barthel index; NIHSS: National Institute of Health Stroke Scale; Treg: Regulatory T cells.
- Citation: Lin FH, Yang YX, Wang YJ, Subbiah SK, Wu XY. Amniotic membrane mesenchymal stromal cell-derived secretome in the treatment of acute ischemic stroke: A case report. World J Clin Cases 2023; 11(27): 6543-6550
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6543.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6543