Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.8018
Peer-review started: February 16, 2022
First decision: June 15, 2022
Revised: June 22, 2022
Accepted: June 30, 2022
Article in press: June 30, 2022
Published online: August 6, 2022
The clinical manifestations of drug eruption are complex and diverse, which can lead to missed diagnosis or misdiagnosis. The clinical manifestations of drug eruption caused by compound honeysuckle have not been reported.
A 20-year-old man was admitted to our department of dermatology due to erythema and papules on the chest and abdomen with pruritus for 3 d. The next day after taking compound honeysuckle granules, the patient suddenly developed a rash and intense itching on his chest and abdomen. Physical examination revealed diffuse red needle-cap size macules and papules with well-defined borders on the chest and abdomen, and discoloration after finger pressure. No abnormality was observed in other areas of the skin. Back skin scratch was positive. White blood cells, eosinophil count and eosinophil ratio were higher than normal. Histopathological examination of the skin lesions on the left abdomen revealed intercellular edema, blurred focal basal cell layers, and focal lymphocyte infiltration in the superficial dermis and perivascular areas. Immunohistochemistry showed CD3+, CD4+ and CD8+ T lymphocytes. The diagnosis was drug eruption with special manifestations induced by compound honeysuckle. The skin lesions completely subsided without pruritus after 2 wk of antihistamine and hormone therapy. Follow-up for > 1 mo showed no recurrence.
Chinese patent medicine compound honeysuckle granules can induce allergic reaction and rare skin damage.
Core Tip: Drug eruption is a common disease in dermatology. The severity of the disease varies, and it can endanger life. The clinical manifestations of drug eruption are complex and varied, and it can imitate any skin disease. Here, we report a case of drug eruption caused by oral administration of Chinese patent medicine compound honeysuckle granules. The clinical manifestations of drug eruption are unique, and the drug eruption is distributed along the Blaschko line on both sides of the chest and abdomen, which is rare clinically.
- Citation: Zhou LF, Lu R. Compound-honeysuckle-induced drug eruption with special manifestations: A case report. World J Clin Cases 2022; 10(22): 8018-8024
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/8018.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.8018
Drug eruption, also known as drug dermatitis, is an inflammatory reaction occurring in the skin and mucous membranes when drugs enter the body through oral administration, intravenous injection and other routes. The severity of the disease varies, and the liver, kidneys, bone marrow and other organs may be involved in severe cases, and may even endanger life[1]. Drug eruption is a common dermatological disease. Drug allergy accounts for 15% of adverse drug reactions, and the incidence of drug eruption in inpatients is 2%–3%[2]. The incidence of drug eruption in the general population is estimated to be between 0.3% and 8%[3,4]. There are many kinds of drugs causing eruptions, and the clinical manifestations of drug eruption are complex and diverse, which can imitate any skin disease[5]. Therefore, it is important to know the clinical manifestations of drug eruption for the diagnosis of skin diseases. We here report a case of drug eruption in a 20-year-old man with specific manifestations induced by oral compound honeysuckle granules.
A 20-year-old man was admitted to our department of dermatology due to erythema and papules on the chest and abdomen with pruritus for 3 d.
The patient felt fatigue and discomfort without headache, pharyngeal pain or fever 5 d ago. Therefore, the patient took compound honeysuckle granules orally, three times a day, 10 g each time. The next day after taking the drug, red rash and severe itching appeared on the chest and abdomen. The patient stopped taking the drug and did not receive diagnosis and treatment. The original rash did not change and pruritus was more obvious after 1 d. There was no fever during the course of the disease.
The patient was previously healthy and denied any history of allergic disease or other diseases.
The patient denied a family history of genetic disease, and there were no family members with similar disease.
