Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12395
Peer-review started: August 21, 2022
First decision: September 26, 2022
Revised: September 28, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: November 26, 2022
Nuclear protein in testis (NUT) carcinoma is a rare aggressive malignant epithelial cell tumor, previously known as NUT midline carcinoma (NMC), characterized by an acquired rearrangement of the gene encoding NUT on chromosome 15q14. Due to the lack of characteristic pathological features, it is often underdiagnosed and misdiagnosed. A variety of methods can be used to diagnose NMC, including immunohistochemistry, karyotyping, fluorescence in situ hybridization, reverse transcription-polymerase chain reaction, and next-generation sequencing. So far, there is no standard treatment plan for NMC and the prognosis is poor, related to its rapid progression, easy recurrence, and unsatisfactory treatment outcome.
A 58-year-old female came to our hospital with a complaint of eye swelling and pain for 8 d. The diagnosis of NMC was confirmed after postoperative pathology and genetic testing. The patient developed nausea and vomiting, headache, and loss of vision in both eyes to blindness after surgery. Magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT) performed after 1.5 mo postoperatively suggested tumor recurrence. The patient obtained remission after radiation therapy to some extent and after initial treatment with anti-angiogenic drugs and sonodynamic therapy (SDT), but cannot achieve long-term stability and eventually developed distant metastases, with an overall survival of only 17 mo.
For patients with rapidly progressing sinus tumors and poor response to initial treatment, the possibility of NMC should be considered and immunohistochemical staining with anti-NUT should be performed as soon as possible, combined with genetic testing if necessary. CT, MRI, and PET/CT imaging are essential for the staging, management, treatment response assessment and monitoring of NMC. This case is the first attempt to apply heat therapy and SDT in the treatment of NMC, unfortunately, the prognosis remained poor.
Core Tip: This case demonstrates the aggressive and recurrence-prone biological behavior of nuclear protein in testis midline carcinoma (NMC). A variety of methods can be used to diagnose NMC, including immunohistochemistry, karyotyping, fluorescence in situ hybridization, reverse transcription-polymerase chain reaction, and next-generation sequencing. The patient achieved some degree of remission after radiation therapy and after initial treatment with anti-angiogenic drugs and sonodynamic therapy (SDT), but cannot achieve long-term stability and eventually developed distant metastases, with an overall survival of only 17 mo. This case is the first attempt to apply heat therapy and SDT in the treatment of NMC. Computed tomography (CT), Magnetic resonance imaging, and positron emission tomography/CT imaging are essential for the staging, management, treatment response assessment and monitoring of NMC.
- Citation: Huang WP, Gao G, Qiu YK, Yang Q, Song LL, Chen Z, Gao JB, Kang L. Multimodality imaging and treatment of paranasal sinuses nuclear protein in testis carcinoma: A case report. World J Clin Cases 2022; 10(33): 12395-12403
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12395.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12395
Nuclear protein in testis (NUT) carcinoma is a rare aggressive malignant epithelial cell tumor, previously known as NUT midline carcinoma (NMC), characterized by an acquired rearrangement of the gene encoding NUT on chromosome 15q14[1]. NMC is most common in children and adolescents and is found in the mediastinum, head and neck and other midline anatomical structures but can also involve the lungs, kidneys, bladder, ilium and other nonmidline sites[2,3]. Due to the lack of characteristic histopathological features, it is often under- and misdiagnosed.
Here, we report the treatment of a patient with paranasal sinus NMC who developed tumor recurrence soon after surgical resection, achieved some degree of remission after radiation therapy and initial treatment with antiangiogenic drugs and sonodynamic therapy (SDT), but never achieved long-term stability and eventually developed distant metastases, with an overall survival of only 17 mo. Heat therapy uses the biothermal effect of nonionizing radiation physical factors to heat up the tissue and kill the tumor tissue or promote the apoptosis of tumor cells[4]. SDT is a noninvasive anticancer treatment method using chemical sonosensitizers and high-intensity focused ultrasound, by which cancerous tissue is destroyed or denatured using a concentrated, high-intensity ultrasound beam[5]. This case is the first attempt to apply heat therapy and SDT in the treatment of NMC.
A 58-year-old female came to our hospital with a complaint of eye swelling and pain for 8 d.
Patient has not undergone any treatment and has no other symptoms.
No special circumstances.
The patient had no family history of hereditary diseases.
No obvious abnormal physical examinations.
Laboratory test results showed that the leukocyte count was 13.21 × 109/L (normal range, 4-10 × 109/L); the neutrophil count 10.78 × 109/L (normal range, 1.8-6.3 × 109/L); D-dimer, 0.65 mg/L (normal range, 0-0.55 mg/L); C-reactive protein, 48.31 mg/L (normal range, 0-5 mg/L); no significant abnormalities in tumor markers.
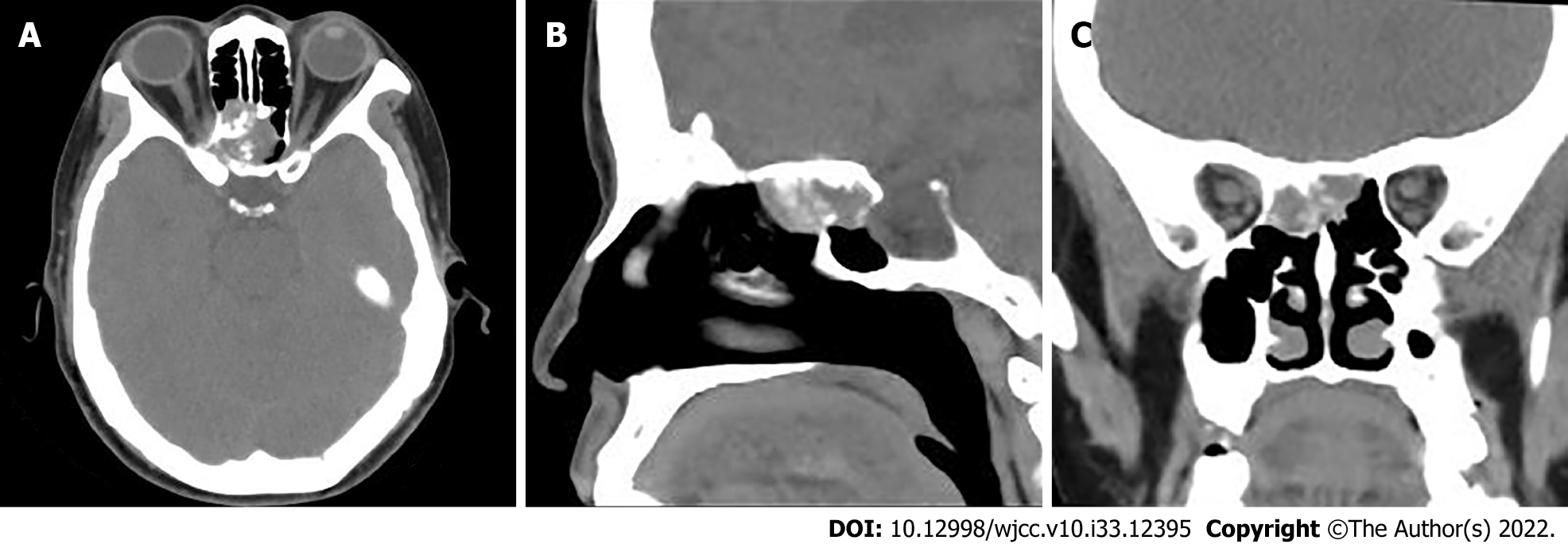
The patient is placed in the supine position for computed tomography (CT) scans. The scanning range was from the inferior orbital wall with the tube voltage at 120 kV and the tube current using the automatic mA technique. CT revealed hypodense masses in the bilateral ethmoid and sphenoid sinuses, with irregular morphology and the plain CT attenuation value of the mass was approximately 44 HU, with a maximum cross-section of about 2.5 cm × 1.8 cm, and osteolytic destruction of surrounding bone (Figure 1). Magnetic resonance imaging (MRI) displayed a mixed-signal mass in the bilateral ethmoid and sphenoid sinuses, with a heterogeneous low signal on T1-weighted imaging (T1WI), a slightly high signal on T2-weighted imaging (T2WI), a slightly low signal on diffusion weighted image. After enhancement, the lesion showed obvious heterogeneous enhancement, with indistinct demarcation with the right internal rectus muscle and shallowing of the right pharyngeal fossa, measuring approximately 2.3 cm × 2.9 cm × 1.3 cm (AP × LR × SI) (Figure 2).
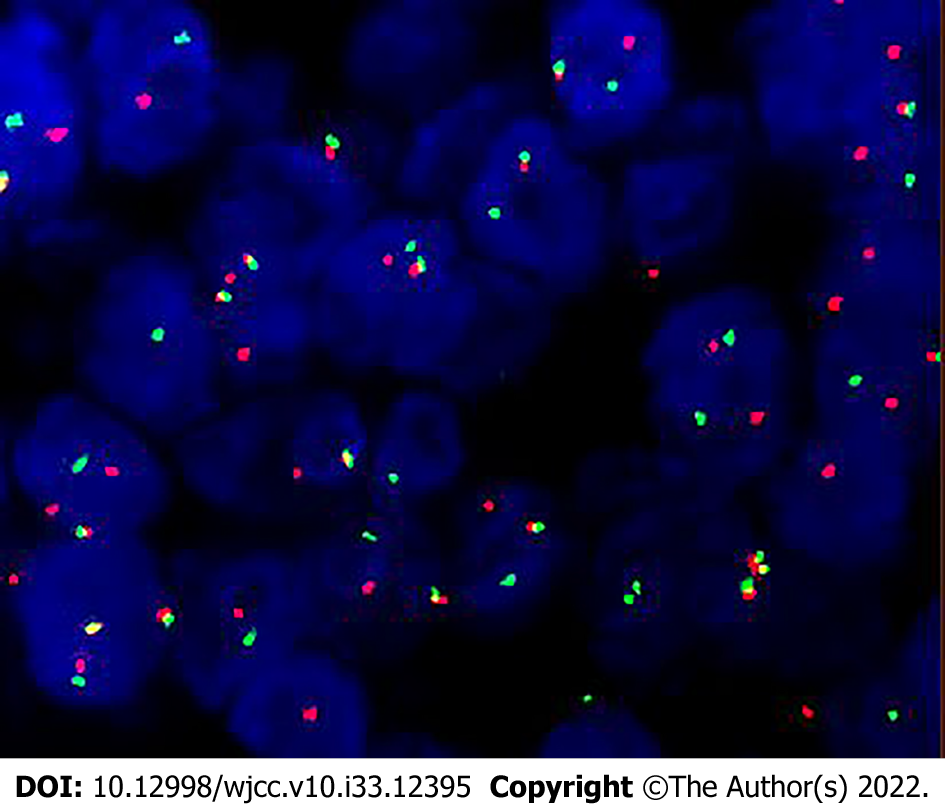
Ultimately, histopathology after surgery showed that diffuse distribution of undifferentiated round tumor cells, lamellar or focal arrangement, submucosal infiltrative growth, visible hemorrhage, necrosis, and invasion of bone. The nuclei were relatively uniform in size, but the karyotype was irregular, heterogeneity was obvious, nucleoli were obvious, nuclear fission images were easily seen, focal eosinophilic apoptotic vesicles were visible, cytoplasm was sparse, and localized squamous epithelial differentiation was visible. Immunohistochemical staining was positive for NUT, AR1/AE3, CK5/6, P63, P40, CK8/18, EGFR and negative for CD56, Syn, SOX-10, S-100, SALL-4, and MPO. NUT isolated probe fluorescence in situ hybridization (FISH): Positive (+), red and green signal separation in the tumor nucleus, suggesting a break in the gene. Combined with immunohistochemistry and FISH results, the diagnosis of NUT carcinoma was made (Figures 3 and 4).
The patient underwent nasal endoscopic excision of a sinus mass under general anesthesia, and the mass was seen to be grayish-white and solid with osteolytic destruction of the adjacent bone.
The patient developed postoperative nausea and vomiting, headache, and loss of vision in both eyes to the point of blindness. Positron emission tomography (PET)/CT examination 1.5 mo after the surgery showed that the septal sinus, pterygoid sinus, right nasal cavity and anterior skull base soft tissue density shadows were radiolucent, maximum standardized uptake value (SUVmax) was about 64.1, localized convexity into the orbits bilaterally, bilateral compression of the internal rectus muscle and optic nerve, and localized bone destruction in the septal sinus and pterygoid sinus (Figure 5A-D). MRI showed tumor size of about 4.5 cm × 3.5 cm × 4.2 cm (AP × LR × SI) (Figure 5E-H). Intensity-modulated radiation therapy (69.96 Gy 2.12Gy/F/33F) with simultaneous cisplatin, temozolomide (75 mg/m2), and deep thermotherapy 3 times/week (42 °C, 40 min) was performed after multidisciplinary consultation. The patient's tumor shrank significantly after 11 radiation treatments, with a size of about 2.5 cm × 2.9 cm × 2.6 cm (AP × LR × SI) (Figure 5I-L), but the radiation treatment was discontinued because of cerebrospinal fluid nasal leakage and grade III radiation oral mucosal reaction, so the dosage of temozolomide was increased (200 mg/m2 for 5 d, 28 d for 1 cycle). 6 mo after surgery, the follow-up evaluation revealed that the disease continued to progress, the tumor size was about 5.0 cm × 3.6 cm × 4.0 cm (AP × LR × SI) (Figure 5M-P), and there was swelling in the left eye and headache, nausea and vomiting, after adding Apatinib (0.25 g/d) for 1 cycle, the lesion was significantly reduced, the size was about 3.3 cm × 3.2 cm × 3.5 cm. The disease progressed after 2 mo of continued treatment and was switched to docetaxel 100 mg d1 + raltitrexed 4 mg d1 + cisplatin 30 mg d1-3 in combination with anlotinib (12 mg/d) for 2 cycles, after which the disease was poorly controlled. It was replaced with gemcitabine 1.0 mg d1, d8 + oxaliplatin 150 mg d1, combined with anlotinib for 2 cycles, during which the swelling was reduced by SDT (ultrasound localization through the eye, SDT device 0.75 W, frequency 50%, 30 min, treatment started after 32 h of input of the acoustically sensitive drug hematoporphyrin, and continuous treatment for 3 d), and the disease was basically stable. After adding nitrozumab and continuing chemotherapy for 1 cycle, the patient developed low back pain and decreased sensation below the 10th thoracic vertebra, and MRI showed thoracic 1-sacral 1 intraspinal metastases. The patient abandoned treatment and was discharged from the hospital, and died 2 mo later. The patient had 15 mo from the onset of symptoms to the development of distant metastases and 17 mo to death.
Approximately one person per 100000 people is diagnosed with sinonasal malignancies each year, which make up 5% of all head and neck cancers[6]. NMC is a highly aggressive malignant epithelial cell tumor that accounts for approximately 2% of nasal tumors[7,8]. It has a unique molecular genetic alteration in which the NUTM1 gene [t(15; 19)(q14; p13.1)] shows rearrangements with the fusion partners bromodomain-containing 4 (BRD4) [t(15; 19)(q13.2; p13.1)], BRD3 (on chromosome 19), nuclear receptor binding SET domain protein 3, and other genes (NUT-variant carcinomas); the most common of these is the BRD4-NUT fusion gene[2]. The specific etiology, pathogenesis and risk factors of NMC are unknown[9]. Its occurrence may be related to MYC, P63, MED24 and the Wnt, MAPK and PI3K signaling pathways[10,11]. The tissue origin of NMC is unknown, and studies have speculated that it may originate from cells of primitive neural sulcus origin[12].
NMC has a sex-neutral onset and can occur at any age, mostly in pediatric and young adult patients, often with aggressive growth involving the orbit and dura mater, and patients usually present with nonspecific symptoms associated with the mass (e.g., nasal leakage, rhinorrhea, nasal congestion, protrusion, decreased vision, difficulty swallowing, or pain)[13,14]. Laboratory tests lack specificity, while there are no specific tumor markers; the tumor marker test was normal in this case. Some patients may have elevated levels of methemoglobin due to osteolytic destruction and will probably develop hypercalcemia.
The morphology of NMC is difficult to differentiate from other poorly differentiated tumors, such as squamous cell carcinoma, lymphoepithelioma-like carcinoma, malignant melanoma, olfactory neuroblastoma, and neuroendocrine carcinoma, with the possibility of misdiagnosis and a challenging diagnosis, but it demonstrates unique NUT gene alterations. Histological features include small to moderate sized, completely undifferentiated or hypodifferentiated tumor cells arranged in diffuse sheets with sparse eosinophilic or basophilic cytoplasm, some with marked mesenchymal connective tissue hyperplasia, irregular nuclear contours, mostly naked nuclei with prominent nucleoli, and frequent nuclear schizophrenia[2,3,10,15]. Immunohistochemical analysis shows positive NUT staining of tumor cells with 100% specificity and 87% sensitivity[16]. Immunostaining for epithelial markers such as AE1/AE3, CK5/6, P63, P40 and EMA is often positive[17]. A variety of methods can be used to diagnose NMC, including immunohistochemistry, karyotyping, FISH, reverse transcription-polymerase chain reaction, and next-generation sequencing[18]. It is rare for NMC to be diagnosed routinely, resulting in a misdiagnosis or a late diagnosis. In this case, the microscopic tumor cells were round in morphology, diffuse or nest-like in distribution, showing hypodifferentiation or undifferentiation. The immunohistochemical epithelial markers AE1/AE3, EGFR, CK8/18, CK5/6 and squamous epithelial markers p63 and P40 were positively expressed to varying degrees, and the NUT gene was diffusely positively expressed. The case characteristics were consistent with those reported previously.
CT, MRI and PET/CT imaging are essential for the staging, management, treatment response assessment and monitoring of NMC[19]. NMC is most commonly associated with lymphatic metastases, followed by bone metastases[20]. CT shows a soft tissue density mass with osteolytic bone destruction, invasive, infiltrative growth, irregular morphology, low density necrotic areas inside the mass, occasional calcification[21], and mostly uneven and mild enhancement, similar to squamous cell carcinoma of the nasal cavity and sinuses and other malignant tumors such as rhabdomyosarcoma; calcification is very rare, however, perhaps a clue to the diagnosis of the disease. The superior soft tissue resolution of MRI facilitates the assessment of detailed anatomical localization of soft tissue masses in the head and neck and skull base and shows the involvement of adjacent areas[22,23]. On MRI, NMC usually shows a low signal on T1WI and a high signal on T2WI. Nonenhancing necrotic areas within the tumor can also be identified, and enhancement is heterogeneous[24]. Since NMC is highly invasive and often accompanied by symptoms and signs caused by distant metastases, patients should be recommended to undergo PET/CT for comprehensive examination to evaluate whether there are other sites of invasion, which can help in tumor staging and early detection of metastases. PET/CT is able to assess the metabolic and anatomical characteristics of the tumor. PET/CT shows increased glucose uptake in the lesion and metastatic areas (standard uptake values can be as high as 18.6), and if the uptake values located in the center of the lesion are decreased, this indicates necrosis within the lesion[7,21,25,26]. In addition, PET/CT has good sensitivity for bone metastases that cannot be detected by bone imaging[10].
To date, there is no standard treatment option for NMC, and the prognosis is poor due to its rapid progression, ease of recurrence and unsatisfactory treatment outcome[1,6]. If the patient is diagnosed early, surgery remains the treatment of choice and will help improve the prognosis[18]. Due to the aggressive growth of the tumor, most patients already have metastases at the time of initial diagnosis and have poor surgical results or missed surgical opportunities[27]. Some studies have shown that systemic therapy should be intensified postoperatively, and patients who receive radiotherapy doses of 50 Gy or more have better survival[8]. Those who have symptoms should be reviewed promptly to detect micrometastases as early as possible, and the target area of radiotherapy should include as many microlesions as possible. According to a retrospective study of 48 patients diagnosed with head and neck NMCs, the survival rate is significantly improved with aggressive surgical resection, regardless of the use of postoperative chemoradiation or radiation, and the median overall survival was 9.7 mo, with a 2-year PFS rate of 26%[28]. It has also been suggested that approximately 40% of patients initially respond to chemotherapy and radiotherapy but always relapse rapidly and do not respond to subsequent therapeutic interventions[9,18]. The patient in this case received multimodal treatment, including surgery, radiation therapy, chemotherapy, heat therapy and SDT. Unfortunately, the prognosis remained poor, with an overall survival of only 17 mo. The overall treatment outcome of NMC is unsatisfactory, and molecularly targeted therapy is currently under investigation as a promising therapeutic approach. Two molecularly targeted drugs targeting the underlying causative mechanisms have emerged as being in clinical trials, with preliminary evidence of efficacy in some patients: Bromodomain inhibitors (BETi) and histone deacetylase inhibitors (HDACi), both of which can induce differentiation and arrest cell growth in NMCs[3]. The BRD4-NUT oncoprotein binds and activates histone acetyltransferases, leading to chromatin acetylation and a feed-forward mechanism leading to tumorigenesis and BETi can inhibit the function of the BRD4-NUT protein, allowing cell differentiation to proceed. HDACis can reverse BRD4-NUT function and restore normal cellular processes and are expected to be a new therapeutic target for lung NUT cancer[3]. However, toxicity and side effects are still issues that need to be addressed[11]. One study found that OTX015/MK-8628, a novel oral BET inhibitor, reduced the amount of BET released into the blood; this NMC-targeting drug demonstrated rapid and impressive antitumor activity in preclinical studies. A significant difference was seen in survival between two patients (19 and 18 mo, respectively) compared with the median survival of 6.7 mo reported in the largest retrospective study of patients with NMC[29]. In this case, the patient relapsed soon after surgery and achieved some degree of remission after radiation therapy and initial treatment with antiangiogenic drugs and SDT but could not achieve long-term stability and eventually developed distant metastases and died soon after.
In summary, we report the multimodal treatment of a patient with paranasal sinus NMC, including surgery, radiation therapy and chemotherapy, but never achieved long-term stability and eventually developed distant metastases. For patients with rapidly progressing sinus tumors and a poor response to initial treatment, the possibility of NMC should be considered, and immunohistochemical staining with anti-NUT should be performed as soon as possible, as well as genetic testing if necessary. CT, MRI and PET/CT imaging are essential for the staging, management, treatment response assessment and monitoring of NMC. This case is the first attempt to apply heat therapy and SDT in the treatment of NMC; unfortunately, the prognosis remains poor.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hwang KH, South Korea; Jain N, Latvia; Kothan S, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Moreno V, Saluja K, Pina-Oviedo S. NUT Carcinoma: Clinicopathologic Features, Molecular Genetics and Epigenetics. Front Oncol. 2022;12:860830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Claudia G, Alexandra G. Challenging Diagnosis in NUT Carcinoma. Int J Surg Pathol. 2021;29:722-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Zhang H, Kong W, Liang W. NUT Midline Carcinoma: A Rare Solid Tumour Characterized by Chromosome Rearrangement. Evid Based Complement Alternat Med. 2022;2022:3369895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Dutz S, Hergt R. Magnetic particle hyperthermia--a promising tumour therapy? Nanotechnology. 2014;25:452001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Yamaguchi T, Kitahara S, Kusuda K, Okamoto J, Horise Y, Masamune K, Muragaki Y. Current Landscape of Sonodynamic Therapy for Treating Cancer. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Youlden DR, Cramb SM, Peters S, Porceddu SV, Møller H, Fritschi L, Baade PD. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 2013;37:770-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Lüer SC, Muhammed S, Evans AG, Sholl LM, Rosai J, Giraldi E, Oakley RP, Rodriguez-Galindo C, London WB, Sallan SE, Bradner JE, French CA. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773-5779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 8. | Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Xie M, Fu X, Wang W. Clinicopathological and molecular characterizations of pulmonary NUT midline carcinoma. Cancer Med. 2021;10:5757-5764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 10. | Li X, Shi H, Zhang W, Bai C, He M, Ta N, Huang H, Ning Y, Fang C, Qin H, Dong Y. Immunotherapy and Targeting the Tumor Microenvironment: Current Place and New Insights in Primary Pulmonary NUT Carcinoma. Front Oncol. 2021;11:690115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Han K, Dong X, Hou Q, Li T, Li L, Zhou G, Liu X, Zhao G, Li W. Case Report and Literature Review: Primary Pulmonary NUT-Midline Carcinoma. Front Oncol. 2021;11:700781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2010;63:492-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Napolitano M, Venturelli M, Molinaro E, Toss A. NUT midline carcinoma of the head and neck: current perspectives. Onco Targets Ther. 2019;12:3235-3244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Wu J, Fang Q, He YJ, Chen WX, Qi YK, Ding J. Local recurrence of sinonasal renal cell-like adenocarcinoma: A CARE compliant case report. Medicine (Baltimore). 2019;98:e14533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Bishop JA. Newly Described Tumor Entities in Sinonasal Tract Pathology. Head Neck Pathol. 2016;10:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Saiki A, Sakamoto K, Bee Y, Izumo T. Nuclear protein of the testis midline carcinoma of the thorax. Jpn J Clin Oncol. 2022;52:531-538. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Li YY, Ke XX, Lu Y. The primary pulmonary NUT carcinomas and some uncommon somatic mutations identified by next-generation sequencing: a case report. AME Case Rep. 2020;4:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Virarkar M, Saleh M, Ramani NS, Morani AC, Bhosale P. Imaging spectrum of NUT carcinomas. Clin Imaging. 2020;67:198-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Harms A, Herpel E, Pfarr N, Penzel R, Heussel CP, Herth FJ, Dienemann H, Weichert W, Warth A. NUT carcinoma of the thorax: Case report and review of the literature. Lung Cancer. 2015;90:484-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Sholl LM, Nishino M, Pokharel S, Mino-Kenudson M, French CA, Janne PA, Lathan C. Primary Pulmonary NUT Midline Carcinoma: Clinical, Radiographic, and Pathologic Characterizations. J Thorac Oncol. 2015;10:951-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Steinbach LS, Palmer WE, Schweitzer ME. Special focus session. MR arthrography. Radiographics. 2002;22:1223-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Sakata K, Hareyama M, Tamakawa M, Oouchi A, Sido M, Nagakura H, Akiba H, Koito K, Himi T, Asakura K. Prognostic factors of nasopharynx tumors investigated by MR imaging and the value of MR imaging in the newly published TNM staging. Int J Radiat Oncol Biol Phys. 1999;43:273-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Nelson BA, Lee EY, French CA, Bauer DE, Vargas SO. BRD4-NUT carcinoma of the mediastinum in a pediatric patient: multidetector computed tomography imaging findings. J Thorac Imaging. 2010;25:W93-W96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Bruzzi JF, Munden RF. PET/CT imaging of lung cancer. J Thorac Imaging. 2006;21:123-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Rosenbaum DG, Teruya-Feldstein J, Price AP, Meyers P, Abramson S. Radiologic features of NUT midline carcinoma in an adolescent. Pediatr Radiol. 2012;42:249-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Baumgartner K, Lauer U, Horger M, Zender L, Kloth C. [Nuclear protein in testis (NUT) midline carcinoma]. Rofo. 2020;192:303-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, Hsi P, Bauer DE, Lathan CS, Rodriguez-Galindo C, Tishler RB, Haddad RI, Sallan SE, Bradner JE, French CA. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. 2016;122:3632-3640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord JP, de La Motte Rouge T, Uro-Coste E, de Braud F, Pelosi G, French CA. Clinical Response of Carcinomas Harboring the BRD4-NUT Oncoprotein to the Targeted Bromodomain Inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 267] [Article Influence: 33.4] [Reference Citation Analysis (0)] |