Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11049
Peer-review started: May 12, 2022
First decision: June 16, 2022
Revised: July 9, 2022
Accepted: September 16, 2022
Article in press: September 16, 2022
Published online: October 26, 2022
Hypophysitis induced by programmed cell death 1 protein (PD-1) immune checkpoint inhibitors is rare and poorly described. We report three patients with non-small cell lung cancer who developed hypophysitis after anti-PD-1 immunotherapy.
Both case 1 and case 2 presented with common symptoms of fatigue, nausea, and vomiting. However, case 3 showed rare acute severe symptoms such as hoarse voice, bucking, and difficulty in breathing even when sitting. Following two cycles of immunotherapy in case 3, the above severe symptoms and pituitary gland enlargement were found on magnetic resonance imaging at the onset of hypophysitis. These symptoms were relieved after 10 d of steroid treatment. Case 3 was the first patient with these specific symptoms, which provided a new insight into the diagnosis of hypophysitis. In addition, we found that the clinical prognosis of patients with hypophysitis was related to the dose of steroid therapy. Case 3 was treated with high-dose hormone therapy and her pituitary-corticotropic axis dysfunction returned to normal after more than 6 mo of steroid treatment. Cases 1 and 2 were treated with the low-dose hormone, and dys
The clinical symptoms described in this study provide a valuable reference for the diagnosis and treatment of immune-related hypophysitis.
Core Tip: Hypophysitis induced by programmed cell death 1 protein (PD-1) inhibitor treatment in non-small cell lung cancer (NSCLC) was rarely reported. In this study, we report three patients with NSCLC who developed hypophysitis induced by PD-1 immune checkpoint inhibitor treatment. Our study suggested that unexpected fatigue, appetite decreases, nausea, vomiting, hoarse voice, bucking, and difficulty breathing were largely correlated with immune-related hypophysitis. We also found that the clinical prognosis of patients with hypophysitis was related to the dose of steroid therapy. This work provided a reference for the diagnosis and timely treatment of hypophysitis in the clinic.
- Citation: Zheng Y, Zhu CY, Lin J, Chen WS, Wang YJ, Fu HY, Zhao Q. Hypophysitis induced by anti-programmed cell death protein 1 immunotherapy in non-small cell lung cancer: Three case reports. World J Clin Cases 2022; 10(30): 11049-11058
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11049.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11049
Immunotherapy of tumors has become a new treatment method in addition to traditional malignant tumor treatment such as surgery, radiotherapy, and chemotherapy. Recently, immune checkpoint inhibitors (ICIs) have attracted more and more attention due to their more favorable clinical outcome than conventional therapy in terminal cancer. ICIs can enhance anti-tumor activity by targeting key points in the immune system, such as cytotoxic T-lymphocyte protein 4 (CTLA4), programmed death 1 (PD-1), and its ligand programmed cell death 1 ligand 1 (PD-L1)[1]. Several immunotherapeutic drugs have been approved for the treatment of melanoma, lung cancer, and urinary tract cancer[2].
Due to the regulatory effect of ICIs on the immune system, they may induce several immune-related adverse events (irAEs) as they lack specificity and result in generalized immune activation. The irAEs occur in various organs such as the skin, gastrointestinal tract, and liver, and induce frequent dysfunction of the endocrine system[3]. Thyroid dysfunction is the most common side effect in the endocrine system; 6.5% of patients treated with ICIs developed hypothyroidism and 2.9% developed hyperthyroidism[4]. Thyroid dysfunction commonly occurs in patients treated with PD-1 or PD-L1 inhibitors. Immune-related hypophysitis (IRH) is the second most common adverse event caused by ICI treatment, which is largely induced by CTLA4 inhibitors with an incidence between 5.6% and 13.6%[5]. However, it is less common with PD-1 inhibitors (0.5%-1.1%)[6] and PD-L1 inhibitors (less than 0.1%).
Hypophysitis induced by CTLA4 inhibitors has been well documented. However, PD-1 and PD-L1 inhibitor-induced hypophysitis has been newly proposed and poorly described, probably because these drugs were approved later than CTLA4 inhibitors[7]. In the present study, we retrospectively analyzed 56 patients with advanced non-small-cell lung cancer (NSCLC) treated with an anti-PD-1 drug from 2019 to 2020 at Shulan (Hangzhou) Hospital. Three cases were diagnosed with IRH. We report these three cases of hypophysitis induced by anti-PD-1 treatment to provide new insights into its diagnosis and treatment.
Case 1: A 57-year-old man was diagnosed with lung squamous cell carcinoma (pT2N3M0, stage IIIB). He then received the anti-tumor treatment but he presented fatigue (grade 1 according to CTCAE version 4.0), decreased appetite (grade 1), and liver damage (grade 1).
Case 2: A 74-year-old man was diagnosed with lung adenosquamous carcinoma (pT2bN2M1, stage IV). After receiving the anti-tumor treatment, he presented severe nausea and vomiting (grade 3).
Case 3: A 54-year-old female patient was diagnosed with lung adenocarcinoma and bone metastases (pT4N2M1, stage IV). After treatment, the patient complained of fatigue, hoarse voice, bucking, and difficulty in breathing even when sitting (grade 3).
Case 1: The patient refused radiotherapy and underwent four cycles of combination treatment with chemotherapy (docetaxel 75 mg/m2 plus nedaplatin 80 mg/m2) and anti-PD-1 drug (toripalimab 240 mg). He then received maintenance therapy with the anti-PD-1 drug only.
Case 2: The patient was treated with adjuvant combination chemotherapy (docetaxel 75 mg/m2 plus nedaplatin 80 mg/m2) and an anti-PD-1 drug (pembrolizumab 200 mg) for 4 cycles. The patient did not receive continuous maintenance therapy with pembrolizumab as he developed immune-related pneumonia. He was then followed every 3 mo without any treatment. Four months later, he was taken to hospital due to severe nausea and vomiting (grade 3).
Case 3: After the patient was diagnosed, she was treated with a combination of chemotherapy (pemetrexed 500 mg/m2 and nedaplatin 80 mg/m2), targeted therapy (bevacizumab 7.5 mg/kg), and anti-PD-1 (toripalimab 240 mg) therapy. Two cycles later, chest computed tomography (CT) reexamination demonstrated that the lung lesions had diminished and the curative effect was considered a significant partial remission. Unfortunately, the patient complained of fatigue, hoarse voice, bucking, and difficulty in breathing even when sitting.
Case 1: The patient had no history of autoimmunity.
Case 2: The patient underwent radical resection of the left lung cancer and presented with pleural metastasis.
Case 3: The patient did not have a history of autoimmunity.
All patients have no family history of autoimmune disorders.
Case 1: The Eastern Cooperative Oncology Group (ECOG) score was 1 and Nutritional Risk Screening (NRS) score was 0. Murphy’s sign was negative. Other physical examination was unremarkable.
Case 2: The ECOG score was 1 and NRS score was 0. Murphy’s sign was negative. Other physical examination was unremarkable.
Case 3: The ECOG score was 1 and NRS score was 1. The psychological reflection was normal and no pathological reflection was induced. Other physical examination was unremarkable.
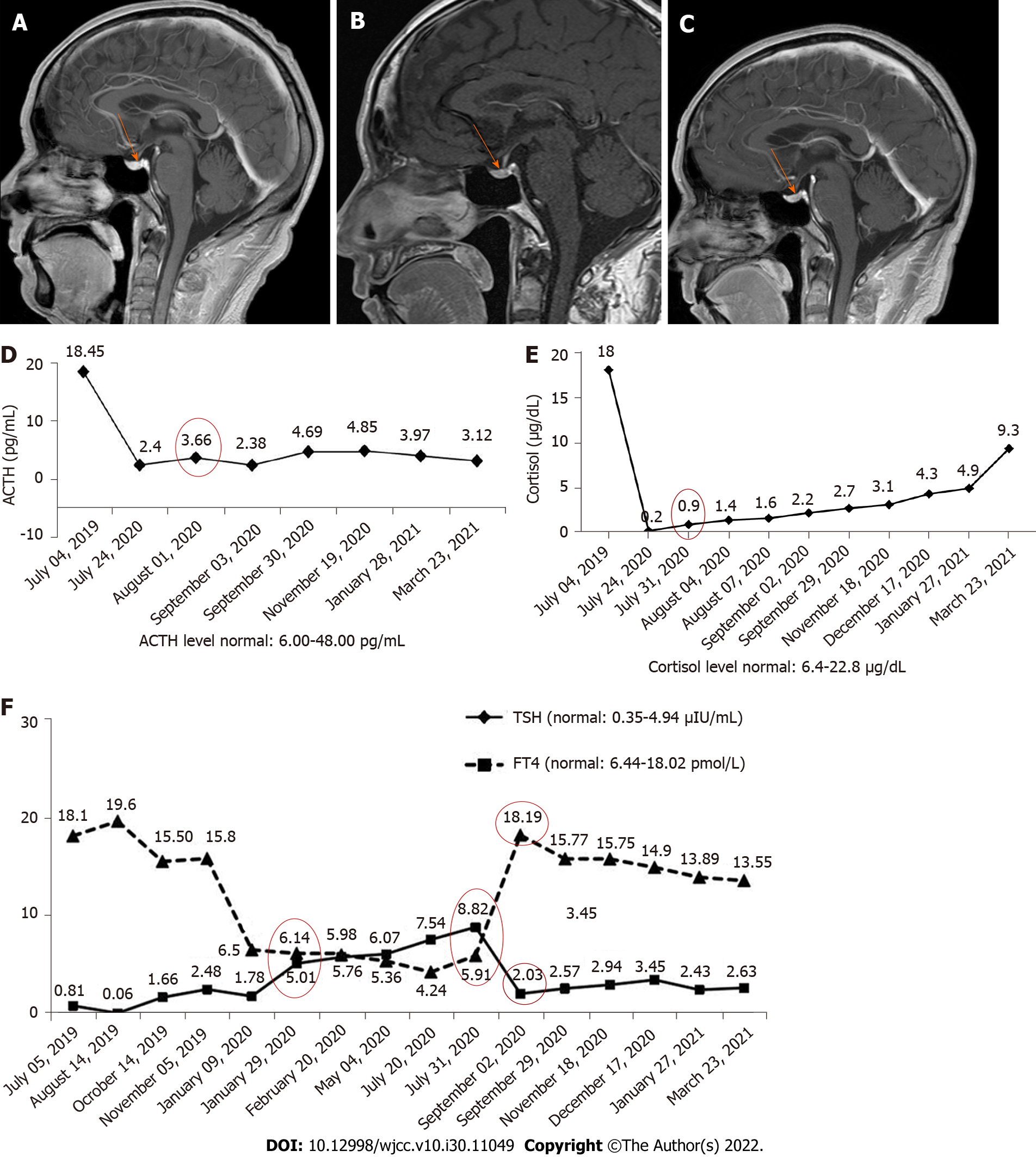
Case 1: The patient had no epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements. Thyroid stimulating hormone (TSH), free thyroxine (FT4), adrenocorticotropic hormone (ACTH), and cortisol levels were all normal before immunotherapy in 2020. The levels of TSH and FT4 were slightly abnormal (Figure 1E, January 29, 2020) after anti-PD-1 treatment.
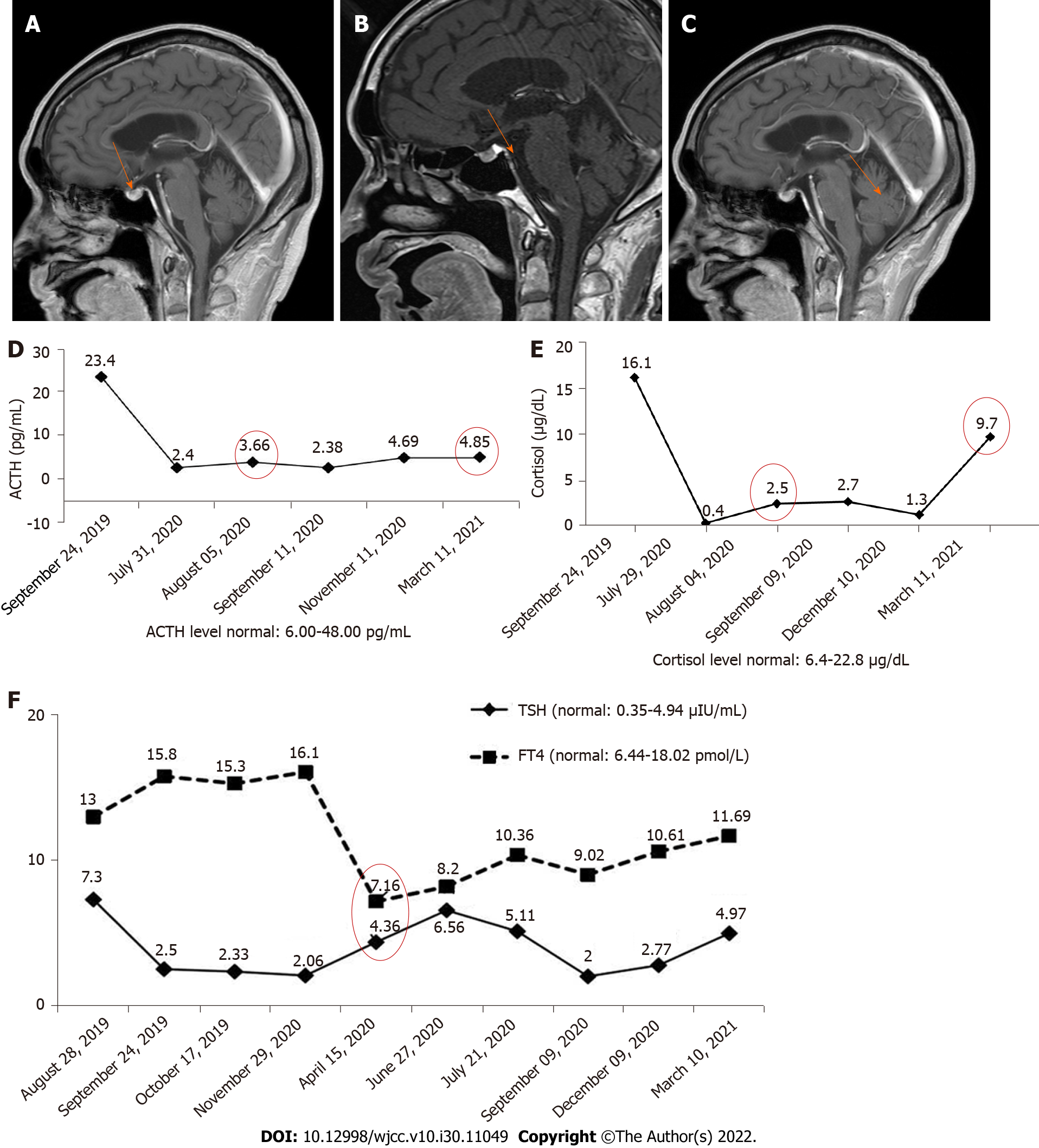
Case 2: The patient had no EGFR mutations or ALK rearrangements and no history of autoimmunity. The PD-L1 tumor proportion score was greater than 50%. The levels of TSH and FT4 were normal (Figure 2E, April 15, 2020).
Case 3: After the patient was diagnosed, the patient presented no EGFR mutations or ALK rearrangements. When the symptoms such as fatigue and hoarse voice appeared, the levels of ACTH (5.37 pg/mL) and cortisol (2 μg/dL) were lower than the normal range (Figure 3F).
Case 1: Magnetic resonance imaging (MRI) showed that the pituitary morphology (Figure 1A) was normal before immunotherapy in 2020. When the patient first exhibited fatigue, decreased appetite, and liver damage, the pituitary enlargement was also not found (Figure 1B).
Case 2: We performed cranial MRI and lumbar puncture, but no pituitary abnormities (Figure 2A) or meningeal metastasis were found.
Case 3: The baseline chest CT is presented in Figure 3A. When the symptoms such as fatigue and hoarse voice appeared, we performed a 3T laryngoscopy but found no vocal cord disorders. We then performed pituitary MRI, and found that the pituitary gland was slightly enlarged (Figure 3D) compared with that at baseline (Figure 3C).
Case 1: The patient was diagnosed with hypothyroidism and secondary adrenal insufficiency induced by hypophysitis due to anti-PD-1 treatment according to his medical history, clinical features, and laboratory measurements.
Case 2: According to his treatment history and clinical symptoms, the patient was diagnosed with hypothyroidism induced by anti-PD-1 treatment.
Case 3: Following a multidisciplinary consultation, the diagnosis of IRH was made after excluding all other possible cause.
Case 1: When the patient first exhibited fatigue, decreased appetite, and liver damage, we decreased the frequency of toripalimab administration. After one cycle of maintenance treatment, he was hospitalized again due to increased fatigue, severe nausea, and vomiting (grade 3). The patient discontinued anti-PD-1 maintenance treatment. The frequency of anti-PD-1 treatment was then adjusted and the patient was continuously treated for 6 mo, but the levels of TSH and FT4 were still abnormal (July 31, 2020). We also found abnormal levels of ACTH (3.66 pg/mL) and cortisol (0.9 μg/dL) (Figure 1D) at this time, suggesting that the symptoms were caused by secondary adrenal insufficiency. The patient was subsequently treated with hormone and thyroxine replacement therapy. He took oral hydrocortisone 50 mg/d and then given oral prednisone 25 mg/d. The dose of prednisone was then tapered to 1 tablet every 14 d, and finally reduced to 1 tablet.
Case 2: After the patient visited our hospital, we treated the patient with an antiemetic agent and his discomfort was relieved but prone to recurrence. After 3 mo of antiemetic treatment, he complained of nausea and vomiting exacerbation and a 5 kg weight loss. Pituitary MRI was performed again, but revealed no enlargement of the pituitary (Figure 2B). However, an empty sella was found on the pituitary MRI. At the same time, the levels of ACTH (3.66 pg/mL) and cortisol (2.5 μg/mL) were abnormally lower than the normal range (Figure 2D). Obvious dysfunction of the pituitary-thyroid axis was not found. He subsequently received hormone therapy. Oral prednisone was administered at a dosage of 2.5 mg bid.
Case 3: The patient received hormone shock therapy. First, 200 mg/d methylprednisolone was injected for 4 d, and then 160 mg/d and 120 mg/d methylprednisolone for 3 d, respectively. After hormone therapy, the patient’s discomfort was gradually relieved. Next, we discontinued anti-PD-1 treatment and the patient received continuous administration of combined chemotherapy and targeted therapy for two cycles. Pemetrexed and bevacizumab were then administered as uninterrupted maintenance therapy. We also adjusted the administration of hormone therapy. Methylprednisolone 80 mg/d was given for 8 d and 5 g/d allotype for 5 d. Although her discomfort completely disappeared, the levels of ACTH and cortisol were not obviously changed. The dose of methylprednisolone was then reduced by 20%-30% every 7 d to 5 mg/d.
Case 1: When the patient took oral hydrocortisone for 1 wk, his discomfort was significantly reduced. After 1 mo of steroid treatment, the levels of TSH and FT4 were found to be in the normal range (September 2, 2020). In addition, dysfunction of the pituitary-gonadal axis and abnormality of pituitary morphology (Figure 1C) were not found. However, function of the pituitary-adrenal axis was abnormal for an extended period (Figure 1D).
Case 2: After 2 wk of hormone treatment, the patient’s symptoms were significantly relieved. Four months later, the pituitary gland was normal (Figure 2C), but the pituitary-corticotropic axis was still abnormal after 7 mo of steroid treatment (ACTH = 4.85 pg/mL).
Case 3: After almost 2 mo of hormone therapy, pituitary enlargement diminished (Figure 3E). Three months later, the level of ACTH (10.36 pg/mL) returned to normal, and cortisol level (10.3 μg/dL) also returned to normal after more than 4 mo of hormone therapy. No dysfunction of the pituitary-thyroid axis (Figure 3G) or the pituitary-gonadal axis was observed during the treatment period.
It is acknowledged that several human cancers can escape immune surveillance through the expression of PD-1. PD-1 is able to inhibit T-cell action against tumor cells; therefore, anti-PD-1 treatment has become an effective therapy by promoting the anti-tumor immune response. However, the main function of PD-1 in the healthy body is to suppress the autoimmune response, and inhibition of this system also triggers the T-cell inflammatory response against any susceptible healthy tissues[8]. Despite anti-PD-1 treatment having a remarkable impact on advanced cancers, it also induces wide-ranging irAEs.
The mechanism of irAEs remains unknown. Dysfunction of the endocrine system is one of the most common irAEs caused by anti-PD-1 treatment. It was reported that hypothyroidism frequently occurs in patients treated with anti-PD-1 drugs. In addition, hypophysitis was commonly found in patients treated with CTLA4 inhibitors[6,9]. This difference may be supported by the fact that there is no ectopic PD-1 expression in normal hypophysis tissue[10,11]. In the present study, only one case presented with hypothyroidism. IRH is a pituitary disorder with one or multiple anterior pituitary hormone deficiencies (mostly ACTH and/or TSH)[12]. In our study, three cases all displayed isolated ACTH insufficiency after anti-PD-1 treatment, which was consistent with a previous study[13]. It is worth mentioning that combination therapy with PD-1 and CTLA4 inhibitors increased the occurrence of hypophysitis, which ranged from 8.8% to 10.5%[14,15]. The frequency of hypophysitis occurrence may be under-estimated in oncological trials that did not systematically screen for this adverse event that has a non-specific clinical presentation[16].
The general symptoms associated with IRH are fatigue, decreased appetite, nausea, and vomiting. These common symptoms were also present in cases 1 and 2 in the present report. However, the symptoms including hoarse voice, bucking, and difficulty in breathing even when sitting in case 3 are rare, and this is the first case showing such specific symptoms in our clinical experience. This may provide oncologists with a new understanding of the symptoms caused by IRH. The adverse event of immune-mediated hypophysitis commonly occurs within 8-12 cycles after the initiation of anti-PD-1 therapy[17]. In the present study, it occurred after eight cycles of immunotherapy in case 1 and after four cycles in case 2. Whereas, case 3 developed hypophysitis after two cycles of immunotherapy. This may be related to the insufficient sample size and heterogeneity between patients. Heterogeneity is derived from the patient’s constitution, treatment profile, psychological pressure, and so on.
MRI is an imaging technique used in the diagnosis of pituitary inflammation. Pituitary enlargement is usually found in patients diagnosed with hypophysitis. However, due to a lack of experience among clinicians, brain or pituitary MRI is not performed at baseline before immunotherapy. Pituitary MRI is generally performed following the development of hypophysitis. Therefore, clinicians are not able to fully assess hypophysitis. It was found that 25% of patients showed a normal pituitary MRI during the acute phase of pituitary inflammation[18-20]; therefore, hypophysitis is easily misdiagnosed if the clinician lacks experience. In this study, the pituitary gland was enlarged in case 3 when hypophysitis developed, but was still considered to be in the normal range. Although pituitary enlargement is not found in most patients at the onset of hypophysitis, pituitary MRI is necessary at baseline before immunotherapy. In addition, the clinician should inform the radiologist of the patient’s medical history in order to make the correct diagnosis.
In terms of treatment for IRH, hormone replacement is commonly administered by clinicians. After treatment, thyroid function can return to normal, but dysfunction of the pituitary-gonadal axis and pituitary-corticotropic axis may still persist[21,22]. Our study also confirmed this situation. Thyroid function in case 1 recovered soon after almost 1 mo of steroid treatment, but function of the pituitary-corticotropic axis in cases 1 and 2 was still abnormal after up to 7 mo of steroid treatment. However, the pituitary-corticotropic axis in case 3 returned to normal after more than 6 mo of steroid treatment. The reason for this difference may be related to the dose of hormone administered, as case 3 was treated with high-dose hormone therapy while cases 1 and 2 were treated with the low-dose hormone therapy. Our study provides a reference for the treatment of IRH. A previous study reported that the overall survival of patients with IRH was not improved by steroid therapy[23]. We speculate that the poor prognosis may be related to the dose of steroid therapy.
Besides the focus on the side effects caused by PD-1 inhibitors, the expression rate of PD-1 and PD-L1 is also important. It has been reported that the expression rate was correlated with the disease types and subtypes[24]. In addition, the inflammatory cytokines can affect the PD-1 or PD-L1 expression. Ribas and Hu-Lieskovan[25] reported that inflammatory cytokines induced the PD-L1 expression, and particularly, the involvement of interferon-γ in immune checkpoint induction has been reported[26]. At present, no research reported the involvement of inflammatory cytokines in related hypophysitis caused by PD-1 expression. The investigation of inflammatory cytokines might be conducive to monitoring the hypophysitis caused by PD-1 inhibitor treatment.
This study describes three patients receiving anti-PD-1 treatment for NSCLC who ultimately developed hypophysitis, simultaneously leading to hypothyroidism and/or isolated ACTH deficiency. PD-1 inhibitor induced-hypophysitis presented with less obvious clinical and radiological signs, and the lack of a clinical presentation can lead to delayed diagnosis. It is necessary to perform a systematic and regular hormonal evaluation in patients treated with ICIs. When unexpected fatigue, decreased appetite, nausea, vomiting, hoarse voice, bucking, and difficulty in breathing develop, IRH should be considered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashimoto K, Japan; Shalaby MN, Egypt S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol. 2017;13:755-763. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Gedye C, van der Westhuizen A, John T. Checkpoint immunotherapy for cancer: superior survival, unaccustomed toxicities. Intern Med J. 2015;45:696-701. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473-486. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:173-182. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Solinas C, Porcu M, De Silva P, Musi M, Aspeslagh S, Scartozzi M, Willard-Gallo K, Mariotti S, Saba L. Cancer immunotherapy-associated hypophysitis. Semin Oncol. 2018;45:181-186. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm Metab Res. 2019;51:145-156. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Levy M, Abeillon J, Dalle S, Assaad S, Borson-Chazot F, Disse E, Raverot G, Cugnet-Anceau C. Anti-PD1 and Anti-PDL1-Induced Hypophysitis: A Cohort Study of 17 Patients with Longitudinal Follow-Up. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Mekki A, Dercle L, Lichtenstein P, Marabelle A, Michot JM, Lambotte O, Le Pavec J, De Martin E, Balleyguier C, Champiat S, Ammari S. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer. 2018;96:91-104. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Seejore K, Giannoudi M, Osborn D, Lynch JM, Al-Qaissi A, Dunwoodie E, Hook J, Marples M, Murray RD. Characterisation of the onset and severity of adrenal and thyroid dysfunction associated with CTLA4-related hypophysitis. Eur J Endocrinol. 2021;186:83-93. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13:195-207. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | González-Rodríguez E, Rodríguez-Abreu D; Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist. 2016;21:804-816. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29-38. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Kitajima K, Ashida K, Wada N, Suetsugu R, Takeichi Y, Sakamoto S, Uchi H, Matsushima T, Shiratsuchi M, Ohnaka K, Furue M, Nomura M. Isolated ACTH deficiency probably induced by autoimmune-related mechanism evoked with nivolumab. Jpn J Clin Oncol. 2017;47:463-466. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kassi E, Angelousi A, Asonitis N, Diamantopoulos P, Anastasopoulou A, Papaxoinis G, Kokkinos M, Giovanopoulos I, Kyriakakis G, Petychaki F, Savelli A, Benopoulou O, Gogas H. Endocrine-related adverse events associated with immune-checkpoint inhibitors in patients with melanoma. Cancer Med. 2019;8:6585-6594. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Spain L, Walls G, Julve M, O'Meara K, Schmid T, Kalaitzaki E, Turajlic S, Gore M, Rees J, Larkin J. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol. 2017;28:377-385. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Faje A, Reynolds K, Zubiri L, Lawrence D, Cohen JV, Sullivan RJ, Nachtigall L, Tritos N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol. 2019;181:211-219. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Ji HH, Tang XW, Dong Z, Song L, Jia YT. Adverse Event Profiles of Anti-CTLA-4 and Anti-PD-1 Monoclonal Antibodies Alone or in Combination: Analysis of Spontaneous Reports Submitted to FAERS. Clin Drug Investig. 2019;39:319-330. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Sekizaki T, Kameda H, Oba C, Yong Cho K, Nakamura A, Miyoshi H, Osawa T, Shinohara N, Atsumi T. Nivolumab-induced hypophysitis causing secondary adrenal insufficiency after transient ACTH elevation. Endocr J. 2019;66:937-941. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Mishima Y, Fukaishi T, Inase N, Isogai S. Nivolumab-induced Hypophysitis, Secondary Adrenal Insufficiency and Destructive Thyroiditis in a Patient with Lung Adenocarcinoma. Intern Med. 2019;58:693-697. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Thapi S, Leiter A, Galsky M, Gallagher EJ. Recovery from secondary adrenal insufficiency in a patient with immune checkpoint inhibitor therapy induced hypophysitis. J Immunother Cancer. 2019;7:248. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Ntali G, Kassi E, Alevizaki M. Endocrine sequelae of immune checkpoint inhibitors. Hormones (Athens). 2017;16:341-350. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33:2092-2099. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, Davis M, Carroll RS, Kaiser UB. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21:749-755. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Hashimoto K, Nishimura S, Ito T, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur J Histochem. 2021;65. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med. 2016;213:2835-2840. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706-2716. [PubMed] [DOI] [Cited in This Article: ] |