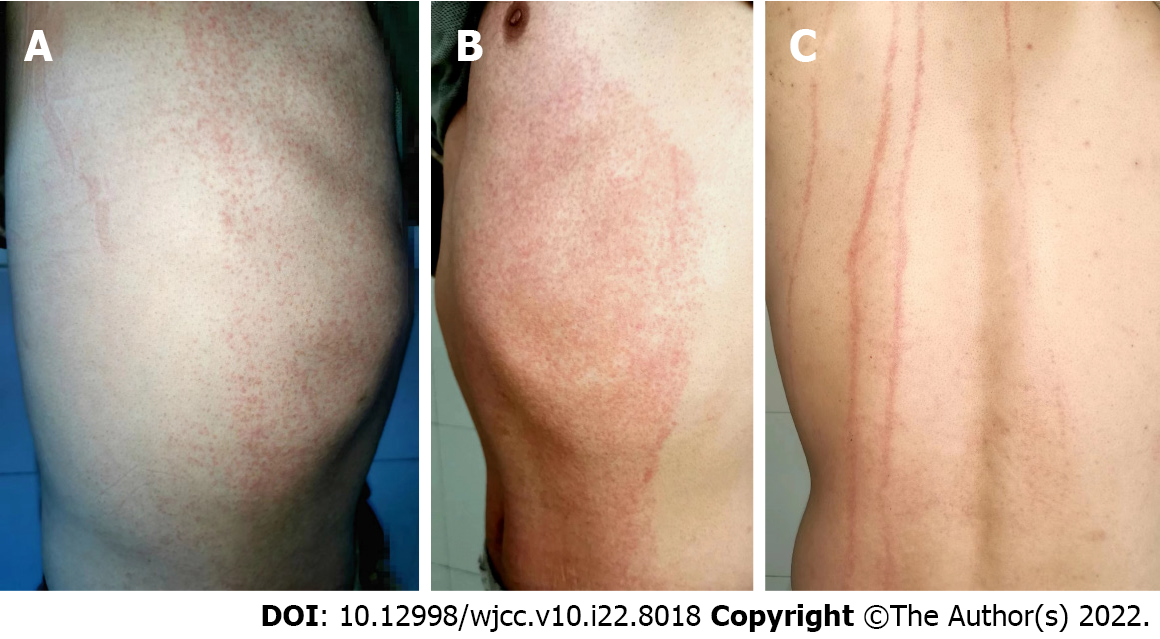
Vital signs were normal. Heart, lung and abdomen examination showed no abnormalities. There was no hyperemia or edema in the pharynx, no hyperplasia in pharyngeal lymphatic follicles, no swelling or hyperemia in the tonsils, no secretions, normal tongue coating, or no oral mucosal spots. No enlarged lymph nodes were palpable behind the ears, in the neck or under the jaw, and the tourniquet test was negative. Dermatological findings showed diffuse distribution of bright red needle-cap size macules and papules with well-defined borders on the chest and abdomen. The rash was discolored after finger pressure (Figure 1A and B). No abnormality was observed on the skin of the face, neck, limbs, axilla and behind the bilateral axillary front. Skin laceration was positive (Figure 1C).
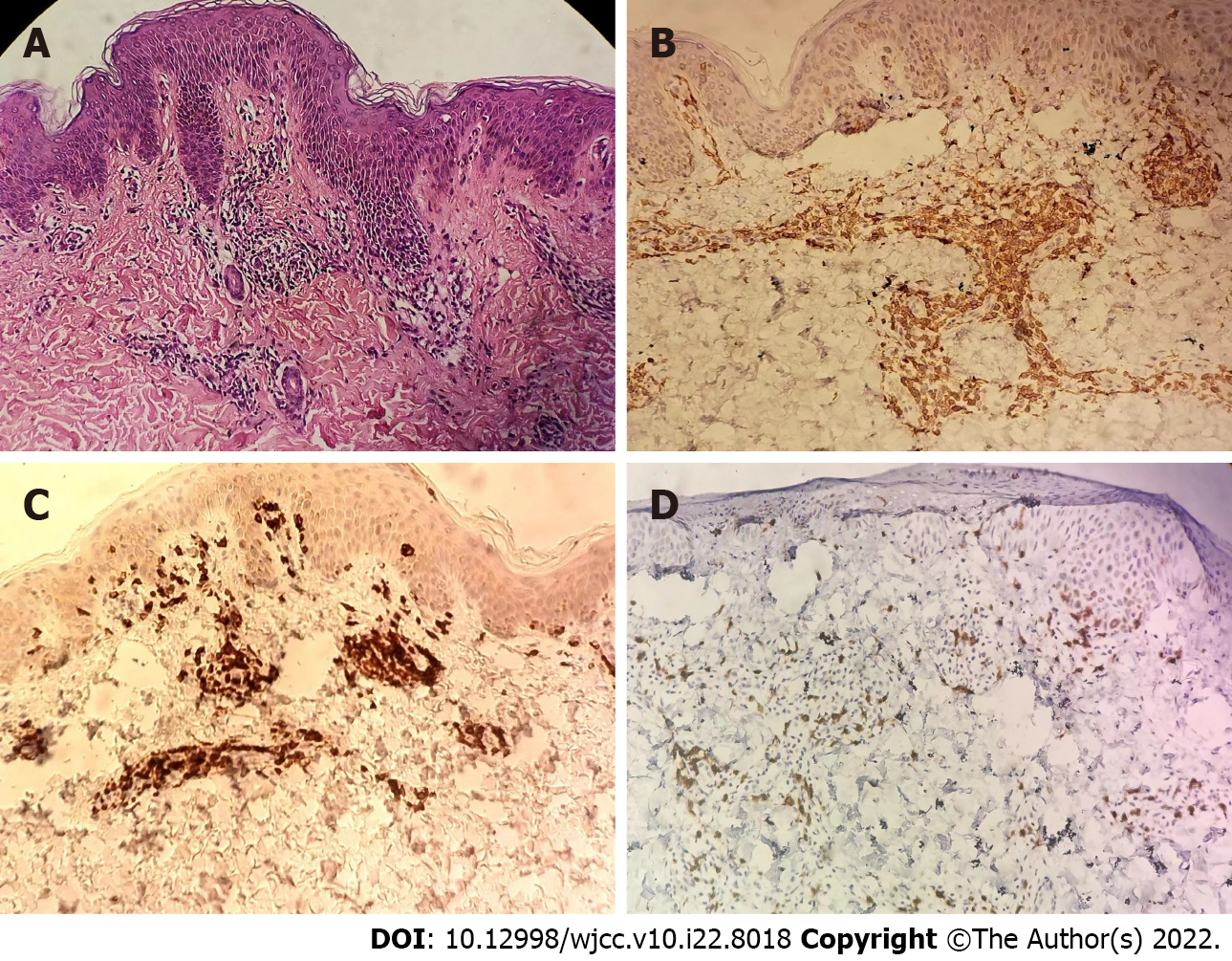
Blood routine examination showed increased white blood cell count (9.82 × 109/L), eosinophil count (0.65 × 109/L) and eosinophil ratio (0.07), and all other indicators were normal. Routine tests of urine, liver and kidney function, blood lipids and electrolytes were all within the normal range. The antinuclear antibody profile and anti-double-stranded DNA were negative. IgA (0.17 g/L) was slightly increased and the remaining immune indexes were normal. Histopathological examination of the skin lesion on the left abdomen showed intercellular edema of the epidermis, blurred focal basal cell layers, and focal lymphocyte infiltration in the superficial dermis and perivascular layers (Figure 2A and B). Immunohistochemistry showed CD3+, CD4+ and CD8+ T lymphocytes.
Chest X-ray and abdominal color ultrasound showed no abnormalities.
The patient was diagnosed with compound-honeysuckle-induced drug eruption with specific appearance.
We asked the patient to drink plenty of water daily. He was treated with oral desloratadine dry suspension granule 1 g daily, oral prednisone acetate tablets 20 mg daily, and oral compound glycyrrhizin tablets 225 mg daily. Seven days after maintenance treatment, the rash was significantly relieved, and itching was also relieved. Desloratadine dry suspension granules 1 g per day were added, prednisone acetate tablets were tapered to 15 mg per day, compound glycyrrhizin tablets were stopped, and calcium carbonate D3 granules 6 g per day were taken orally for another 1 wk. Subsequently, the skin lesions and itching completely disappeared, and no scale or pigmented spots were found. All drugs were then withdrawn.
After 2 wk of antihistamine and glucocorticoid treatment, the thoracic and abdominal rash and the itching disappeared completely. The patient was followed up for > 1 mo and no recurrence was found.
Drug eruption is a common dermatological disease. Most patients have acute onset and severe symptoms. Some cases may involve the liver, kidneys, and gastrointestinal and other visceral systems, and severe cases may endanger life. The occurrence of drug eruption is related to genetic factors, functional conditions and enzyme defects.
The pathogenesis of drug eruption is complex. Studies have found that T-cell-mediated immune response is involved in the occurrence of drug eruption, and the dominant subsets of T lymphocytes vary in different types of drug eruption[6]. CD4+ T cells play a predominant role in drug eruption[7]. Activated CD4+ T cells mainly secrete a variety of cytokines and inflammatory mediators, such as interferon-A, tumor necrosis factor receptor, interleukin-2 and other Th1 cytokines, which play a role in drug eruption[8]. Hari et al[6] reported maculopapular exanthema drug eruption with almost only CD4+ T cell infiltration. Yawalkar et al[9] demonstrated that maculopapular exanthema drug eruption was mainly infiltrated by CD3+ T cells and also marked by CD4+ T cells. In our case, the rash was mainly infiltrated by CD4+ T cells, with more CD3+ T cells and a small number of CD8+ T cells, which was similar to the previous studies on T lymphocyte infiltration with drug eruption.
In recent years, new discoveries have been made about the pathogenesis of drug eruption. Studies have shown that HLA polymorphism is the main genetic factor of drug sensitization[10]. HLA-B alleles may activate T cells by expressing peptides that bind to drugs or drug metabolites[11]. The main chemical components of compound Honeysuckle are phenolic acids, including chlorogenic acid, neochlorogenic acid and cryptochlorogenic acid. Both chlorogenic acid and cryptochlorogenic acid can cause allergic reactions, but neochlorogenic acid cannot[12]. In our case, skin rash appeared on the chest and abdomen after oral administration of compound Honeysuckle granules, which was probably related to genetic polymorphism. The peptide expressed by specific alleles was combined with drugs or drug metabolites to activate CD4+ T cells, resulting in allergic reaction and drug eruption.
Drug eruption needs to be distinguished from eruptive skin diseases caused by infectious diseases such as measles, rubella and scarlet fever. In our patient, there was no fever, cough, runny nose or other symptoms before the eruption, no congestion in the pharynx, no Koplik spot in the buccal mucosa, and no skin rash on the face and neck. The itching was severe, and the rash subsided slowly after drug withdrawal. No desquamation or pigmentation was observed at the subsided area, so measles were ruled out. There was no fever, runny nose, cough, pharyngeal pain or other symptoms of upper respiratory tract infection, no skin rash on the face and neck, no lymph node enlargement behind the occiput, behind the ears and on the neck, and there was no abnormal lymphocyte count before the eruption, so rubella was excluded. Before the eruption, the patient had no fever, no pharyngeal congestion, no tonsil enlargement, no cervical lymph node enlargement, no pallor around the mouth, normal lingual papilla, negative tourniquet test, severe itching, and no abnormal neutrophil count, which excluded scarlet fever. One day after the patient took compound Honeysuckle granules orally, diffuse red macules suddenly appeared on the chest and abdominal wall, and the rash distribution was symmetrical. The color disappeared after finger pressing, and the itching was obvious. The rash did not increase after withdrawal of compound Honeysuckle granules. Skin scratch was positive, the number and ratio of eosinophils were higher than normal, and the rash was infiltrated with CD4+ T lymphocytes. Skin diseases with similar rashes and exanthemic infectious skin diseases were excluded, and drug eruption was finally diagnosed.
Blaschko line was described by Alfred Blaschko in 1901 and refers to the linear distribution of various nevi and acquired skin diseases on human skin mucosa[13]. Later, Happle et al[14] gave a supplementary description of the distribution of skin lesions on the head and neck. Its distribution pattern is S-shaped on the side and front of the trunk, V-shaped in the middle of the back, spiral on the head and occiput, arc-shaped on the side of the head and neck, and linear on the limbs. The Blaschko line represents the pathway of epidermal cell migration and proliferation during fetal development, reflecting the existence of skin mosaicism[15]. Skin lesions along the Blaschko line are clinical manifestations caused by skin chimerism[16]. Many congenital skin diseases as well as some acquired skin diseases can be distributed along the Blaschko line, which may be the rapid response of the chimera of skin susceptibility genes to systemic immune factors[17]. Munro et al[18] believe that mutated cells arranged along the Blaschko line could cause disease under the excitation of some epigenetic factors. Gene mosaicism may play an important role in the occurrence of diseases distributed along the Blaschko line. Under some external stimuli, the mosaicism and cloning expression of genes encoding skin antigen determinants caused the loss of immune tolerance, which leads to skin inflammation at the T-cell stage distributed along the Blaschko line. This results in dermatitis distributed along the Blaschko line[19]. In our case, the rash only appeared on the chest and abdomen. The rash boundary was unusually clear, and the rash distribution was consistent with the Blaschko-line pattern of mosaicism described by Happle et al[14]. The pathogenesis may be mosaicism of genes encoding this skin region, loss of immune tolerance, and inflammatory response in this region under the stimulation of drugs or drug metabolites.
The histopathology of Blaschko line dermatitis is mainly characterized by spongiform dermatitis[20-22], and there are also reports of interfacial dermatitis[23]. The histopathology of our patient showed changes in spongiform dermatitis, as well as interfacial dermatitis.
Some lesions distributed along the Blaschko line can spontaneously resolve, which is difficult to explain by genetic pathogenesis, and some scholars believe that it may be related to the enhanced peripheral nerve response to foreign stimuli caused by neurological dysfunction[24]. In our case, the rash did not increase after discontinuing the sensitizing drugs, indicating that the external stimulant drugs play an important role in the occurrence of inflammation. The patient was treated with antihistamines and glucocorticoids, and the rash subsided completely, indicating that the rash was caused by an allergic reaction to drugs rather than by pathogenic microorganisms.
Drug eruption along the Blaschko line caused by drugs is rare. Sigal-nahum et al[25] reported linear fixed drug eruption in the left lower limb after intramuscular injection of cefazoline. Ozkaya-Bayazit et al[26] found that trimethoprim induced linear fixed drug eruption on the right arm. Coskun et al[27] found that calcium acetate induced Blaschko line drug eruption, extending from the right shoulder to the flexor surface of the right forearm. Das et al[28] reported that azithromycin induced linear fixed drug eruption over the midline of lower back. Brinkmeier et al[29] showed that metronidazole induced Blaschko line drug eruption on the right chest and abdomen and right upper limb. Couderc et al[30] showed that cestuzumab induced Blaschko line drug eruption on the left lower limb. In our case, drug eruption occurred after oral administration of Chinese patent medicine compound Honeysuckle granules, and the rash was distributed along the Blaschko line on the chest and abdomen, with no separation at the midline. No clinical reports on the similar case have been reported.
There are few drug-induced eruptions along the Blaschko line. A rash along the Blaschko line has been reported mainly on one side of the trunk or limb and is induced by western medicine. The rash in the present case was caused by oral Chinese patent medicine compound Honeysuckle granules and appeared on both sides of the thorax and abdomen along the Blaschko line. To our knowledge, this is the first report of drug eruption along the Blaschko line caused by Chinese patent medicine compound Honeysuckle.
We sincerely thank all the medical staff who treated this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM, Poland; Chae HB, South Korea S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Yawalkar N. Drug hypersensitivity. Acta Clin Belg. 2009;64:529-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Lerch M, Pichler WJ. The immunological and clinical spectrum of delayed drug-induced exanthems. Curr Opin Allergy Clin Immunol. 2004;4:411-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Chem Immunol Allergy. 2012;97:1-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Huang HY, Luo XQ, Chan LS, Cao ZH, Sun XF, Xu JH. Cutaneous adverse drug reactions in a hospital-based Chinese population. Clin Exp Dermatol. 2011;36:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Patel RM, Marfatia YS. Clinical study of cutaneous drug eruptions in 200 patients. Indian J Dermatol Venereol Leprol. 2008;74:430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Hari Y, Frutig-Schnyder K, Hurni M, Yawalkar N, Zanni MP, Schnyder B, Kappeler A, von Greyerz S, Braathen LR, Pichler WJ. T cell involvement in cutaneous drug eruptions. Clin Exp Allergy. 2001;31:1398-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Zanni MP, Mauri-Hellweg D, Brander C, Wendland T, Schnyder B, Frei E, von Greyerz S, Bircher A, Pichler WJ. Characterization of lidocaine-specific T cells. J Immunol. 1997;158:1139-1148. [PubMed] [Cited in This Article: ] |
| 8. | Cornejo-Garcia JA, Fernandez TD, Torres MJ, Carballo M, Hernan I, Antunez C, Blanca M, Mayorga C. Differential cytokine and transcription factor expression in patients with allergic reactions to drugs. Allergy. 2007;62:1429-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Yawalkar N, Egli F, Hari Y, Nievergelt H, Braathen LR, Pichler WJ. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, Hu SL, Wu MT, Chen GS, Wong TW, Hsiao PF, Chen WH, Shih HY, Fang WH, Wei CY, Lou YH, Huang YL, Lin JJ, Chen YT. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 453] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 12. | Wang F, Li C, Zheng Y, Li Y, Peng G. Study on the anaphylactoid of three phenolic acids in Honeysuckle. J Ethnopharmacol. 2015;170:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Jackson R. The lines of Blaschko: a review and reconsideration: Observations of the cause of certain unusual linear conditions of the skin. Br J Dermatol. 1976;95:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 179] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Happle R, Assim A. The lines of Blaschko on the head and neck. J Am Acad Dermatol. 2001;44:612-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Grosshans EM. Acquired blaschkolinear dermatoses. Am J Med Genet. 1999;85:334-337. [PubMed] [Cited in This Article: ] |
| 16. | Molho-Pessach V, Schaffer JV. Blaschko lines and other patterns of cutaneous mosaicism. Clin Dermatol. 2011;29:205-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Moss C. Cytogenetic and molecular evidence for cutaneous mosaicism: the ectodermal origin of Blaschko lines. Am J Med Genet. 1999;85:330-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Munro CS, Wilkie AO. Epidermal mosaicism producing localised acne: somatic mutation in FGFR2. Lancet. 1998;352:704-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Lipsker D, Cribier B, Girard-Lemaire F, Flori E, Grosshans E. Genetic mosaicism in an acquired inflammatory dermatosis following the lines of Blaschko. Arch Dermatol. 2000;136:805-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Grosshans E, Marot L. [Blaschkitis in adults]. Ann Dermatol Venereol. 1990;117:9-15. [PubMed] [Cited in This Article: ] |
| 21. | Megahed M, Reinauer S, Scharffetter-Kochanek K, Milde P, Hölzle E, Goerz G, Ruzicka T. Acquired relapsing self-healing Blaschko dermatitis. J Am Acad Dermatol. 1994;31:849-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Lee HJ, Kang WH, Hann SK. Acquired Blaschko dermatitis: acquired relapsing self-healing Blaschko dermatitis. J Dermatol. 1996;23:639-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Johnson M, Walker D, Galloway W, Gardner JM, Shalin SC. Interface dermatitis along Blaschko's lines. J Cutan Pathol. 2014;41:950-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Gupta S, Gupta S, Thomas M, Mahendra A. Unilateral lichen planus along the lines of Blaschko: a rare clinical presentation. Acta Med Indones. 2012;44:163-164. [PubMed] [Cited in This Article: ] |
| 25. | Sigal-Nahum M, Konqui A, Gaulier A, Sigal S. Linear fixed drug eruption. Br J Dermatol. 1988;118:849-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Ozkaya-Bayazit E, Baykal C. Trimethoprim-induced linear fixed drug eruption. Br J Dermatol. 1997;137:1028-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Coskun B, Saral Y, Ozturk P, Karincaoglu Y, Cobanoglu B. Calcium acetate-induced linear fixed drug eruption. Dermatology. 2005;210:244-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Das A, Ghosh S, Coondoo A, Kumar P. Azithromycin-Induced Linear Fixed Drug Eruption: A Rare Instance. Indian Dermatol Online J. 2021;12:353-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Brinkmeier T, Herbst RA, Schaller J, Kuegler K, Pirker C, Beiteke U, Grosshans E, Frosch PJ. Drug-induced blaschkitis. Acta Derm Venereol. 2004;84:314-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Couderc M, Soubrier M. Blaschkitis under certolizumab for rheumatoid arthritis. Joint Bone Spine. 2014;81:372-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |