Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.255
Peer-review started: September 9, 2022
First decision: November 22, 2022
Revised: November 29, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 16, 2023
Processing time: 125 Days and 0.6 Hours
The cortical bone trajectory (CBT) is a novel technique in lumbar fixation and fusion. The unique caudocephalad and medial-lateral screw trajectories endow it with excellent screw purchase for vertebral fixation via a minimally invasive method. The combined use of CBT screws with transforaminal or posterior lumbar interbody fusion can treat a variety of lumbar diseases, including spondylolisthesis or stenosis, and can also be used as a remedy for revision surgery when the pedicle screw fails. CBT has obvious advantages in terms of surgical trauma, postoperative recovery, prevention and treatment of adjacent vertebral disease, and the surgical treatment of obese and osteoporosis patients. However, the concept of CBT internal fixation technology appeared relatively recently; consequently, there are few relevant clinical studies, and the long-term clinical efficacy and related complications have not been reported. Therefore, large sample and prospective studies are needed to further reveal the long-term complications and fusion rate. As a supplement to the traditional pedicle trajectory fixation technique, the CBT technique is a good choice for the treatment of lumbar diseases with accurate screw placement and strict indications and is thus deserving of clinical recommendation.
Core Tip: The cortical bone trajectory (CBT) has obvious advantages in terms of surgical trauma, postoperative recovery, prevention and treatment of adjacent vertebral disease, and the surgical treatment of obese and osteoporosis patients. This review presents the biomechanical characteristics, the perioperative osteoporosis management of midle line fusion (MIDLF) surgery, the clinical effect of MIDLF when comparing to other lumbar fusion surgery, the clinical effect of MIDLF for the treatment of lumbar spondylolisthesis, the advantages about MIDLF for spinal revision surgery, and the computer navigation-assisted CBT technique.
- Citation: Peng SB, Yuan XC, Lu WZ, Yu KX. Application of the cortical bone trajectory technique in posterior lumbar fixation. World J Clin Cases 2023; 11(2): 255-267
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/255.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.255
Since its first use for stabilizing the spine segment six decades ago[1], pedicle screw (PS) fixation has been widely applied and popularized due to its desirable biomechanical properties and high fusion success rate. It is considered to be the main surgical method for spinal fusion and fixation[2-4]. The traditional trajectory for PS insertion is from lateral to medial, which corresponds to the anatomical axis of the pedicle and is parallel to the endplate in the vertebral body. However, to longitudinally arrange the screws on the left and right sides with a focus on convergence, the muscles and soft tissues must be widely retracted, which will increase muscle injury and prolong the recovery time. Especially in patients with osteoporosis, maintaining the stability of internal fixation is a major challenge. When PSs are embedded in cancellous bone with low pedicle density, they can be easily loosened, removed or broken, resulting in poor surgical effects and even complications. The fixation strategies for low bone mass are mainly divided into: (1) Modifying the implant design, such as changing the length, pitch, and thread depth or expanding the diameter of the screw; and (2) Strengthening the vertebral body with reinforcing materials, such as assisted fixation with bone cement, to improve the structural strength of the osteoporotic bone. However, the use of screws with larger diameters and lengths will lead to a doubling of the risk of injury to nerves and vessels, and the use of bone cement is associated with risks of bone cement leakage and spinal canal nerve compression, leading to more disastrous consequences. A computed tomography (CT) study of pedicle bone density conducted by Hirano et al[5] revealed that the pyramidal cortex of osteoporotic bone is thinner than the normal cortex, and the bone mineral density of subcortical bone is low. Increasing the screw diameter does not improve the stability of the screw and may even lead to extraneous fracture of the thinned pedicle cortex. Therefore, a new internal fixation system is urgently needed to solve this problem.
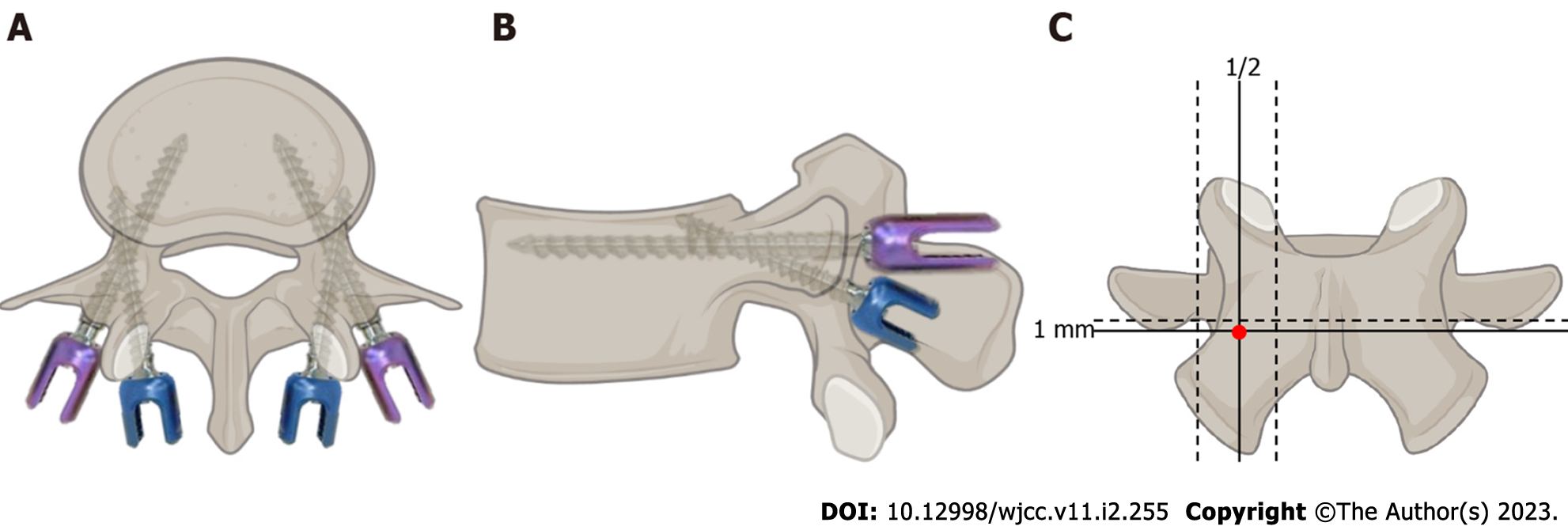
To solve the major problem of effectively fixing vertebrae with low bone mass, based on the idea proposed by Buck and attempted by a group of surgeons[6-8], an alternative screw trajectory was reported by Santoni et al[9] and gained increasing attention in 2009. This novel trajectory has a caudocephalad direction in the sagittal plane and a medial-lateral path in the transverse plane (Figure 1). With this new trajectory, the mechanical stability and pullout strength of the screws were significantly improved by including 4 Layers of cortical bone, including the starting point, the inner wall of the pedicle, the upper wall of the pedicle and the outer-upper wall of the vertebral body; hence, the trajectory was called the “cortical bone trajectory (CBT)”. The screw designed by Santoni et al[9] is shorter and has a lower diameter and a tighter thread than the traditional PS, all of which aim to maximize the thread contact with this higher density bone surface to increase vital biomechanical parameters.
Based on the application of CBT screw fixation, Mizuno et al[10] first proposed the concept of midline lumbar fusion (MIDLF) in 2014. In this original procedure, single segment spinal canal decompression, discectomy, interbody fusion and cortical screw fixation are all completed through a small midline incision, minimizing the injury related to the approach and providing another choice of fusion internal fixation for the clinical treatment of lumbar diseases. Over time, surgeons have applied CBT screws through a middle incision to fix two or more levels, which, combined with transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF), has also been defined as MIDLIF[11-13]. Although many researchers have described techniques with names such as CBT-PLIF or CBT-TLIF, they were in fact instances of MIDLIF[14-18].
Although MIDLF also involves separation of the paravertebral muscles along both sides of the spinous process, unlike in traditional TLIF or PLIF, only exposure of the inner edge of the articular process and the vertebral lamina is required, and the relatively large distance between the screw entry point and the articular process helps to protect the articular process joints.
In 1986, Steffee et al[19] proposed the term “force nucleus”, which refers to the focus point of the pedicle, lateral pars, lamina, transverse process and superior articular process. This area has a high concentration of cortical bone, so it can provide a strong screw holding force. In 2004, Li et al[20] found that the medial and superior pedicle isthmus cortex (more than 2 mm) was thicker than the lateral and inferior pedicle isthmus cortex (less than 2 mm), providing a stronger holding force for the screw than the lower and outer walls of the pedicle. In 2009, Santoni et al[9] proposed the CBT fixation technique and first analyzed its biomechanical properties. The results showed that CBT screws provide a 30% increase in uniaxial yield pullout load over traditional PSs and have similar flexion and extension resistance to PSs. Another notable result was that the type of CBT screw required did not depend on bone quality, which means it was suitable for osteoporosis patients, similar to the findings of other studies[21-25]. In 2014, Matsukawa et al[26] first reported that the insertional torque of CBT screws is approximately 1.71 times higher than that of traditional PSs.
Ueno et al[27] found that the maximum pullout strength of CBT screws in pig lumbar vertebrae was significantly higher than that of traditional pedicle cancellous screws. Oshino et al[28] concluded that the intervertebral stability after CBT fixation was similar to that after PS fixation. Delgado-Fernandez et al[29] found that CBT screws exhibit greater stiffness in cephalocaudal and medio-lateral loading, better flexion and extension resistance and better screw-holding stability in the pullout strength than traditional PSs; however, CBT screws were inferior in terms of lateral bending and axial rotation; these findings were confirmed in recent studies[24,30-34].
Mai et al[24] found a higher bone mineral density (BMD) for the CBT path than for the traditional pedicle trajectory path through CT examination of the lumbar vertebrae of 180 patients. Therefore, CBT screw purchase may be sufficient to stabilize the spine in osteoporotic patients. Another radiological evaluation performed by Kojima arrived at a similar conclusion[35]. The mean CT number [Hounsfield Unit (HU)] for CBT was more than four times that of the traditional pedicle trajectory. This is in keeping with previous hypotheses that the new trajectory offers. A previous study found that among 470 adult lumbar vertebral specimens, the CBT screw bone channels showed no significant differences in the diameter, length, or angle with the sagittal and transverse positions of the vertebral body; thus, the researchers concluded that the lumbar CBT screw bone cortex channel has a stable anatomical structure and small dissection variation[36]. Perez et al[37] found that the strength of CBT screws in lateral bending was significantly lower than that of PSs. Ninomiya et al[38] showed that the average insertion torque of the vertebral bodies of patients with spondylolysis was significantly lower than that of the vertebral bodies of patients without spondylolysis. In addition, the authors found that the average insertion torque of CBT screws in women over 75 years old was low and that CBT internal fixation was not suitable for 75-year-old women with spondylolysis. Matsukawa et al[36] performed a biomechanical study and showed that CBT is not suitable for patients with lumbar spondylolysis, mainly due to anti-flexion stretching and poor rotation compared with traditional PS fixation[39].
Given that CBT screws demonstrate lower lateral bending and axial rotation than traditional PSs, new attempts at developing combination strategies have been reported. Cornaz et al[40] suggested that a cross-connector could be beneficial for increasing the anti-lateral bending or axial rotation properties of CBT screws; however, there is no increased necessity to use a cross-connector in a CBT construct. Matsukawa et al[41] found that the combined use of traditional trajectory and CBT screws offered superior fixation strength over the traditional trajectory and CBT techniques in each plane of motion. Kahaer et al[42] and Mullin et al[43] also found that using a combined CBT and traditional pedicle trajectory offered superior fixation strength over that of the individual trajectories alone. This may be because the shorter length of the original CBT screw designed by Santoni et al[9] cannot provide sufficient fixation strength for the vertebral body, whereas the intervertebral space is the main structure for bending or rotation. Therefore, the original CBT screws not only provides less strength for anti-lateral bending or axial rotation but also may decrease the fusion rate for the interverbal body. This was demonstrated by Matsukawa et al[44] in 2 studies. In the first study conducted in 2016, they found that the screw length within the vertebral body (% length) was more important than the total screw length and that the screw should be placed sufficiently deep into the vertebral body. Then, in a study conducted in 2021, they identified that %depth > 39.2% was a predictor of bone fusion (sensitivity 90.9%, specificity 75.0%), that is, that the depth of the screw in the vertebral body should be at least 39.2% to achieve a stable fusion rate[45].
The CBT screw placement method differs from the traditional trajectory screw internal fixation method in a number of ways. The first difference is the screw entry point, which in the CBT is located much more medially than in the traditional trajectory. Mobbs’s study first described the nail placement path in the CBT[46]: After the nail insertion point is selected inside the isthmus, holes are drilled with a 2 mm grinding drill to establish the nail insertion channel, and the screws are inserted to point from the tail side to the head side and finally end at the rear 1/3 of the upper endplate to minimize the risk of isthmus fracture. However, this study did not indicate the standard trajectory for CBT screw implantation. Matsukawa et al[47,48] showed that the ideal insertion point of a CBT screw is the intersection of the vertical line passing through the center of the superior articular process and the horizontal line 1 mm below the lower edge of the transverse process. They found no significant difference in the angle of insertion from Lumbar 1 (L1) to L5; the head angle was 8° to 9°, and the abduction angle was 25° to 26°. The diameter of the screw varies according to the width and shape of the L1 to L5 pedicle and is generally smaller than that of the traditional PS. The diameter of the screw channel is 6.2 to 8.4 mm, and the depth of the screw is 36.8 to 38.3 mm.
In conclusion, most researchers believe that the ideal starting point is at the junction of the 1 mm horizontal line at the lower edge of the transverse process and the vertical line in the middle of the superior articular process. The point was specifically located projecting in the 5-o’clock orientation in the left pedicle and the 7-o’clock orientation in the right pedicle under fluoroscopic imaging[26,36]. Another difference is the trajectory path. Most researchers have concluded that the ideal screw path is at a 25° to 30° inclination from caudal to cephalad and at an 8° to 20° inclination from medial to lateral[9,10,49,50]. Additionally, the length of the CBT screw is 25 to 40 mm, and the diameter is 4.0 to 7.8 mm[44,51-54]. The angle and path vary greatly, possibly because of variations in the lumbar vertebrae from person to person due to, for example, ethnicity and the degree of lumbar degeneration, which make it difficult to develop an ideal, standardized trajectory.
To date, few articles have discussed the perioperative osteoporosis management of MIDLF surgery; therefore, we will describe existing management methods combined with our experience. For osteoporosis patients who undergo MIDLF surgery, it is very important to avoid excessive lying-in bed, as it will result in accelerated bone loss. Based on the excellent purchase of CBT screws and the reduced tissue damage, patients are advised to perform out-of-bed activities 48 h postoperatively and avoid standing or sitting for long periods of time during the first month[55]. The thoracolumbosacral orthosis should be worn for 6 mo postoperatively[56], and the patients will be able to resume normal activities 3 mo postoperatively. Postoperative intravenous analgesia is typically unnecessary due to the low amount of intraoperative trauma; for those patients who do experience postoperative pain, celecoxib is sufficient. However, when considering the poor anti-lateral bending and axial rotation properties of CBT screw fixation, patients should avoid lumbar lateral bending and rotation, especially when getting up from a seated or supine position. Patients are advised to wear the thoracolumbar orthosis when lying in bed and then get up from bed. Other common management strategies are similar to traditional lumbar fusion surgery, such as standard anti-osteoporosis treatment, prevention of deep venous thrombosis, straight leg lifting exercises to avoid adhesion of the nerve root in the spinal canal, incision care and postoperative radiographic imaging examinations.
Although MIDLF has been widely used in recent years and has achieved good clinical efficacy in lumbar spondylolisthesis, it was initially controversial. From 2014-2016, some of the first articles highlighted good results from this procedure, including better safety and effectiveness[57,58], less multifidus muscle damage and blood loss[59], shorter operative duration and hospital stays and less postoperative pain than traditional pedicle trajectory surgeries[61,62]. Conversely, others have focused on postoperative complications, including pseudarthrosis caudal adjacent segment failure, screw loosening[63], intraoperative pars fracture[64], and pedicle fracture[65]. The contrasting results may be related not only to the small size of the studies, the necessary learning curve and the experience of the surgeon but also to the patient's individual factors, such as nerve and surrounding tissue adhesion and local anatomical structure variation. Therefore, it is urgent to perform a randomized comparative study to identify the effectiveness of MIDLIF.
The first prospective, randomized, noninferiority, comparative study between MIDLIF and traditional lumbar fusion was published by Lee et al[66] in 2015. A total of 79 patients were enrolled and randomly assigned to the PS group or CBT group. Similar fusion rates were observed in both groups at the 6- and 12-mo follow-ups. According to the clinical outcomes, CBT provided similar improvements in pain amelioration and functional status over traditional PSs. Additionally, CBT was superior to PS in terms of blood loss, operative duration and incision length. The same authors then published a consecutive study on the same group of patients at the 2-year follow-up in 2018. The results demonstrated that there was no statistically significant difference between the groups in terms of clinical outcomes, radiologic outcomes and related complications[67].
Over time, some large series on MIDLIF have been published, mostly regarding lumbar spond
Most of the literature on MIDLF for lumbar spondylolisthesis aimed to analyze grade I to II spondylolisthesis, and there have been few publications about high-grade spondylolisthesis. Although low-grade spondylolisthesis seems more common than high-grade spondylolisthesis, this may be due to a lack of suitable instruments for reducing severe spondylolisthesis.
Takenaka et al[71] studied 119 consecutive patients with a minimum 1-year follow-up. There were no significant differences in operative duration or fusion rates, whereas the MIDLIF group experienced significantly less blood loss and lower postoperative creatine kinase levels than the PLIF group. Matsukawa et al[72] performed a retrospective cohort study aiming to find a predictor of screw loosening. He found that the regional HU values of the screw trajectory were more strongly correlated with the insertional torque than the femoral BMD and lumbar BMD, and the incidence of screw loosening was 4.6%. Multivariate logistic regression analysis revealed that the regional HU value was an independent risk factor that significantly affected screw loosening. Lee et al[73] compared the CBT and conventional pedicle trajectory techniques in terms of proximal adjacent segment pathology after lumbar fusion. Among 53 patients enrolled in this study, the postoperative fusion rate was not significantly different at the 1-year follow-up, while CBT exhibited superior satisfaction at 1 mo and lower pain intensity within 1 mo, blood loss, operative time, hospital stay and incision length. This study suggests that CBT can be a viable alternative to conventional PS surgery.
Other comparative studies have aimed to investigate the results between MIDLIF and other traditional minimally invasive surgeries, such as percutaneous PS placement[74-76], microendoscopic laminotomy[77] and minimally invasive (MIS)-PLIF or MIS-TLIF[78].
Bonis et al[74] reviewed 72 consecutive patients treated with percutaneous PSs (PPSs) and CBT screws and showed that pain significantly improved in both groups. The Charlson Comorbidity Index was the only variable associated with an increased risk of complications. Patients with a body mass index (BMI) ≥ (median value) and patients with percutaneous screws had an increased risk of a worse Smiley-Webster Score. Patients with a BMI ≥ 27.4, patients with percutaneous screws and patients with more comorbidities showed a higher risk of presenting with a severe/crippling ODI. Maruo et al[75] compared the clinical outcomes after TLIF using CBT or PPSs in 77 patients and found that the CBT group showed significantly lower serum creatine kinase (CK) levels and numeric rating scale scores on postoperative days 1 and 3 than the PPS group. There were no significant differences in cage subsidence, screw loosening, or fusion rates between the groups at the 1-year follow-up. Another study performed by Inoue et al[76] compared traditional PSs, CBT-PSs, and PPSs for PLIF and found that neither the operative time nor blood loss was significantly different among them. However, the postoperative drainage volume in the PPS-PLIF group was significantly lower than that in the PS-PLIF and CBT-PLIF groups. Elmekaty et al[78] found that CBT-TLIF led to a shorter operative time, less blood loss, and lower CK and C-reactive protein levels than MIS-TLIF and MIS-PLF, while there was no significant difference in functional outcomes among the three techniques. Additionally, the fusion rate was 100% with CBT-TLIF and MIS-TLIF but 90% with MIS-PLF. Screw loosening occurred in 10% of the MIS-PLF group, 7.14% of the MIS-TLIF group and 4.76% of the CBT-TLIF group. Ding et al[55] performed a prospective randomized controlled trial study that aimed to compare the results with TLIF using CBT and traditional PSs for treating osteoporosis patients with lumbar degenerative disease. The results indicated similar fusion rates between the two techniques at 6 and 12 mo, while CBT resulted in a significantly lower incidence of screw loosening and better ODIs and JOA scores at 3 mo postoperatively.
Compared with traditional lumbar fusion surgery, the advantages and disadvantages of MIDLF are as follows: Advantages: (1) Strong screw purchase, especially for osteoporosis patients; (2) Minimal invasiveness: The CBT screw is inserted near the middle line in the lumbar posterior approach, and less paravertebral muscle stripping is required. Compared with traditional PS insertion, CBT screw insertion results in less operative blood loss, shorter hospitalization time, lower postoperative CK level, less fat infiltration, and a larger postoperative lumbar dorsal muscle cross-sectional area; (3) Safety: CBT screws are inserted distant from the spinal canal and nerves; and (4) Effectiveness: Combined with various lumbar fusion procedures, CBT can treat a variety of lumbar diseases, lumbar spondylolisthesis, spinal traumas, and infections and produce effective outcomes, especially in the early postoperative period. Disadvantages: (1) It is difficult to place the screw by hand, and the manual feel with the CBT is notably different from that of the traditional pedicle trajectory, which results in a steep learning curve; (2) For patients with thin pedicles, pedicle fracture can easily occur; (3) It is difficult to connect screws and rods when fixing long segments; and (4) Relevant imaging equipment is required to place the screw accurately, and intraoperative fluoroscopy must be performed repeatedly.
MIDLIF is advantageous in the treatment of lumbar spondylolisthesis with osteoporosis due to its superior biomechanics and minimally invasive nail placement. With the maturity and popularization of computer navigation system technology, three dimensional (3D) printing navigation, 3D navigation and robot navigation-assisted CBT screw placement can reduce the complications caused by screw placement errors, address the shortcomings of CBT screw internal fixation technology, and increase the effectiveness of CBT screw internal fixation technology in the treatment of spinal surgical diseases.
With the application of posterior lumbar interbody fusion, ASDs caused by fusion have become increasingly prominent, with 5%-16% and 10%-26% of patients with symptomatic ASDs requiring revision surgery 5 and 10 years after lumbar posterior interbody fusion, respectively[79-81]. Compared with the first surgery, revision surgery is more difficult due to the obstruction of the PSs and the influence of surgical scars. The risk of dural sac tears and other complications during revision surgery are increased 1.7 times, and the bleeding volume is increased by 16% in traditional posterior fusion surgery fixed by vertebral arch screws[82,83]. MIDLIF can be used to perform decompression, fusion and fixation on the basis of less soft tissue dissection while retaining the original PSs, providing a new option for posterior revision of ASD.
In revision surgeries, CBT screws can be placed without removing the original PS so that one PS and one CBT screw can be accommodated in the same segment at the same time[84]. In addition, the insertion point of the CBT screw is closer to the midline near the isthmus; it is not necessary to expose the outer edge of the articular process when placing the screw, significantly reducing surgical trauma and bleeding.
Zhang et al[85] performed a human cadaveric biomechanical study and found that both CBT screws and PSs can be applied in a revision operation to salvage each other. The biomechanical stability of the traditional PS in revision with CBT is equivalent to that of the original PS, while the stability of the CBT screw in revision with a traditional PS is significantly lower than that of the original CBT screw. The original PS has a great influence on the modified CBT screw; however, the original CBT screw has little influence on the traditional pedicle revision screw[25]. He et al[63] used MIDLF with 3D-printed navigation templates in revision surgery to treat ASDs and obtained good clinical efficacy with a short operation duration and little blood loss. Recently, Wang et al[86] found that CBT screws were feasible for bridging fixation in ASD revision surgery. Melikian et al[87] published a case report on unilateral cortical trajectory screw instrumentation, allowing for posterior instrumentation without having to remove the existing PSs in the setting of ASD. Rho et al[88] first applied robotic placement of a CBT screw in the same pedicle as a prior traditional PS for ASD.
With the development and progress of computer navigation systems and related equipment, robot navigation technology, 3D-guided plate navigation, and preoperative 3D CT planning trajectory-assisted placement of CBT screws have addressed the lack of accuracy in manual screw placement and improved the accuracy and safety of CBT screw internal fixation technology in spinal surgery.
To better standardize and increase the accuracy of the trajectory, Matsukawa et al[36] attempted to improve the perspective during CBT screw surgery using a CT guide, which acts as a "pedicle diagram" similar to a clock; that is, in the left pedicle, the screw starts in the direction of 5 o'clock and is aimed at the direction of 11-12 o'clock, and in the right pedicle, the starting point is located in the direction of 7 o'clock, and the screw is aimed at the direction of 12-1 o'clock. The authors also believed that the starting point of the sacral CBT screw should be located at the junction of the center of the S1 upper articular process and approximately 3 mm below the lowest edge of the L5 Lower articular process[89]. In the axial plane, the direction is vertical and forward, and in the sagittal plane, the angle is 30°, and the screw directly penetrates the middle of the sacral endplate. Although penetrating the sacral endplate seems dangerous, it produces more stability against pullout forces and loosening[90]. However, Spirig et al[91] did not recommend placing CBT screws that penetrate the lumbar vertebral endplate, as no relevant biomechanical advantage is gained, while the potential risk for iatrogenic injury to structures anterior to the spine is increased.
Intraoperative CT (o-arm) image navigation technology combined with CBT screw fixation technology was first used to treat symptomatic adjacent vertebral diseases by Rodriguez et al[84] in 2014; CBT screws were placed again in the pedicle in which traditional PSs had been previously placed. This technique avoids the disadvantage of removing the connecting rod in traditional revision surgery and can reduce the operation time and trauma. Similarly, Kotheranurak et al[92] used CT-guided and image-guided unilateral CBT screw fixation to treat L5/S1 intervertebral disc diseases in an anterior approach assisted by endoscopy. The results showed that a variety of minimally invasive combined technologies with the assistance of a navigation system can improve the ease and accuracy of screw placement and reduce the amount of surgical trauma. Larata et al[93] used intraoperative cone beam CT to insert 618 CBT screws and showed that the accuracy rate of the overall navigation was as high as 98.3%. Kumar et al[94] compared the accuracy and complication rate of CBT screw placement under traditional fluoroscopy and CT navigation during the operation. The results showed that the destruction rate of the medial wall of the pedicle and the incidence of cerebrospinal fluid leakage and postoperative infection-related complications in the fluoroscopy group were higher than those in the navigation group.
In designing a 3D-printed navigation template, the surgeon first obtains images through preoperative CT scanning and then uses computer-aided, preset nail tracks to transfer the data to the 3D printer to create an individualized and accurate nail placement navigation template that allows CBT screws to be accurately placed during the operation. First, this technique was limited to cadaveric research[95-96]. Recently, some researchers have used this technique in clinical surgery. Kim et al[97] used this technique to treat an L4 spondylolisthesis patient, and the postoperative recovery was satisfactory without any related complications. Marengo et al[98] also used this technique to treat 11 patients in the same year. The results showed that the average deviation between the actual position of the screw and the postoperative position was 0.91 mm, and 85.2% of the screw deviation angles were < 2°. Mastsukawa et al[99] found that the accuracy rate of the 3D-printed template reached up to 97.5%, and the screw size used in this study was as large as 6.0 mm × 40 mm, which potentially increases the fixation strength. Similarly, Maruo et al[100] studied the accuracy of CBT screw placement by surgeons without free-hand experience using 3D-printed navigation template technology. The results showed that the overall accuracy was 91%, which increased to 97% after 10 operations.
Compared with traditional CT navigation technology, robots can avoid the interference of personnel and human errors caused by fatigue and emotion. Le et al[101] compared the accuracy of CBT screw placement under robot assistance and traditional fluoroscopy assistance. According to the improved Gertzbein-Robbins classification, the accuracy of the robot-assisted group was as high as 87.2%, while the accuracy of the traditional fluoroscopy-assisted group was only 66.9%. However, the operation time, blood loss and cumulative radiation time were greater than those in the traditional fluoroscopy-assisted group. Le et al[101] also found that the use of robots could reduce the rate of facet joint invasion and concluded that a measurement ≥ 45° was a significant risk factor for facet joint invasion in both groups. Khan et al[102] compared the accuracy of CBT screw placement assisted by the Mazor X robot and intraoperative 3D CT navigation technology[102]. The results showed that 92 screws in the robot group and 69 screws in the CT group were accurately placed, but there was no significant difference in operation time or bleeding volume.
CBT is a technique that can enhance the stability of screw fixation of osteoporotic vertebral bodies without the use of additional materials. It provides a new selection for lumbar internal fixation, especially for osteoporosis and revision cases. According to the anatomical characteristics of the resulting screw channels, the exposure range of the CBT technique is small and provides a safe passage distant from the nerve root and facet joint, which reduces the potential risk of neurovascular and facet joint injury and the incidence of ASDs and can also achieve a minimally invasive effect. Additionally, the CBT screw technique can be used in conjunction with the traditional PS technique and is expected to play an increasingly important role in treating lumbar diseases.
At present, large-scale, high-quality randomized controlled trials on CBT and traditional trajectory technology in lumbar degenerative diseases are being carried out[103,104], and more evidence-based medical evidence will be produced. Of course, with further basic research and clinical practice, clinicians and researchers will achieve a deeper understanding of the CBT technique. In short, the application prospects of CBT technology are worth considering.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mehren C, Germany; Nath SG, India S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Judet R, Judet J, Roy-Camille R, Lord G, Letournel E. [MacMurray osteotomy. Fixation technic]. Presse Med (1893). 1963;71:573. [PubMed] |
| 2. | Roy-Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop Relat Res. 1986;7-17. [PubMed] |
| 3. | Reid PC, Morr S, Kaiser MG. State of the union: a review of lumbar fusion indications and techniques for degenerative spine disease. J Neurosurg Spine. 2019;31:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Cheng LM, Wang JJ, Zeng ZL, Zhu R, Yu Y, Li C, Wu ZR. Pedicle screw fixation for traumatic fractures of the thoracic and lumbar spine. Cochrane Database Syst Rev. 2013;CD009073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Hirano T, Hasegawa K, Takahashi HE, Uchiyama S, Hara T, Washio T, Sugiura T, Yokaichiya M, Ikeda M. Structural characteristics of the pedicle and its role in screw stability. Spine (Phila Pa 1976). 1997;22:2504-9; discussion 2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52:432-437. [PubMed] |
| 7. | Lehman RA Jr, Polly DW Jr, Kuklo TR, Cunningham B, Kirk KL, Belmont PJ Jr. Straight-forward versus anatomic trajectory technique of thoracic pedicle screw fixation: a biomechanical analysis. Spine (Phila Pa 1976). 2003;28:2058-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Sterba W, Kim DG, Fyhrie DP, Yeni YN, Vaidya R. Biomechanical analysis of differing pedicle screw insertion angles. Clin Biomech (Bristol, Avon). 2007;22:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Santoni BG, Hynes RA, McGilvray KC, Rodriguez-Canessa G, Lyons AS, Henson MA, Womack WJ, Puttlitz CM. Cortical bone trajectory for lumbar pedicle screws. Spine J. 2009;9:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 10. | Mizuno M, Kuraishi K, Umeda Y, Sano T, Tsuji M, Suzuki H. Midline lumbar fusion with cortical bone trajectory screw. Neurol Med Chir (Tokyo). 2014;54:716-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Silva F, Silva PS, Vaz R, Pereira P. Midline lumbar interbody fusion (MIDLIF) with cortical screws: initial experience and learning curve. Acta Neurochir (Wien). 2019;161:2415-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Ver MLP, Gum JL, Crawford CH, Djurasovic M, Owens RK, Brown M, Steele P, Carreon LY. Index episode-of-care propensity-matched comparison of transforaminal lumbar interbody fusion (TLIF) techniques: open traditional TLIF versus midline lumbar interbody fusion (MIDLIF) vs robot-assisted MIDLIF. J Neurosurg Spine. 2020;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kovalenko RA, Kashin VA, Cherebillo VY. Individual Navigation Templates for Subcortical Screw Placement in Lumbar Spine. Sovrem Tekhnologii Med. 2021;13:41-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Sakaura H, Ikegami D, Fujimori T, Sugiura T, Yamada S, Mukai Y. Surgical outcomes after posterior lumbar interbody fusion using traditional trajectory screw fixation or cortical bone trajectory screw fixation: A comparative study between the polyetheretherketone cage and the same shape titanium-coated polyetheretherketone cage. Clin Neurol Neurosurg. 2021;209:106945. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Sakaura H, Miwa T, Yamashita T, Kuroda Y, Ohwada T. Cortical bone trajectory screw fixation versus traditional pedicle screw fixation for 2-level posterior lumbar interbody fusion: comparison of surgical outcomes for 2-level degenerative lumbar spondylolisthesis. J Neurosurg Spine. 2018;28:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Yamagishi A, Sakaura H, Ishii M, Ohnishi A, Ohwada T. Postoperative Loss of Lumbar Lordosis Affects Clinical Outcomes in Patients with Pseudoarthrosis after Posterior Lumbar Interbody Fusion Using Cortical Bone Trajectory Screw Fixation. Asian Spine J. 2021;15:294-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Sakaura H, Ikegami D, Fujimori T, Sugiura T, Mukai Y, Hosono N, Fuji T. Early cephalad adjacent segment degeneration after posterior lumbar interbody fusion: a comparative study between cortical bone trajectory screw fixation and traditional trajectory screw fixation. J Neurosurg Spine. 2019;32:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Kasukawa Y, Miyakoshi N, Hongo M, Ishikawa Y, Kudo D, Shimada Y. Short-Term Results of Transforaminal Lumbar Interbody Fusion Using Pedicle Screw with Cortical Bone Trajectory Compared with Conventional Trajectory. Asian Spine J. 2015;9:440-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Steffee AD, Biscup RS, Sitkowski DJ. Segmental spine plates with pedicle screw fixation. A new internal fixation device for disorders of the lumbar and thoracolumbar spine. Clin Orthop Relat Res. 1986;45-53. [PubMed] |
| 20. | Li B, Jiang B, Fu Z, Zhang D, Wang T. Accurate determination of isthmus of lumbar pedicle: a morphometric study using reformatted computed tomographic images. Spine (Phila Pa 1976). 2004;29:2438-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Zhang R, Gao H, Li H, Xing T, Jia C, Zhang J, Dong F, Shen C. Differences in bone mineral density of trajectory between lumbar cortical and traditional pedicle screws. J Orthop Surg Res. 2019;14:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Wray S, Mimran R, Vadapalli S, Shetye SS, McGilvray KC, Puttlitz CM. Pedicle screw placement in the lumbar spine: effect of trajectory and screw design on acute biomechanical purchase. J Neurosurg Spine. 2015;22:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Sansur CA, Caffes NM, Ibrahimi DM, Pratt NL, Lewis EM, Murgatroyd AA, Cunningham BW. Biomechanical fixation properties of cortical versus transpedicular screws in the osteoporotic lumbar spine: an in vitro human cadaveric model. J Neurosurg Spine. 2016;25:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Mai HT, Mitchell SM, Hashmi SZ, Jenkins TJ, Patel AA, Hsu WK. Differences in bone mineral density of fixation points between lumbar cortical and traditional pedicle screws. Spine J. 2016;16:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Li HM, Zhang RJ, Gao H, Jia CY, Xing T, Zhang JX, Dong FL, Shen CL. Biomechanical Fixation Properties of the Cortical Bone Trajectory in the Osteoporotic Lumbar Spine. World Neurosurg. 2018;119:e717-e727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976). 2014;39:E240-E245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 27. | Ueno M, Sakai R, Tanaka K, Inoue G, Uchida K, Imura T, Saito W, Nakazawa T, Takahira N, Mabuchi K, Takaso M. Should we use cortical bone screws for cortical bone trajectory? J Neurosurg Spine. 2015;22:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Oshino H, Sakakibara T, Inaba T, Yoshikawa T, Kato T, Kasai Y. A biomechanical comparison between cortical bone trajectory fixation and pedicle screw fixation. Journal of orthopaedic surgery and research. 2015; 10: 125.. |
| 29. | Delgado-Fernandez J, García-Pallero MÁ, Blasco G, Pulido-Rivas P, Sola RG. Review of Cortical Bone Trajectory: Evidence of a New Technique. Asian Spine J. 2017;11:817-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Zhang S, Liu Z, Lu C, Zhao L, Feng C, Wang Y, Zhang Y. Oblique lateral interbody fusion combined with different internal fixations for the treatment of degenerative lumbar spine disease: a finite element analysis. BMC Musculoskelet Disord. 2022;23:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Zhang L, Li HM, Zhang R, Zhang H, Shen CL. Biomechanical Changes of Adjacent and Fixed Segments Through Cortical Bone Trajectory Screw Fixation versus Traditional Trajectory Screw Fixation in the Lumbar Spine: A Finite Element Analysis. World Neurosurg. 2021;151:e447-e456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Liu CW, Wang LL, Xu YK, Chen CM, Wang JC, Tsai WT, Lin SC. Traditional and cortical trajectory screws of static and dynamic lumbar fixation- a finite element study. BMC Musculoskelet Disord. 2020;21:463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Tobing SDAL, Wisnubaroto RP. Pull-Out Strength Comparison Among Conventional Pedicle Screw, Cortical Infero-Superior, and Cortical Supero-Inferior Trajectories in Yorkshire Porcine Lumbar Spines: A Biomechanical Study. Int J Spine Surg. 2020;14:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Rexiti P, Aierken G, Wang S, Abudurexiti T, Abuduwali N, Deng Q, Guo H, Sheng W. Anatomical research on strength of screw track fixation in novel cortical bone trajectory for osteoporosis lumbar spine. Am J Transl Res. 2019;11:6850-6859. [PubMed] |
| 35. | Kojima K, Asamoto S, Kobayashi Y, Ishikawa M, Fukui Y. Cortical bone trajectory and traditional trajectory--a radiological evaluation of screw-bone contact. Acta Neurochir (Wien). 2015;157:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Matsukawa K, Yato Y, Nemoto O, Imabayashi H, Asazuma T, Nemoto K. Morphometric measurement of cortical bone trajectory for lumbar pedicle screw insertion using computed tomography. J Spinal Disord Tech. 2013;26:E248-E253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Perez-Orribo L, Kalb S, Reyes PM, Chang SW, Crawford NR. Biomechanics of lumbar cortical screw-rod fixation vs pedicle screw-rod fixation with and without interbody support. Spine (Phila Pa 1976). 2013;38:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Ninomiya K, Iwatsuki K, Ohnishi YI, Ohkawa T, Yoshimine T. Significance of the Pars Interarticularis in the Cortical Bone Trajectory Screw Technique: An In Vivo Insertional Torque Study. Asian Spine J. 2016;10:901-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 39. | Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Asazuma T, Chiba K. Biomechanical evaluation of lumbar pedicle screws in spondylolytic vertebrae: comparison of fixation strength between the traditional trajectory and a cortical bone trajectory. J Neurosurg Spine. 2016;24:910-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Cornaz F, Widmer J, Fasser MR, Snedeker JG, Matsukawa K, Spirig JM, Farshad M. Is a cross-connector beneficial for single level traditional or cortical bone trajectory pedicle screw instrumentation? PLoS One. 2021;16:e0253076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Asazuma T, Nemoto K. Biomechanical Evaluation of Cross Trajectory Technique for Pedicle Screw Insertion: Combined Use of Traditional Trajectory and Cortical Bone Trajectory. Orthop Surg. 2015;7:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Kahaer A, Zhou Z, Maitirouzi J, Wang S, Shi W, Abuduwaili N, Maimaiti X, Liu D, Sheng W, Rexiti P. Biomechanical Investigation of the Posterior Pedicle Screw Fixation System at Level L4-L5 Lumbar Segment with Traditional and Cortical Trajectories: A Finite Element Study. J Healthc Eng. 2022;2022:4826507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Mullin JP, Perlmutter B, Schmidt E, Benzel E, Steinmetz MP. Radiographic feasibility study of cortical bone trajectory and traditional pedicle screw dual trajectories. J Neurosurg Spine. 2016;25:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Abe Y, Asazuma T, Chiba K. Biomechanical evaluation of fixation strength among different sizes of pedicle screws using the cortical bone trajectory: what is the ideal screw size for optimal fixation? Acta Neurochir (Wien). 2016;158:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Matsukawa K, Yanai Y, Fujiyoshi K, Kato T, Yato Y. Depth of vertebral screw insertion using a cortical bone trajectory technique in lumbar spinal fusion: radiological significance of a long cortical bone trajectory. J Neurosurg Spine. 2021;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Mobbs RJ. The "medio-latero-superior trajectory technique": an alternative cortical trajectory for pedicle fixation. Orthop Surg. 2013;5:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Matsukawa K, Yato Y. Lumbar pedicle screw fixation with cortical bone trajectory: A review from anatomical and biomechanical standpoints. Spine Surg Relat Res. 2017;1:164-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Matsukawa K, Yato Y, Hynes RA, Imabayashi H, Hosogane N, Asazuma T, Matsui T, Kobayashi Y, Nemoto K. Cortical Bone Trajectory for Thoracic Pedicle Screws: A Technical Note. Clin Spine Surg. 2017;30:E497-E504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Dabbous B, Brown D, Tsitlakidis A, Arzoglou V. Clinical outcomes during the learning curve of MIDline Lumbar Fusion (MIDLF®) using the cortical bone trajectory. Acta Neurochir (Wien). 2016;158:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Matsukawa K, Taguchi E, Yato Y, Imabayashi H, Hosogane N, Asazuma T, Nemoto K. Evaluation of the Fixation Strength of Pedicle Screws Using Cortical Bone Trajectory: What Is the Ideal Trajectory for Optimal Fixation? Spine (Phila Pa 1976). 2015;40:E873-E878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Zhang H, Ajiboye RM, Shamie AN, Wu Q, Chen Q, Chen W. Morphometric measurement of the lumbosacral spine for minimally invasive cortical bone trajectory implant using computed tomography. Eur Spine J. 2016;25:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Senoglu M, Karadag A, Kinali B, Bozkurt B, Middlebrooks EH, Grande AW. Cortical Bone Trajectory Screw for Lumbar Fixation: A Quantitative Anatomic and Morphometric Evaluation. World Neurosurg. 2017;103:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Kaye ID, Prasad SK, Vaccaro AR, Hilibrand AS. The Cortical Bone Trajectory for Pedicle Screw Insertion. JBJS Rev. 2017;5:e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Gao H, Zhang R, Jia C, Xing T, Zhang J, Dong F, Ge P, Song P, Xu P, Zhang H, Li H, Shen C. Novel Placement of Cortical Bone Trajectory Screws in the Lumbar Spine: A Radiographic and Cadaveric Study. Clin Spine Surg. 2018;31:E329-E336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Ding H, Hai Y, Liu Y, Guan L, Pan A, Zhang X, Han B, Li Y, Yin P. Cortical Trajectory Fixation Versus Traditional Pedicle-Screw Fixation in the Treatment of Lumbar Degenerative Patients with Osteoporosis: A Prospective Randomized Controlled Trial. Clin Interv Aging. 2022;17:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Miyakoshi N, Maekawa S, Urayama M, Shimada Y. Utilizing a Cortical Bone Trajectory Pedicle Screw for Lumbar Flexion-Distraction Injury. Case Rep Orthop. 2018;2018:8185051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 57. | Ohkawa T, Iwatsuki K, Ohnishi Y, Ninomiya K, Yoshimine T. Isthmus-guided Cortical Bone Trajectory Reduces Postoperative Increases in Serum Creatinine Phosphokinase Concentrations. Orthop Surg. 2015;7:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Cheng WK, İnceoğlu S. Cortical and Standard Trajectory Pedicle Screw Fixation Techniques in Stabilizing Multisegment Lumbar Spine with Low Grade Spondylolisthesis. Int J Spine Surg. 2015;9:46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Pacione D, Kim I, Wilson TA, Frempong-Boadu A. Cortical screw trajectory for instrumentation and fusion in the setting of osteopathic compression fracture allows for percutaneous kyphoplasty for adjacent level compression fractures. J Clin Neurosci. 2015;22:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Ninomiya K, Iwatsuki K, Ohnishi Y, Ohkawa T, Yoshimine T. Clear Zone Formation around Screws in the Early Postoperative Stages after Posterior Lumbar Fusion Using the Cortical Bone Trajectory Technique. Asian Spine J. 2015;9:884-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Mori K, Nishizawa K, Nakamura A, Imai S. Short-Term Clinical Result of Cortical Bone Trajectory Technique for the Treatment of Degenerative Lumbar Spondylolisthesis with More than 1-Year Follow-Up. Asian Spine J. 2016;10:238-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Hung CW, Wu MF, Hong RT, Weng MJ, Yu GF, Kao CH. Comparison of multifidus muscle atrophy after posterior lumbar interbody fusion with conventional and cortical bone trajectory. Clin Neurol Neurosurg. 2016;145:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Glennie RA, Dea N, Kwon BK, Street JT. Early clinical results with cortically based pedicle screw trajectory for fusion of the degenerative lumbar spine. J Clin Neurosci. 2015;22:972-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Patel SS, Cheng WK, Danisa OA. Early complications after instrumentation of the lumbar spine using cortical bone trajectory technique. J Clin Neurosci. 2016;24:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Snyder LA, Martinez-Del-Campo E, Neal MT, Zaidi HA, Awad AW, Bina R, Ponce FA, Kaibara T, Chang SW. Lumbar Spinal Fixation with Cortical Bone Trajectory Pedicle Screws in 79 Patients with Degenerative Disease: Perioperative Outcomes and Complications. World Neurosurg. 2016;88:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Lee GW, Son JH, Ahn MW, Kim HJ, Yeom JS. The comparison of pedicle screw and cortical screw in posterior lumbar interbody fusion: a prospective randomized noninferiority trial. Spine J. 2015;15:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Lee GW, Ahn MW. Comparative Study of Cortical Bone Trajectory-Pedicle Screw (Cortical Screw) Versus Conventional Pedicle Screw in Single-Level Posterior Lumbar Interbody Fusion: A 2-Year Post Hoc Analysis from Prospectively Randomized Data. World Neurosurg. 2018;109:e194-e202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Sakaura H, Miwa T, Yamashita T, Kuroda Y, Ohwada T. Posterior lumbar interbody fusion with cortical bone trajectory screw fixation versus posterior lumbar interbody fusion using traditional pedicle screw fixation for degenerative lumbar spondylolisthesis: a comparative study. J Neurosurg Spine. 2016;25:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 69. | Mori K, Yayama T, Nishizawa K, Nakamura A, Saito H, Kitagawa M, Imai S. Incidence of Cranial Adjacent Segment Disease after Posterior Lumbar Interbody Fusion Using the Cortical Bone Trajectory Technique for the Treatment of Single-Level Degenerative Lumbar Spondylolisthesis; More than a 2-Year Follow-Up. Spine Surg Relat Res. 2021;5:98-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 70. | Menon N, Turcotte J, Speciale A, Patton CM. Cortical bone trajectory instrumentation provides favorable perioperative outcomes compared to pedicle screws for single-level lumbar spinal stenosis and degenerative spondylolisthesis. J Orthop. 2020;22:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Takenaka S, Mukai Y, Tateishi K, Hosono N, Fuji T, Kaito T. Clinical Outcomes After Posterior Lumbar Interbody Fusion: Comparison of Cortical Bone Trajectory and Conventional Pedicle Screw Insertion. Clin Spine Surg. 2017;30:E1411-E1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 72. | Matsukawa K, Abe Y, Yanai Y, Yato Y. Regional Hounsfield unit measurement of screw trajectory for predicting pedicle screw fixation using cortical bone trajectory: a retrospective cohort study. Acta Neurochir (Wien). 2018;160:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Lee GW, Shin JH. Comparative Study of Two Surgical Techniques for Proximal Adjacent Segment Pathology after Posterior Lumbar Interbody Fusion with Pedicle Screws: Fusion Extension using Conventional Pedicle Screw vs Cortical Bone Trajectory-Pedicle Screw (Cortical Screw). World Neurosurg. 2018;117:e154-e161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | DE Bonis P, Chiccoli M, Visani J, Cavallo MA, Scerrati A. Functional outcome of patients with unstable single-level/two-level lumbar stenosis treated with decompression plus divergent screws (cortical bone trajectory) or percutaneous convergent pedicle screws. J Neurosurg Sci. 2022;66:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 75. | Maruo K, Arizumi F, Kusuyama K, Yoshie N, Tomoyuki K, Tachibana T. Comparison of Clinical Outcomes After Transforaminal Interbody Fusion Using Cortical Bone Trajectory versus Percutaneous Pedicle Screw Fixation. World Neurosurg. 2021;151:e821-e827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 76. | Inoue T, Mizutamari M, Hatake K. Surgical Invasiveness of Single-Segment Posterior Lumbar Interbody Fusion: Comparing Perioperative Blood Loss in Posterior Lumbar Interbody Fusion with Traditional Pedicle Screws, Cortical Bone Trajectory Screws, and Percutaneous Pedicle Screws. Asian Spine J. 2021;15:856-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Hayashi K, Toyoda H, Terai H, Hoshino M, Suzuki A, Takahashi S, Tamai K, Ohyama S, Hori Y, Yabu A, Nakamura H. Comparison of minimally invasive decompression and combined minimally invasive decompression and fusion in patients with degenerative spondylolisthesis with instability. J Clin Neurosci. 2018;57:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Elmekaty M, Kotani Y, Mehy EE, Robinson Y, Tantawy AE, Sekiguchi I, Fujita R. Clinical and Radiological Comparison between Three Different Minimally Invasive Surgical Fusion Techniques for Single-Level Lumbar Isthmic and Degenerative Spondylolisthesis: Minimally Invasive Surgical Posterolateral Fusion versus Minimally Invasive Surgical Transforaminal Lumbar Interbody Fusion versus Midline Lumbar Fusion. Asian Spine J. 2018;12:870-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 79. | Virk SS, Niedermeier S, Yu E, Khan SN. Adjacent segment disease. Orthopedics. 2014;37:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 80. | Zhang C, Berven SH, Fortin M, Weber MH. Adjacent Segment Degeneration Versus Disease After Lumbar Spine Fusion for Degenerative Pathology: A Systematic Review With Meta-Analysis of the Literature. Clin Spine Surg. 2016;29:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 81. | Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015;15:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 82. | Louie PK, Haws BE, Khan JM, Markowitz J, Movassaghi K, Ferguson J, Lopez GD, An HS, Phillips FM. Comparison of Stand-alone Lateral Lumbar Interbody Fusion Versus Open Laminectomy and Posterolateral Instrumented Fusion in the Treatment of Adjacent Segment Disease Following Previous Lumbar Fusion Surgery. Spine (Phila Pa 1976). 2019;44:E1461-E1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Tormenti MJ, Maserati MB, Bonfield CM, Gerszten PC, Moossy JJ, Kanter AS, Spiro RM, Okonkwo DO. Perioperative surgical complications of transforaminal lumbar interbody fusion: a single-center experience. J Neurosurg Spine. 2012;16:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 84. | Rodriguez A, Neal MT, Liu A, Somasundaram A, Hsu W, Branch CL Jr. Novel placement of cortical bone trajectory screws in previously instrumented pedicles for adjacent-segment lumbar disease using CT image-guided navigation. Neurosurg Focus. 2014;36:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Zhang RJ, Li HM, Gao H, Jia CY, Xing T, Dong FL, Shen CL. Cortical bone trajectory screws used to save failed traditional trajectory screws in the osteoporotic lumbar spine and vice versa: a human cadaveric biomechanical study. J Neurosurg Spine. 2019;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Wang L, Zhao YH, Cai XB, Liang JL, Luo HT, Ma YL, Xu YQ, Lu S. Feasibility of cortical bone trajectory screws for bridging fixation in revision surgery for lumbar adjacent segment degeneration. Medicine (Baltimore). 2021;100:e26666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 87. | Melikian R, Yeremian S. Placement of Unilateral Cortical Bone Trajectory Screws in Previously Instrumented Pedicle without Removal of Existing Hardware for Adjacent Segment Disease. Case Rep Orthop. 2021;2021:9994539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 88. | Rho K, OConnor TE, Lucas JM, Pollina J, Mullin J. Minimally Invasive Robot-Guided Dual Cortical Bone Trajectory for Adjacent Segment Disease. Cureus. 2021;13:e16822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 89. | Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K. Cortical bone trajectory for lumbosacral fixation: penetrating S-1 endplate screw technique: technical note. J Neurosurg Spine. 2014;21:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Grigoryan G, Inceoglu S, Danisa OA, Cheng W. Sacral Endplate Penetrating Screw for Lumbosacral Fixation: A Cadaveric Biomechanical Study. Oper Neurosurg (Hagerstown). 2019;17:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 91. | Spirig JM, Winkler E, Cornaz F, Fasser MR, Betz M, Snedeker JG, Widmer J, Farshad M. Biomechanical performance of bicortical versus pericortical bone trajectory (CBT) pedicle screws. Eur Spine J. 2021;30:2292-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Hahn P, Oezdemir S, Komp M, Giannakopoulos A, Heikenfeld R, Kasch R, Merk H, Godolias G, Ruetten S. A New Electromagnetic Navigation System for Pedicle Screws Placement: A Human Cadaver Study at the Lumbar Spine. PLoS One. 2015;10:e0133708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Laratta JL, Shillingford JN, Pugely AJ, Gupta K, Gum JL, Djurasovic M, Crawford CH. Accuracy of cortical bone trajectory screw placement in midline lumbar fusion (MIDLF) with intraoperative cone beam navigation. J Spine Surg. 2019;5:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Kumar KK, Parikh B, Jabarkheel R, Dirlikov B, Singh H. Fluoroscopic versus CT-guided cortical bone trajectory pedicle screw fixation: Comparing trajectory related complications. J Clin Neurosci. 2021;89:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Kim SB, Rhee JM, Lee GS, Lee HY, Kim T, Won Y. Computer-assisted Patient-specific Prototype Template for Thoracolumbar Cortical Bone Trajectory Screw Placement: A Cadaveric Study. Tech Orthop. 2018;33:246-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Wang K, Zhang ZJ, Chen JX, Wu AM, Wang XY, Sheng SR. Design and Application of Individualized, 3-Dimensional-Printed Navigation Template for Placing Cortical Bone Trajectory Screws in Middle-Upper Thoracic Spine: Cadaver Research Study. World Neurosurg. 2019;125:e348-e352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Kim J, Rajadurai J, Choy WJ, Cassar L, Phan K, Harris L, Fiechter M, Mobbs RJ. Three-Dimensional Patient-Specific Guides for Intraoperative Navigation for Cortical Screw Trajectory Pedicle Fixation. World Neurosurg. 2019;122:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Marengo N, Matsukawa K, Monticelli M, Ajello M, Pacca P, Cofano F, Penner F, Zenga F, Ducati A, Garbossa D. Cortical Bone Trajectory Screw Placement Accuracy with a Patient-Matched 3-Dimensional Printed Guide in Lumbar Spinal Surgery: A Clinical Study. World Neurosurg. 2019;130:e98-e104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 99. | Matsukawa K, Kaito T, Abe Y. Accuracy of cortical bone trajectory screw placement using patient-specific template guide system. Neurosurg Rev. 2020;43:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Maruo K, Arizumi F, Kusuyama K, Kishima K, Tachibana T. Accuracy and safety of cortical bone trajectory screw placement by an inexperienced surgeon using 3D patient-specific guides for transforaminal lumbar interbody fusion. J Clin Neurosci. 2020;78:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Le X, Tian W, Shi Z, Han X, Liu Y, Liu B, He D, Yuan Q, Sun Y, Xu Y. Robot-Assisted Versus Fluoroscopy-Assisted Cortical Bone Trajectory Screw Instrumentation in Lumbar Spinal Surgery: A Matched-Cohort Comparison. World Neurosurg. 2018;120:e745-e751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 102. | Khan A, Rho K, Mao JZ, O'Connor TE, Agyei JO, Meyers JE, Mullin JP, Pollina J. Comparing Cortical Bone Trajectories for Pedicle Screw Insertion using Robotic Guidance and Three-Dimensional Computed Tomography Navigation. World Neurosurg. 2020;141:e625-e632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 103. | Tschugg A, Kavakebi P, Hartmann S, Lener S, Wipplinger C, Löscher WN, Neururer S, Wildauer M, Thomé C. Clinical and radiological effect of medialized cortical bone trajectory for lumbar pedicle screw fixation in patients with degenerative lumbar spondylolisthesis: study protocol for a randomized controlled trial (mPACT). Trials. 2018;19:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Feng Z, Li X, Tang Q, Wang C, Zheng W, Zhang H, Wu AM, Tian N, Wu Y, Ni W. Transforaminal lumbar interbody fusion with cortical bone trajectory screws versus traditional pedicle screws fixation: a study protocol of randomised controlled trial. BMJ Open. 2017;7:e017227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |