Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3323
Peer-review started: February 13, 2023
First decision: March 14, 2023
Revised: March 22, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 16, 2023
Left ventricular thrombus is a rare condition, for which appropriate treatments are not extensively studied. Although it can be treated by thrombectomy, such surgery can be difficult and risky, and not every patient can tolerate the surgery.
We report a case of a middle-aged man receiving veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for acute myocardial infarction who developed left ventricular thrombus despite systemic anticoagulation. After systemic thrombolysis with urokinase, the left ventricular thrombus disappeared, ECMO was successfully withdrawn 9 days later, and the patient recovered and was discharged from hospital.
Systemic thrombolysis is a treatment option for left ventricular thrombus in addition to anticoagulation and thrombectomy.
Core Tip: Urokinase is a useful medicine for thrombolysis. This is the first case report of urokinase administration for left ventricular thrombus occurring during veno-arterial extracorporeal membrane oxygenation therapy. Systemic thrombolytic therapy with urokinase can be considered when the effect of anticoagulant therapy is poor or the risk of surgical treatment is high after left ventricular thrombus formation.
- Citation: Wang YD, Lin JF, Huang XY, Han XD. Successful treatment of veno-arterial extracorporeal membrane oxygenation complicated with left ventricular thrombus by intravenous thrombolysis: A case report. World J Clin Cases 2023; 11(14): 3323-3329
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3323.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3323
Extracorporeal membrane oxygenation (ECMO) is an important treatment for severe cardiopulmonary insufficiency. In recent years, particularly after coronavirus disease 2019, ECMO has rapidly developed in China. Recently, ECMO has become more reliable due to improvements in equipment and increased experience, which is reflected in improved results[1]. However, many complications can occur during ECMO treatment, such as bleeding and thrombosis. Of these complications, intracardiac thrombosis is rare but can be life-threatening. For intracardiac thrombosis, the current guidelines and expert consensus recommend anticoagulation and thrombectomy, but there are no reports on intravenous thrombolysis. We report a case of successful intravenous thrombolysis after left ventricular thrombus which occurred during VA-ECMO therapy.
The patient had undergone cardiopulmonary resuscitation twice in half a day.
A 48-year-old man who suffered from persistent angina pectoris for 2 h was diagnosed with acute extensive anterior wall myocardial infarction in a local hospital on September 19, 2022. Coronary angiography showed total occlusion of the left main ostium. Following implantation of a Medtronic 3.0 mm × 2.6 mm drug-eluting stent (10 atm, 10 s) in the left anterior descending artery, the blood flow returned to TIMI3. The patient was then admitted to the intensive care unit (ICU). Sudden cardiac arrest occurred twice in the ICU, and cardiopulmonary resuscitation was given. The patient required 15 min resuscitation during the first arrest, and 5 min resuscitation during the second arrest. However, his blood pressure was extremely unstable, and the dose of norepinephrine was raised to 120 µg/min. After consultation, VA-ECMO treatment was initiated. The speed of the ECMO centrifugal pump was 3500 rpm, the air flow rate was 2.5 L/min, the oxygen concentration was 100%, and the blood flow rate was approximately 2.4 L/min. His arterial blood pressure increased to 125/114 mmHg, and the dose of norepinephrine was decreased to 40 µg/min. The patient was then transferred to our department.
The patient had no previous medical history.
All family members were healthy and denied any history of genetic disease and genetic predisposition.
The patient’s temperature was 36℃, heart rate was 86 bpm, blood pressure was 125/114 mmHg with norepinephrine 40 µg/min, and respiratory rate was 15 breaths/min. The patient was in a state of subcoma, moist rales were heard in both lungs, arrhythmia was identified, heart sounds were low and weak, and bowel sounds were not heard.
Laboratory examinations indicated leucocytosis (white blood cell count, 15.96 × 109/L); glutamic oxaloacetic transaminase 906 U/L; cardiac troponin I 50 ng/mL; N-Terminal pro-brain natriuretic peptide 2000 pg/mL.
Echocardiography revealed segmental wall dyskinesia and hypofunction of the left heart.
Acute extensive anterior wall myocardial infarction and cardiogenic shock.
He was given 100 mg/d aspirin, 180 mg/d ticagrelor and 600 U/h heparin. Bedside echocardiography showed that systolic function of the left ventricle was extremely poor, there was no obvious thrombus in the left ventricle, and the patient’s blood was in a turbulent state. The mean arterial pressure was 72 mmHg when the dose of norepinephrine was 20 µg/min and the blood flow rate was 3.2 L/min. The active partial thromboplastin time (APTT) was 54.6 s.
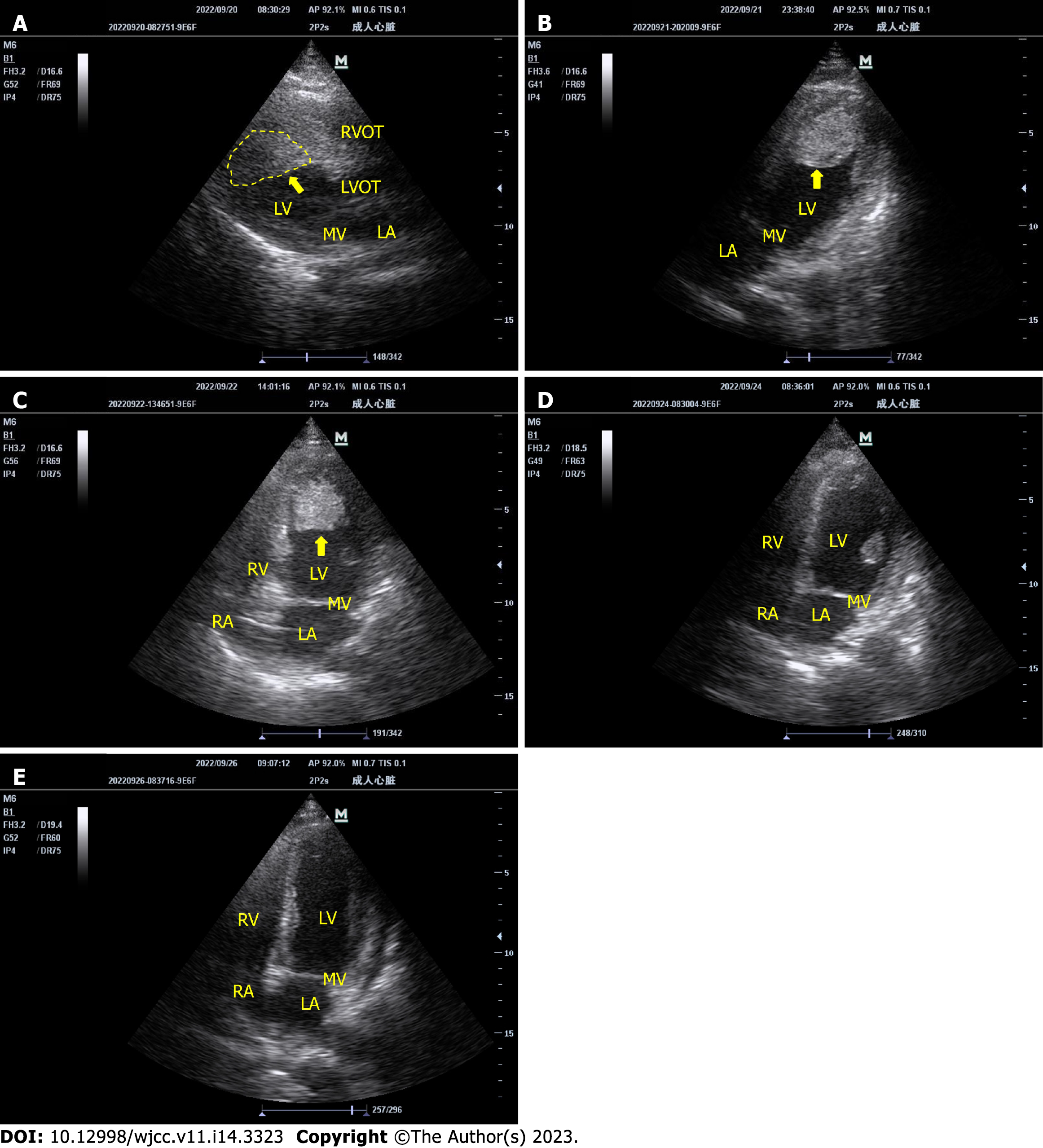
Eight hours later, bedside echocardiography showed a large left ventricular thrombus (Figure 1A). However, the Antithrombin-Ⅲ was only 46.6%, which did not improve after plasma infusion. We administered argatroban 1 mg/h for anticoagulation instead of heparin. After one day of treatment, bedside echocardiography showed that the thrombus had not decreased (Figure 1B). We added urokinase 50000 U/h for thrombolysis. Six hours later, there was no obvious bleeding sign, and the dosage of urokinase was increased to 100000 U/h. Twenty-four hours after thrombolysis, the thromboelastogram showed that the patient’s R time was 10.3 min, K time was 6.2 min, alpha angle was 50.9°, max amplitude was 35.2 mm, and LY30 was 0.0%. Thirty-four hours after thrombolysis, blood leakage increased at the ECMO catheter in the femoral artery. The dose of argatroban was reduced to 0.6 mg/h, the dose of urokinase was reduced to 50000 U/h, and local compression was added to stop the bleeding. However, the bleeding was not controlled. Therefore, urokinase was stopped 46 h after initiation, and the total dose of urokinase was 4.6 million U (changes in the patient’s coagulation function during thrombolysis are shown in Table 1). During thrombolysis, the left ventricular thrombus gradually decreased (Figure 1C) and then disappeared on the second day after urokinase was stopped (Figure 1D and E). No signs of thromboembolism were found on head, chest and abdominal computed tomography (CT). We successfully removed ECMO on the ninth day.
| Thrombolysis time | -8 h | 0 h | 8 h | 16 h | 24 h | 32 h | 40 h | 48 h | 56 h | 64 h |
| PT (s) | 24 | 16.5 | 20.4 | 19.7 | Null | 19.9 | 21 | 14 | 18.5 | 19.5 |
| APTT (s) | 64.1 | 59.9 | 40.8 | 50.9 | Null | 55.3 | 53.1 | 44.1 | 56.1 | 51.3 |
| FIB (g/L) | 3.4 | 3.55 | 4.18 | 4.34 | Null | 3.3 | 2.69 | 1.92 | 2.37 | 2.72 |
| AT-Ⅲ (%) | 46.6 | 48.1 | 49.9 | 56 | Null | 66.9 | 62.1 | 59.2 | 70.7 | 68.1 |
| FDP (mg/L) | 18 | 8.21 | 16.1 | 34.1 | Null | 129.2 | 184.4 | 365.3 | 141.8 | 99 |
| D-D (mg/L) | 6.27 | 2.59 | 7.61 | 18.55 | Null | 53.28 | 75.92 | 85.36 | 55.58 | 36.67 |
One month later, the left anterior descending branch stent was found to be blocked, the stent was re-inserted, and the patient was successfully discharged from hospital with New York Heart Association class II-III.
VA-ECMO provides good hemodynamic support for adults with cardiogenic shock. However, thrombosis is an important complication during VA-ECMO support and can occur in lines, oxygenators, pumps, and ventricles. The reported incidence of these complications is as high as 17%[2]. Among them, left ventricular thrombus is a serious complication. According to the registration report of Extracorporeal Life Support Organization in 2017, left ventricular thrombus accounts for 5%-6% of all complications of VA-ECMO. Left ventricular thrombus leads to increased mortality.
The formation of left ventricular thrombus in ECMO is mainly related to the primary disease (such as myocardial infarction)[3], decreased myocardial contractility[4], critical illness, sedation, frequent blood transfusion[5], ECMO tubing and the membrane oxygenator in contact with blood leading to fibrinogen and thrombin activation[6], etc. The patient in this report had the abovementioned high-risk factors for thrombosis. At the same time, the patient's antithrombin-Ⅲ (AT-III) was only 40%-60%, and the anticoagulant efficiency of heparin decreased[7], which eventually led to the formation of a left ventricular thrombus.
The fundamental way to prevent left ventricular thrombus formation is anticoagulant therapy and the prevention of left ventricular dilatation[8]. However, there are few guidelines for the treatment of left ventricular thrombus. At present, the guidelines of the American Heart Association (AHA)[9], American Stroke Association and American College of Cardiology (ACC)[10] mainly recommend anticoagulation. In this case, heparin was initially administered for anticoagulation. However, the patient’s AT-III was low, and did not increase significantly after plasma transfusion. At the same time, plasma transfusion may bring the risk of coagulation. According to the literature reports, 27% of patients developed intracardiac thrombus after plasma transfusion[11]. Therefore, in this case, argatroban anticoagulation was administered when left ventricular thrombus occurred, but anticoagulation alone failed to decrease the thrombus.
There are also many reports on surgical thrombectomy or thrombus removal[12,13]. However, this approach exposes patients to additional surgical risks and further complications[14]. Ventriculotomy negatively affects the contractility of an already failing heart[15], and anticoagulation after surgery is a huge challenge. Installing a left ventricular decompression device such as the Impella reduces thrombosis by decreasing blood stasis in the left ventricle[16]. Use of the AngioVac system (AngioVac®) to remove ECMO-associated ventricular thrombi has also been reported[17]. Local thrombolysis in the left ventricle has been performed using a left ventricular decompression tube[18], and the thrombus was even removed from the cut aorta through a bronchoscope[15].
At present, there is no convincing evidence in the guidelines that a thrombolytic drug is superior to other drugs[19]. The best thrombolytic agent is still under debate[20]. At present, in most infants and young children, systemic thrombolysis is used to treat ECMO-related left ventricular thrombus[18,21]. The thrombolytic drug used is alteplase. There are few reports on the application of urokinase in left ventricular thrombus. Although the advantages of alteplase have become increasingly prominent in recent years, clinicians have a better understanding of the dose, time, safety and efficacy of urokinase[19]. It has been reported that compared with alteplase, urokinase has a higher thrombus lysis rate[22], lower bleeding risk and better safety[23]. In the meta-analysis conducted by Kharel et al[24] it was shown that the efficacy and safety of urokinase in thrombolysis were not inferior to those of alteplase. The 2019 Chinese Stroke Association guidelines indicate that urokinase is still a safe and effective thrombolytic agent[25]. Therefore, in this case, urokinase was used for thrombolysis.
Urokinase is extracted from human urine and is without antigenicity. It acts directly on the endogenous fibrinolytic system to drive the conversion of fibrinogen into fibrinolytic enzyme, thereby rapidly dissolving fresh thrombi. Following 24 h of thrombolytic therapy in our patient, the thromboelastogram showed a hypocoagulable state, and bedside echocardiography showed that the thrombus had decreased. At the same time, fibrinogen degradation product and D dimer initially increased and then decreased, while fibrinogen did not significantly decrease. As there was thrombus dissolution at this time, rather than coagulation activation, thrombolysis was considered effective. However, 34 h after thrombolysis, hemorrhage occurred at the catheter site. Following treatment measures such as local compression which were ineffective, urokinase was stopped, argatroban anticoagulation was continued, and the bleeding was controlled. Seventy-two hours after thrombolytic therapy, the left ventricular thrombus had disappeared. At that time, we were also worried that the thrombus would break off and cause embolism in other areas. However, no clinical manifestations of embolism were observed, and no obvious signs of vascular embolism were seen on head, chest and abdominal CT. The patient recovered and was discharged from hospital.
The cornerstone of left ventricular thrombosis prevention in ECMO is systemic anticoagulation and prevention of left ventricular dilation. Systemic thrombolysis with urokinase is a treatment option following the formation of left ventricular thrombus.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Emam AMA, Saudi Arabia; Bonacchi M, Italy S-Editor: Liu JH L-Editor: A P-Editor: Chen YX
| 1. | Makdisi G, Hashmi ZA, Wozniak TC, Wang IW. Left ventricular thrombus associated with arteriovenous extra corporeal membrane oxygenation. J Thorac Dis. 2015;7:E552-E554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 2. | Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, Davis AK. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015;29:90-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 3. | Rabbani LE, Waksmonski C, Iqbal SN, Stant J, Sciacca R, Apfelbaum M, Sayan OR, Giglio J, Homma S. Determinants of left ventricular thrombus formation after primary percutaneous coronary intervention for anterior wall myocardial infarction. J Thromb Thrombolysis. 2008;25:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Lee KS, Jung Y, Jeong IS, Song SY, Na KJ, Oh SG. Acute biological mitral valve thrombosis after the left atrial venting in a patient with a venoarterial extracorporeal membrane oxygenator. J Card Surg. 2022;37:437-439. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Doyle AJ, Hunt BJ. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. Front Med (Lausanne). 2018;5:352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Chen T, Yao L, Fan X, Zhu C. Massive hollow catheter thrombus in venovenous extracorporeal membrane oxygenation assisted lung transplantation: A case report. Medicine (Baltimore). 2021;100:e24235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Sievert A, Uber W, Laws S, Cochran J. Improvement in long-term ECMO by detailed monitoring of anticoagulation: a case report. Perfusion. 2011;26:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Takei Y, Ejima Y, Toyama H, Takei K, Ota T, Yamauchi M. A case of a giant cell myocarditis that developed massive left ventricular thrombus during percutaneous cardiopulmonary support. JA Clin Rep. 2016;2:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 507] [Cited by in F6Publishing: 1050] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 10. | Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 958] [Article Influence: 319.3] [Reference Citation Analysis (0)] |
| 11. | Williams B, Wehman B, Mazzeffi MA, Odonkor P, Harris RL, Kon Z, Tanaka KA. Acute Intracardiac Thrombosis and Pulmonary Thromboembolism After Cardiopulmonary Bypass: A Systematic Review of Reported Cas. Anesth Analg. 2018;126:425-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Alhussein M, Moayedi Y, Posada JD, Ross H, Hickey E, Rao V, Billia F. Ventricular Thrombosis Post-Venoarterial Extracorporeal Membrane Oxygenation. Circ Heart Fail. 2017;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Kim BJ, Song SH, Shin YR, Park HK, Park YH, Shin HJ. Intracardiac Thrombosis Involving All Four Cardiac Chambers after Extracardiac Membranous Oxygenation Associated with MTHFR Mutations. Korean J Thorac Cardiovasc Surg. 2016;49:207-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Ogawa S, Richardson JE, Sakai T, Ide M, Tanaka KA. High mortality associated with intracardiac and intrapulmonary thromboses after cardiopulmonary bypass. J Anesth. 2012;26:9-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Perri JL, Wieselthaler GM. Left ventricular thrombus with extracorporeal membrane oxygenation: Novel technique of bronchoscope-guided thrombus retrieval. JTCVS Tech. 2022;15:130-132. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 16. | Imada T, Shibata SC, Okitsu K, Fujino Y. Unexpected bioprosthetic mitral valve thrombus during left ventricular assist device implantation. JA Clin Rep. 2017;3:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Griffith KE, Jenkins E, Copenhaver W, Williams DM. Novel use of the AngioVac® system to remove thrombus during simultaneous extracorporeal membrane oxygenation life support. Perfusion. 2016;31:164-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Sangalli F, Greco G, Galbiati L, Formica F, Calcinati S, Avalli L. Regional thrombolysis with tenecteplase during extracorporeal membrane oxygenation: a new approach for left ventricular thrombosis. J Card Surg. 2015;30:541-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Gong M, He X, Song J, Zhao B, Shi W, Chen G, Gu J. Catheter-Directed Thrombolysis With a Continuous Infusion of Low-Dose Alteplase for Subacute Proximal Venous Thrombosis: Efficacy and Safety Compared to Urokinase. Clin Appl Thromb Hemost. 2018;24:1333-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Righini M, Le Gal G, Bounameaux H. Venous thromboembolism diagnosis: unresolved issues. Thromb Haemost. 2015;113:1184-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Gunnarsson B, Heard CM, Martin DJ, Brecher ML, Steinhorn RH. Successful lysis of an obstructive aortic and renal artery thrombus in a neonate on extracorporeal membrane oxygenation. J Perinatol. 2000;20:555-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Keric N, Döbel M, Krenzlin H, Kurz E, Tanyildizi Y, Heimann A, König J, Kempski O, Ringel F, Masomi-Bornwasser J. Comparative analysis of fibrinolytic properties of Alteplase, Tenecteplase and Urokinase in an in vitro clot model of intracerebral haemorrhage. J Stroke Cerebrovasc Dis. 2020;29:105073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Bao H, Gao HR, Pan ML, Zhao L, Sun HB. Comparative study on the efficacy and safety of alteplase and urokinase in the treatment of acute cerebral infarction. Technol Health Care. 2021;29:85-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Kharel S, Nepal G, Joshi PR, Yadav JK, Shrestha TM. Safety and efficacy of low-cost alternative urokinase in acute ischemic stroke: A systematic review and meta-analysis. J Clin Neurosci. 2022;106:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 25. | Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, Xu W, Huang L, Zheng H, Liu J, Liu H, Wei Y, Xu J, Wang Y; Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. 2020;5:159-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |