Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3330
Peer-review started: February 15, 2023
First decision: March 14, 2023
Revised: March 18, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 16, 2023
Chronic obstructive pulmonary disease (COPD) is associated with high morbidity and mortality rates worldwide. Older patients have a degenerative cardiopulmonary function, weak compensatory capacity, and poor surgical tolerance. Therefore, the mode of anesthesia must be optimized. Remimazolam is a new ultrashort-acting benzodiazepine with a rapid onset of action, rapid metabolism, and mild effects on pulmonary circulation. Remimazolam sedation combined with an epidural block has not been reported in hypertensive older adults with severe COPD and inguinal mass resection.
We report the case of a 73-year-old man with hypertension and severe COPD, who underwent resection of an enlarged inguinal mass that he had noticed more than 7 mo before presentation. The patient presented with a “right inguinal mass” and was recommended to undergo an enlarged inguinal mass resection. Surgery was relatively challenging, due to the large mass (13 cm × 8 cm × 7 cm), hard texture, and poor mobility. Considering the advanced age of the patient, grade III hypertension, and severe COPD, we administered remimazolam combined with an epidural block for anesthesia to ensure perioperative safety and careful consideration. The anesthetic effect was precise; the procedure was performed smoothly without any complications, and the patient was successfully anesthetized. However, anesthetic management in such cases has not yet been reported by previous studies.
Remimazolam sedation combined with an epidural block is safe and effective in older patients with hypertension and severe COPD.
Core Tip: Chronic obstructive pulmonary disease (COPD) is associated with high morbidity and mortality rates. Older patients have degenerative systems with weak compensatory capacity. Therefore, their status must be carefully evaluated to optimize the anesthesia plan when choosing an anesthetic agent for surgery. Remimazolam sedation combined with an epidural block in older adults with hypertension and severe COPD has rarely been reported. Our management experience shows that remimazolam sedation combined with an epidural block is safe and effective in older adults with hypertension and severe COPD.
- Citation: Yu JJ, Pei HS, Meng Y. Successful remimazolam sedation-epidural block in an older patient with severe chronic obstructive pulmonary disease: A case report. World J Clin Cases 2023; 11(14): 3330-3339
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3330.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3330
Chronic obstructive pulmonary disease (COPD) has high morbidity and mortality rates worldwide. Older patients have degenerative systems and weak compensatory capacity. Therefore, careful assessment of patient status and optimization of anesthesia is required when selecting an anesthetic agent for surgery. The sedative effect of remimazolam is mild in the pulmonary circulation, and a long-term intravenous infusion is nonaccumulative. Flumazenil can reverse this sedative effect by facilitating the response to intraoperative emergencies. Remimazolam sedation combined with an epidural block in older patients with hypertension and severe COPD has not been reported.
In this case report, we describe the anesthetic management of a 73-year-old man with hypertension and severe COPD, who underwent an enlarged inguinal mass resection with remimazolam sedation combined with an epidural block. Based on our experience with this patient, we conclude that remimazolam sedation combined with an epidural block is safe and effective in older adults with hypertension and severe COPD. This report provides new ideas for the safe administration of anesthesia in older patients with severe COPD.
The patient was a 73-year-old man with a height of 169 cm and weight of 48 kg, who presented to the clinic with a complaint of > 7 mo right inguinal mass.
The patient had undergone radical distal major gastrectomy under general anesthesia 7 years ago and was transferred to the intensive care unit for transitional treatment after the operation. The patient recovered well and was discharged.
The patient had a history of hypertension for > 7 years. He was receiving oral reserpine, and blood pressure (BP) control was stable 2 wk before the operation; when reserpine was discontinued, BP control was fair. The patient had no history of coronary heart disease or diabetes mellitus.
The patient denied any relevant family history of cancer.
On physical examination, the patient’s vital signs were as follows: Body temperature (T), 36.6 ℃; BP, 145/86 mmHg; heart rate (HR), 62 beats per min; respiratory rate (RR), 16 breaths per min. The patient was conscious, cooperated with physical examinations, and was admitted to the ward. He presented with a barrel chest, low-breath sounds in both lungs, and no wheezing was heard. There was no precordial bulging, with a regular heart rhythm, and no extra heart or pericardial friction sounds were heard. A 13 cm × 8 cm × 7 cm mass with a hard texture, and poor mobility was observed in the right inguinal region.
The results of routine preoperative blood tests, biochemistry tests, coagulation function tests, and other laboratory tests were normal.
Chest computed tomography (CT) revealed subpleural inflammation in the lower lobe of the right lung, emphysema, pulmonary bullae, increased translucency in the lower lung fields of both lungs, non-smooth and depressed diaphragms on both sides, a blunt costophrenic angle, and aortic calcification.
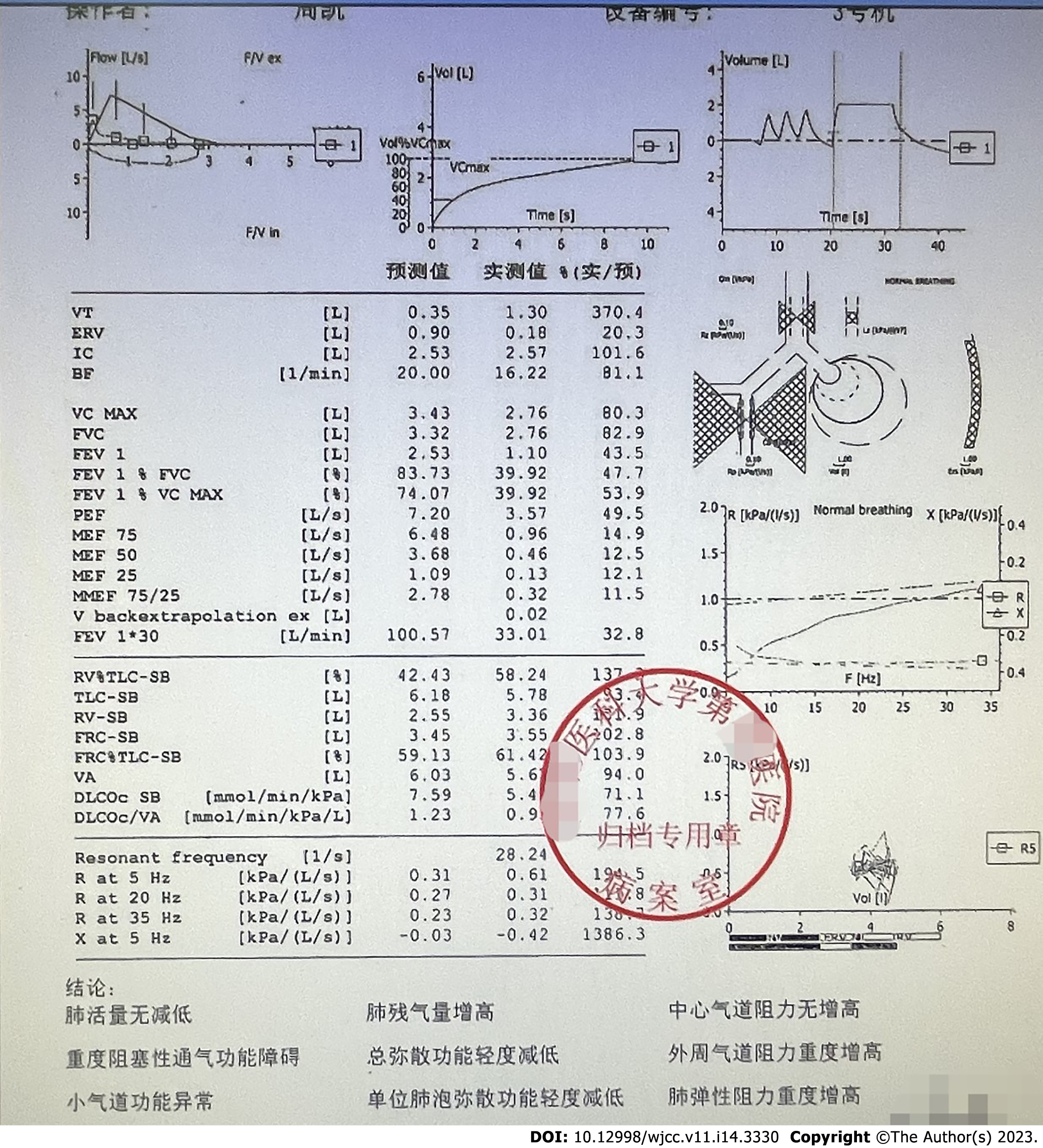
Electrocardiography (ECG) showed sinus rhythm, normal ECG, and a 72 beats/min ventricular rate. Echocardiography revealed degenerative aortic valve changes combined with mild regurgitation, reduced left ventricular diastolic function, and a left ventricular ejection fraction of 65%. Pulmonary function showed a forced expiratory volume in 1 s (FEV1): 1.10 L, with a measured value of 43.5% of the expected value, a FEV1/forced vital capacity (FVC) of 62%, and a lung carbon monoxide diffusion to alveolar ventilation ratio of 0.95 L·min-1·mmHg-1, with a measured value of 77.6% of the expected value. The pulmonary function has been reported to include severe obstructive ventilation dysfunction, mildly reduced total diffusion function, severely increased peripheral airway resistance, abnormal small airway function, and severely increased pulmonary elastic resistance (Figure 1).
ASA grade III, class II cardiac function, metabolic equivalent of 4 metabolic equivalents, clinical frailty scale grade 4, thyromental distance > 6 cm, mouth opening > 3 fingers, and mallampati grade II.
The patient was diagnosed with a right inguinal mass, radical distal gastrectomy, high-risk grade III hypertension, and severe COPD.
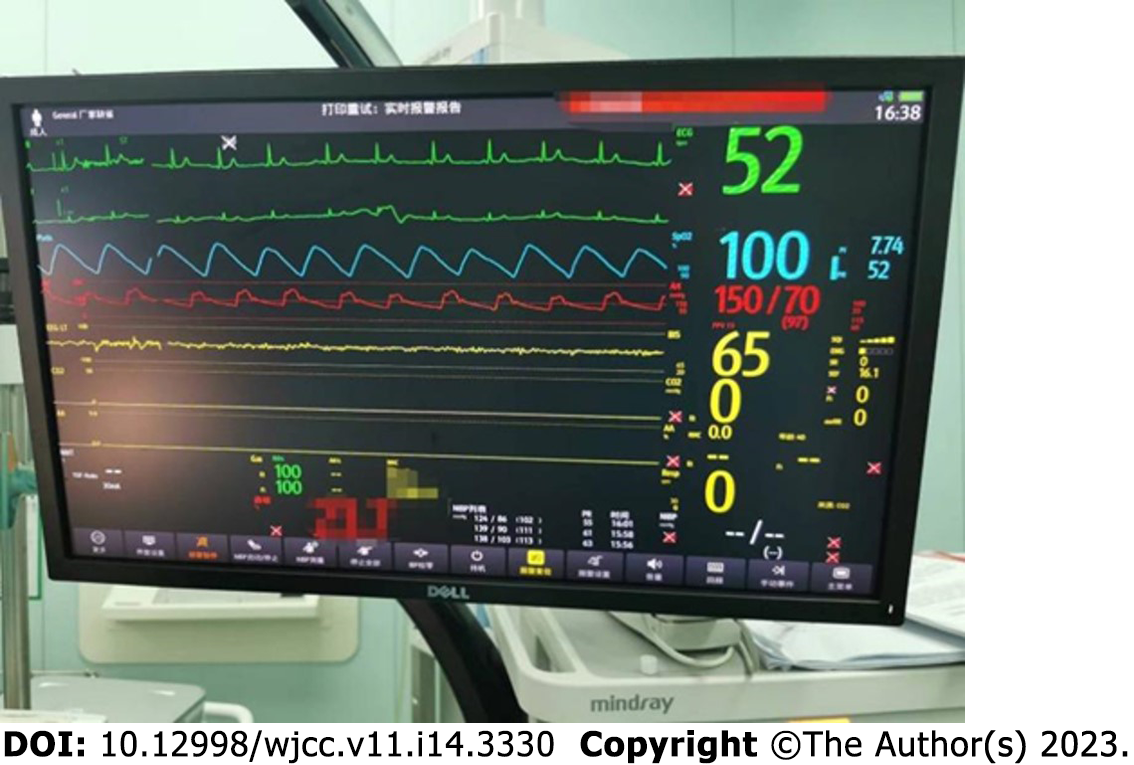
The patient was admitted at 15:40, complaining of extreme nervousness and strongly requesting to be kept asleep during the operation. With the patient’s previous history of hypertension and emotional stress following admission, the operator was eager to determine whether the anesthesiologist could provide the patient with moderate sedation to maintain perioperative circulatory stability. Left upper extremity venous access was secured after admission, and cuff BP, ECG, Peripheral capillary oxygen saturation (SpO2), and bispectral index (BIS) were monitored (BP, 165/85 mmHg; HR, 85 beats/min; RR, 16–18 beats/min; SpO2, 96%; and BIS, 98). Oxygen was administered at a rate of 6 L/min using a face mask, and sedation was provided with 2 mg remimazolam (ambulatory blood pressure [ABP], 180/90 mmHg; HR, 82 beats/min; RR, 16–18 beats/min; SpO2, 95%; and BIS, 97). Arterial blood gas metrics on admission were as follows: fraction of inspiration O2 (FiO2), 0.21; potential of hydrogen, 7.43; pressure of carbon dioxide (PaCO2), 36.3 mmHg; and pressure of oxygen (PaO2), 69.8 mmHg. At 15:52, BP was 150/84 mmHg, HR was 72 beats/min, SpO2 was 100%, and BIS was 75–80, with stable spontaneous breathing. During the 8-min observation period, the patient’s vital signs remained stable. Induction of anesthesia: epidural puncture and tube placement were performed at 16:00. The patient was placed in the right lateral position, and the median approach was taken. An 18-gauge Tuohy needle was used to puncture the L1-2 intervertebral space, and a negative pressure test was positive. After confirming entry of the puncture needle into the epidural cavity, the epidural catheter was placed headward, the puncture needle was withdrawn, the epidural catheter was fixed at an 8-cm scale, and the patient was placed supine. At 16:10, the epidural catheter was connected to a 5-mL syringe. Blood-free cerebrospinal fluid was withdrawn and injected into the 2% lidocaine test at 16:15. The patients were asked about any complaints of discomfort. His ABP was 140/80 mmHg, 75 beats/min, 16–18 beats/min, SpO2 was 95%, and BIS was 87. The level of anesthesia block was determined, and an additional 2% lidocaine (6.5 mL was injected via the epidural catheter). At 16:25, the level of the anesthesia block was determined again, and 5 mg of remimazolam was injected to sedate the patient. Surgery was initiated at 16:30. To ensure stable intraoperative sedation, remimazolam (40 mg/h) was infused intravenously using a micropump. At 17:05, 5 mL of 2% lidocaine was injected epidurally. At 18:00, the operation was completed, and the intravenous infusion of remimazolam was stopped. Subsequently, 50 mg flurbiprofen was administered. The operation lasted 90 min, with 650 mL crystalloid infusion and blood loss of 50 mL. The operation was performed under rimazolam sedation combined with an epidural block. During the operation, the patient was in a continuous sleep state, during which the BIS was stabilized at shallow sedation, circulation was stable, spontaneous breathing was stable, no hypotension or respiratory depression was observed, no vasoactive drugs were applied, no jaw-supporting assisted breathing was performed, the airway tools prepared before the operation were not used, the anesthetic effect was exact, and the operation was completed successfully with complete resection of the right inguinal mass. The intraoperative management metrics were as follows: ABP was maintained at 135–150/80–90 mmHg; HR, 65-75 beats/min; pure oxygen, 5 L/min (administered using a face mask under spontaneous breathing); SpO2 96–100%; RR, 16–18 beats/min; and BIS fluctuated between 65–80 (Figures 2 and 3). If the systolic BP decreased by > 20% of the basal value or < 100 mmHg, 4 µg of norepinephrine was administered; if the systolic BP increased by > 20% of the basal value or > 180 mmHg, 10–25 mg of uradil was administered; if the HR was < 50 beats/min, 0.3–0.5 mg of atropine was administered, and if the HR was > 100 beats/min, 0.5 mg/kg of esmolol was administered with repeat dosing if necessary. Remimazolam dosage was adjusted according to the BIS, maintaining the BIS fluctuating between 65 and 80 and avoiding too shallow or deep sedation.
The patient gained clear consciousness at 18:02; ABP was maintained between 136–148/80–85 mmHg, HR between 70 and 75 beats/min, with stable spontaneous respiration; SpO2 up to 96% under air inhalation; BIS between 92 and 96; no respiratory distress, nausea, or vomiting; and no complaints of incisional pain, injection pain, dizziness, headache, or other discomforts. The patient’s vital signs were stable for 30 min, and he returned to the ward at 18:32. He was discharged 6 d after surgery and followed up for 2 mo after the operation.
COPD remains one of the most common and deadly diseases, and is the third leading cause of death globally[1]. COPD is a progressive inflammatory disease characterized by persistent and irreversible airflow limitation (AL), and respiratory symptoms are usually associated with exposure to harmful particles or gases. Patients with COPD are prone to dyspnea due to AL during expiration and inspiration caused by pulmonary lesions or narrowing of the airways in the lungs, generally defined as AL, associated with small airway obstruction and low FEV. COPD is diagnosed in patients with chronic bronchitis and emphysema when AL is present and is not fully reversible[2]. COPD is usually accompanied by an abnormal decline in lung function, and chronic respiratory failure is the leading cause of death. Because patients with COPD have limited lung function, the severity of COPD should be carefully evaluated preoperatively, including comprehensive history, spirometry, and arterial blood gas analysis. Our patient had an FEV1/FVC ratio of 62% and an FEV1 of 1.10 L. As a percentage of the expected value, the measured value was 43.5%, AL was present, chest CT suggested emphysema, and the diagnosis of severe COPD was confirmed.
Surgical operations can cause stress, anxiety, and fear in patients, and hormonal and metabolic changes that affect the autonomic and immune systems of the body, leading to elevated BP, increased HR, elevated blood glucose, and enhanced protein and lipid metabolism and can even lead to postoperative myocardial injury and cognitive dysfunction, affecting the prognosis of patients[3]. In older patients with hypertension, these stress responses are more likely to lead to dramatic hemodynamic fluctuations, increase the risk of perioperative cardiovascular and cerebrovascular accidents, increase the incidence of postoperative cognitive dysfunction in older patients, and affect patient prognosis. Previous studies have demonstrated a strong link between stress responses and neuronal injury. Excessive inflammatory responses can be generated under intense stress, which in turn, can stimulate neurological injury. This cascade affects homeostasis and aggravates stress responses in patients. Subsequently, an excessive inflammatory response activates the oxidative stress response by expressing various catalytic enzymes[4]. This enhanced inflammatory response induces the acute exacerbation of COPD and aggravates its severity in COPD patients.
This case involved an older adult with hypertension grade III and severe COPD, ASA grade III; a 13 cm × 8 cm × 7 cm mass in the right inguinal region, hard and poorly mobile; and an enlarged right inguinal mass proposed for resection. Patients are at high risk because they are highly stressed after admission. The selection and management of the anesthesia protocol require extra attention to maintain circulatory stability and avoid drastic hemodynamic fluctuations; therefore, it is necessary to provide continuous and precise analgesia and stable sedation to minimize the stress response. As patients also suffer from severe COPD and have a poor pulmonary function, the selection and management of anesthesia should focus on avoiding COPD-triggering factors, reducing the occurrence of perioperative hypoxemia, minimizing the occurrence of perioperative cardiopulmonary disease complications, and improving patient prognosis. Therefore, accurate analgesia and moderate sedation are key to the successful management of this case. For this reason, the design of the anesthesia plan should ensure that the anesthesia method chosen can provide precise and continuous analgesia and can respond to the adjustment of the operation style and operation time; sedation treatment chosen should meet the needs of the patient and the operator while maintaining the stability of the patient's perioperative pulmonary circulation; anesthesia plan chosen should minimize the perioperative COPD triggering factors, avoid the acute exacerbation of COPD and aggravation of COPD, and minimize the perioperative pulmonary complications as the top priority; and strengthen the hemodynamic and depth of sedation monitoring to facilitate individualized and precise anesthesia management.
How do you receive good analgesia? Local, regional blocks, and general and intralesional anesthesia can provide analgesia for inguinal mass resection; however, each has its advantages and disadvantages. For smaller masses, this procedure is routinely performed under local anesthesia. In older patients, local anesthesia can maintain hemodynamic stability and voluntary respiratory function; reduce the risk of tracheal extubation, bronchoconstriction, and respiratory depression after general anesthesia; reduce the need for postoperative mechanical ventilation; reduce postoperative pulmonary complications; and facilitate the reduction of postoperative cognitive dysfunction. However, in this case, the inguinal mass was large, hard, and poorly mobile, and surgery was relatively difficult. Therefore, the analgesic effect of simple local anesthesia is unsatisfactory and significantly reduces patient comfort. Regional nerve block anesthesia slightly affects the body’s pulmonary circulation, but its blocking range is limited; maintaining a long operation time after a single injection is challenging, and it can easily cause tissue damage and complications during the puncture process. General anesthesia has the advantages of rapid onset, high comfort during anesthesia, and no memory of the whole procedure. However, it has a strong inhibitory effect on the patient’s central nervous system, which can easily lead to postoperative cognitive dysfunction and increase the incidence of delirium in older adult patients; general anesthetic drugs have a particular inhibitory effect on cardiovascular vessels. In older adults, owing to degenerative changes in the central nervous and cardiovascular systems, the elasticity of blood vessels becomes poor, which can easily cause hypotension; stimulation, such as tracheal intubation and extubation, can lead to violent circulatory fluctuations, and hemodynamics are very unstable under stress; when endotracheal intubation is performed under general anesthesia, which bypasses the defensive protection of the mouth and nose, the bronchus is directly connected to the outside world. The bacteria and secretions in the oral cavity can easily invade the respiratory tract. The gastrointestinal tract can be easily damaged by muscle relaxation or misuse. When general anesthesia is administered, the respiratory system is affected by using anesthetic drugs that inhibit the efficiency of postoperative sputum excretion and increase the probability of pulmonary infection[5]. Patients with advanced COPD experience adverse consequences following general anesthesia, tracheal intubation, and intermittent positive pressure ventilation. This increases the incidence of postoperative pulmonary complications and the risk of hypoxemia, laryngospasm, pneumatic injuries, bronchospasm, and compromised circulatory stability. In particular, circulatory fluctuations are more likely to occur in older patients with combined underlying diseases, which significantly increase the risk of cardiovascular complications.
Endotracheal anesthesia is advantageous in patients with COPD. However, subarachnoid block anesthesia can cause a series of physiological disturbances, the severity of which is closely related to the block level. A high block level can lead to respiratory and circulatory depression, causing severe hypotension and respiratory muscle paralysis, especially in older patients with hypertension and poor cardiopulmonary function. This makes it challenging to meet the need for prolonged surgery after a single injection. In contrast, epidural block anesthesia provides both definitive analgesia and mild effects on pulmonary circulation, and additional local anesthetics can be added as needed to accommodate prolonged surgery. The patient was diagnosed with an enlarged inguinal mass. Although the mass was superficial, it was large (13 cm × 8 cm × 7 cm), hard, and poorly mobile. Combined with the fact that this patient was an older adult with grade III hypertension and severe COPD, poor responsiveness to cardiopulmonary function, and weak self-replacement ability, considering the relative difficulty of the operation, the operation time might have been longer, and the operation technique might have to be changed intraoperatively. We concluded that epidural block anesthesia was more advantageous than other anesthetic modalities for resolving analgesia in this patient.
How can good sedation be achieved? Implementation of safe and effective sedation in older patients with hypertension and severe COPD should be considered. Sedation that is too deep increases the risk of respiratory depression, aggravates the degree of COPD, and increases the inhibition of circulation, which is unfavorable for older adult patients with combined hypertension. Sedation that is too shallow results in poor patient cooperation, and the patient is in a state of stress, which is not conducive to circulatory stability and can easily induce an acute COPD attack. Therefore, choosing the appropriate sedative drugs is crucial in maintaining an appropriate sedation depth and ensuring safe and effective sedation. Commonly used sedative drugs, such as propofol and midazolam, and the new drug, dexmedetomidine, have some problems with clinical application. Propofol has a rapid onset of action, short elimination half-life, rapid sleep after intravenous administration, rapid awakening, no post-awakening euphoria, and low incidence of postoperative nausea and vomiting. It can induce amnesia, without any behavioral impairment. However, it causes intravenous pain and can increase stress reactions due to injection pain; it has dose-dependent circulatory and respiratory depressant effects and can cause hypotension, hypoventilation, and even apnea[6]. Because this patient was an older adult with hypertension and severe COPD, propofol could have increased the risk of respiratory and circulatory depression, increased stress response, and precipitated an acute COPD attack. Midazolam is a drug representing the benzodiazepine with sedative, anxiolytic, anticonvulsant, and amnesic effects. However, midazolam may lead to the loss of airway reflexes, increase the risk of respiratory depression or aspiration, and induce delirium to some extent. Prolonged administration can lead to accumulation and delayed sedative effects, which must be antagonized by administering flumazenil, if necessary[7]. Here, we report the case of an older patient with a high risk of delirium who also had severe COPD and poor pulmonary function. The application of midazolam increased the difficulty of respiratory management. Dexmedetomidine is a highly specific alpha-2 agonist with anesthetic, analgesic, and antisympathetic properties. It exerts sedative and weak analgesic effects, without significant respiratory depression. It can produce a unique sedative effect similar to physiological sleep, making it the drug of choice for superficial sedation. However, it is prone to the side-effects of hypotension and bradycardia[8]. Continuous intraoperative intravenous infusions of larger doses of dexmedetomidine can significantly attenuate the body’s physiological regulatory response to hypovolemia[9], especially in older patients with hypertension and a vertebral canal. Furthermore, hypotension and sinus bradycardia are more likely to increase in older patients with hypertension and endovascular anesthesia. Additionally, the onset of action of dexmedetomidine is 10–15 min, which is slow and has a long elimination half-life and accumulation effect. Its application in older patients can prolong the action time and increase the safety risks of anesthesia.
Remimazolam is a new ultrashort-acting benzodiazepine, a derivative of midazolam, a drug obtained by introducing a metabolizable methyl propionate side chain to the structure of midazolam, which also acts on γ-aminobutyric acid type A receptors, exerts inhibitory effects on neurons and reduces neuronal excitability[10]. Remimazolam is metabolized by nonspecific plasma esterase hydrolysis and has no cumulative effects. Wiltshire et al[11] reported that the average peak blood concentration of 0.01–0.30 mg/kg of remimazolam can be reached in about 1 min with rapid metabolism. Continuous intravenous infusion of remimazolam for 2 h has a maximum half-life of 7–8 min, and flumazenil rapidly reverses its sedative effect. Compared with propofol, remimazolam has a rapid onset of action but does not cause significant respiratory and circulatory depression. Remimazolam has a faster onset of action, stronger sedation, and shorter recovery time. Previous studies have demonstrated that remimazolam is safe for sedation in patients undergoing gastrointestinal endoscopy, with minimal effects on the pulmonary circulation. According to clinical observations, the incidence of hypotension and hypoxemia due to remimazolam administration is extremely low. In addition to its sedative effects, recent animal experiments have suggested that remimazolam also has anti-inflammatory effects. Liu et al[12] first found that remimazolam inhibited mitogen-activated protein kinase and phosphoinositide 3-kinase signaling pathway activation and interfered with TLR4 expression associated with Rab5a in the cell surface response to lipopolysaccharides, attenuating the inflammatory response in mouse macrophages. Fang et al[13] reported that remimazolam acts dose-dependently by activating peripheral ben-zodiazepine receptors and inhibiting macrophage p38 phosphorylation, attenuating the inflammatory response in rats with acute liver injury associated with sepsis. These animal studies broadened our understanding of the pharmacological effects of remimazolam, suggesting that it may have anti-inflammatory effects in clinical settings. However, further clinical trials are required to confirm our findings. Future studies on the anti-inflammatory effects of remimazolam in a clinical setting are needed to investigate whether its anti-inflammatory effects can reduce the triggers of acute exacerbations in patients with COPD and produce a more positive effect on perioperative sedation in such patients.
The administration of remimazolam with epidural block anesthesia in older patients with hypertension and severe COPD has not yet been reported. A well-controlled sedative drug is more beneficial for this patient because the intraoperative dosage of sedative drugs can be regulated according to BIS monitoring, especially when intraoperative sedation is too deep and sudden respiratory depression occurs. It is easy to reduce the anesthesia depth immediately and wake up the patient at any time. The administration of effective antagonist drugs can reverse the sedative effect at any time, which was the best choice for this patient. Therefore, the pharmacological effects of remimazolam are theoretically beneficial.
The anesthetic protocol used in the present case involved remimazolam sedation combined with an epidural block. Immediately after entering the room and connecting to the intravenous access, 2 mg remimazolam was injected intravenously to sedate the patient. After testing the level of the anesthetic block, 5 mg remimazolam was added to relieve the patient’s nervousness, reduce the stress response, and stabilize pulmonary circulation. Invasive arterial BP and BIS monitoring were performed to facilitate individualized circulation management and regulation of sedation depth. Epidural block anesthesia was selected for the L1-2 interval. A trial dose of 3 mL of 2% lidocaine was injected, and an additional 2% lidocaine (6.5 mL was added after the anesthetic level was determined). Five milliliters of 2% lidocaine were administered epidurally 50 min after epidural block anesthesia, which induced definite analgesia. Continuous intraoperative intravenous infusion of remimazolam (40 mg/h) using a micropump ensured stable intraoperative sedation. Intraoperatively, the patient’s vital signs were stable, with BP maintained at 135–150/80–90 mmHg, HR at 65–75 beats/min, SpO2 between 96% and 100%, and BIS fluctuating between 65 and 80 beats/min. Intraoperative arterial blood gas metrics were as follows: FiO2, 0.40; pH, 7.40; PaCO2, 41.1 mmHg; and PaO2, 288.8 mmHg. The operative time was 90 min, and the intravenous infusion of remimazolam was stopped at the end of the operation. The patient gained clear consciousness 2 min after surgery, with a BIS score of 92, stable spontaneous breathing, and stable circulation. Arterial blood gas metrics at the end of the operation were as follows: FiO2, 0.21; pH, 7.40; PaCO2, 39.2 mmHg; and PaO2, 139.9 mmHg. After 30 min of observation, the patient’s vital signs were stable, and he returned to the ward. His vital signs stabilized after returning to the room. Airway tools and vasoactive drugs prepared before the induction of anesthesia were not used. Upon returning to the ward, the BP was 147/74 mmHg, HR was 70 beats/min, and SpO2 was 99%. No postoperative anesthesia-related complications were observed. The patient recovered well and was discharged. At the 2-mo postoperative follow-up, the patient did not complain of discomfort. In this case, the successful application of remimazolam sedation and epidural block anesthesia, ensured perioperative safety and provided comfortable anesthesia (Table 1).
| Item | Timeline | |
| Preoperative | 1 | Discovery of right inguinal mass for > 7 mo |
| 2 | History of hypertension for > 7 years | |
| 3 | Pulmonary function was reported to have severe obstructive ventilation dysfunction, mildly reduced total diffusion function, severely increased peripheral airway resistance, abnormal small airway function, and severely increased pulmonary elastic resistance | |
| 4 | The operation was performed under remimazolam sedation combined with epidural block anesthesia | |
| Perioperative | 5 | The patient was admitted to the room at 15:40. The cuff blood pressure, electrocardiograph, saturation of pulse oximetry, and bispectral index was monitored |
| 6 | Invasive blood pressure was monitored and arterial blood gas analysis was conducted. Sedation was provided with 2 mg remimazolam | |
| 7 | Induction of anesthesia: epidural puncture and tube placement were performed at 16:00 | |
| 8 | At 16:25, the level of anesthesia block was determined again, and 5 mg remimazolam was injected to sedate the patient | |
| 9 | Surgery was initiated at 16:30 | |
| 10 | To ensure stable intraoperative sedation, 40 mg/h remimazolam was infused intravenously using a micropump. At 17:05, 5 mL of 2% lidocaine was injected epidurally | |
| 11 | At 18:00, the operation was completed | |
| Postoperative | 12 | The patient gained clear consciousness at 18:02, the patient’s vital signs were stable |
| 13 | The patient’s vital signs were stable for 30 min, and he was returned to the ward at 18:32 | |
| 14 | The patient was discharged 6 d after surgery | |
| 15 | The patient was followed up 2 mo after the operation | |
In conclusion, in the case of an older patient with hypertension and severe COPD who underwent inguinal mass resection, we chose regimazolam sedation combined with an epidural block anesthesia protocol. Under BIS monitoring, a single dose of regimazolam was administered preoperatively, and a continuous intravenous infusion of regimazolam was used intraoperatively, which provided the patient with continuous and stable intraoperative sedation and definitive analgesia, preserving perioperative safety, meeting the patient's comfort needs, and ensuring the successful completion of the surgery. This is a case report, and further clinical trials are required. We expect that the superior pharmacological effects of remimazolam will provide new ideas for developing protocols to induce comfortable anesthesia in such patients.
Remimazolam sedation combined with an epidural block is safe and effective in older adult patients with hypertension and severe COPD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Han JH, South Korea S-Editor: Liu XF L-Editor: Filipodia P-Editor: Chen YX
| 1. | Sandelowsky H, Weinreich UM, Aarli BB, Sundh J, Høines K, Stratelis G, Løkke A, Janson C, Jensen C, Larsson K. COPD - do the right thing. BMC Fam Pract. 2021;22:244. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Fazleen A, Wilkinson T. Early COPD: current evidence for diagnosis and management. Ther Adv Respir Dis. 2020;14:1753466620942128. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Xiong MY, Fu TT, Liu JD, Wang XH, Tao Z, Chen SB. Effect of serratus anterior plane block on stress response and postoperative analgesia in patients undergoing thoracoscopic cardiac surgery under cardiopulmonary bypass. J Clin Anesthesiol. 2022;38:1066-1070. [Cited in This Article: ] |
| 4. | Fang Q, Wang YL, Zhang ZZ, Wang HY, Luo H, Song XM. Correlation between different anesthesia methods and outcomes after hip replacement in elderly patients. J Clin Anesthesiol. 2020;36:971-974. [DOI] [Cited in This Article: ] |
| 5. | Chen W, Chen JJ, Kong JH. Effects of transversus abdominis plane block combined with intravenous injection of hydromorphone on stress response and postoperative analgesia in patients undergoing laparoscopic colorectal cancer surgery. J Clin Anesthesiol. 2022;38:1025-1030. [DOI] [Cited in This Article: ] |
| 6. | Veselis RA, Pryor KO, Reinsel RA, Mehta M, Pan H, Johnson R Jr. Low-dose propofol-induced amnesia is not due to a failure of encoding: left inferior prefrontal cortex is still active. Anesthesiology. 2008;109:213-224. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | DAS-Taskforce 2015, Baron R, Binder A, Biniek R, Braune S, Buerkle H, Dall P, Demirakca S, Eckardt R, Eggers V, Eichler I, Fietze I, Freys S, Fründ A, Garten L, Gohrbandt B, Harth I, Hartl W, Heppner HJ, Horter J, Huth R, Janssens U, Jungk C, Kaeuper KM, Kessler P, Kleinschmidt S, Kochanek M, Kumpf M, Meiser A, Mueller A, Orth M, Putensen C, Roth B, Schaefer M, Schaefers R, Schellongowski P, Schindler M, Schmitt R, Scholz J, Schroeder S, Schwarzmann G, Spies C, Stingele R, Tonner P, Trieschmann U, Tryba M, Wappler F, Waydhas C, Weiss B, Weisshaar G. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. Ger Med Sci. 2015;13:Doc19. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Bailard NS, Ortiz J, Flores RA. Additives to local anesthetics for peripheral nerve blocks: Evidence, limitations, and recommendations. Am J Health Syst Pharm. 2014;71:373-385. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Wu XM, Xue ZG, Ma H, Wang G, Shi XY, Huang SQ, Li WZ, Wang TL, Li LH, Zhang MZ, Yu WF, Li J, Huang WQ. Expert consensus on the clinical application of dexmedetomidine (2018). J Clin Anesthesiol. 2018;34:820-823. [DOI] [Cited in This Article: ] |
| 10. | Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33:506-511. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115:284-296. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Liu X, Lin S, Zhong Y, Shen J, Zhang X, Luo S, Huang L, Zhang L, Zhou S, Tang J. Remimazolam Protects Against LPS-Induced Endotoxicity Improving Survival of Endotoxemia Mice. Front Pharmacol. 2021;12:739603. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Fang H, Zhang Y, Wang J, Li L, An S, Huang Q, Chen Z, Yang H, Wu J, Zeng Z. Remimazolam reduces sepsis-associated acute liver injury by activation of peripheral benzodiazepine receptors and p38 inhibition of macrophages. Int Immunopharmacol. 2021;101:108331. [PubMed] [DOI] [Cited in This Article: ] |