Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7906
Peer-review started: October 13, 2021
First decision: January 11, 2022
Revised: January 24, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 6, 2022
Latamoxef shows excellent antibacterial activity against anaerobic bacteria such as Bacteroides fragilis. Reports of thrombocytopenic toxicity of latamoxef are limited. This report presents a case of severe thrombocytopenia possibly induced by latamoxef, an infrequent adverse drug reaction in a young patient with tuber
We reported a case of severe thrombocytopenia induced by latamoxef in a 28-year-old man with tuberculosis and Crohn's disease. On admission, the patient presented with a cough productive of bloody sputum, a chest computed tomogram suggested scattered mottled, high-density shadows in both lungs. Laboratory tests indicated a platelet count of 140000/μL. Considered a pulmonary bacterial infection, the patient received anti-infection therapy with latamoxef (dose: 2.0 g) intravenously Q12h. On the 9th day of treatment, the platelet count decreased to 44000/μL. On the 12th day, scattered purpura and ecchymosis appeared on the patient’s limbs and trunk, and the platelet count decreased to 9000/μL after latamoxef treatment for 15 d. Three days after discontinuation of latamoxef, the platelet count recovered to 157000/μL, and the area of scattered purpura and ecchymosis on the limbs and trunk decreased. The platelet counts remained in the normal range, and no thrombocytopenia was found at follow-up 15 mo after discharge.
For patients treated with latamoxef, platelet counts should be carefully followed, and caregivers should be vigilant for the appearance of scattered ecchymosis.
Core Tip: We described a case of severe thrombocytopenia likely induced by latamoxef, an infrequent adverse drug reaction in a young patient with tuberculosis and Crohn's disease. We followed the changes in platelet counts and the appearance of purpura during latamoxef treatment and after drug withdrawal and excluded other possible causes of thrombocytopenia. Our findings suggested that the patient's thrombocytopenia was caused by latamoxef. This is the first reported case of severe thrombocytopenia induced by latamoxef in a young Chinese patient to the best of our knowledge.
- Citation: Zhang RY, Zhang JJ, Li JM, Xu YY, Xu YH, Cai XJ. Latamoxef-induced severe thrombocytopenia during the treatment of pulmonary infection: A case report. World J Clin Cases 2022; 10(22): 7906-7912
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7906.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7906
Latamoxef is a new semisynthetic oxacephem antibiotic structurally similar to third-generation cephalosporins. Latamoxef has excellent antibacterial activity against anaerobic bacteria such as Bacteroides fragilis. It is stable against β-lactamases produced by most Enterobacteriaceae, mediated by plasmids or partially by chromosomes[1]. The primary associated adverse reactions are rash, drug fever, hepatic and renal dysfunction, neutropenia, and eosinophilia, followed by coagulation dysfunction, with an incidence of 12.45%[2,3]. Thrombocytopenia is a common blood disorder characterized by the destruction of circulating platelets and inhibition of platelet production[4]. Although several studies have reported that latamoxef could cause thrombocytopenia[5-7], thrombocytopenia induced by latamoxef in the Chinese population is rare and clinicians often overlook latamoxef-induced thrombocytopenia. This case report presents the first case of severe thrombocytopenia and multiple ecchymoses caused by latamoxef in a young Chinese patient. We defined thrombocytopenia as a platelet count less than 100000/μL[8].
A 28-year-old male patient presented to the hospital with a fever for one month and a cough for more than ten days.
He developed a fever at about 38℃ without obvious inducement one month prior and went to another hospital’s emergency department. He received a diagnosis of upper respiratory tract infection. The symptoms subsided after symptomatic treatment. More than 10 days before presentation, the patient had a paroxysmal cough with white sticky sputum and was diagnosed with pneumonia. Symptoms did not improve after expectorant treatment. In the days before the presentation, he had developed yellow and bloody sputum accompanied by night sweats.
The patient had a history of Crohn’s disease for more than five years and took mesalazine sustained-release tablets. Half a year prior, he stopped the mesalazine and switched to adalimumab injection once every two weeks, and he was in stable condition at presentation.
There is no specific family history of illness.
Several enlarged lymph nodes were found on the left and right sides of the patient's neck. The skin color was normal without ecchymosis, and respiratory rate and vital signs were normal.
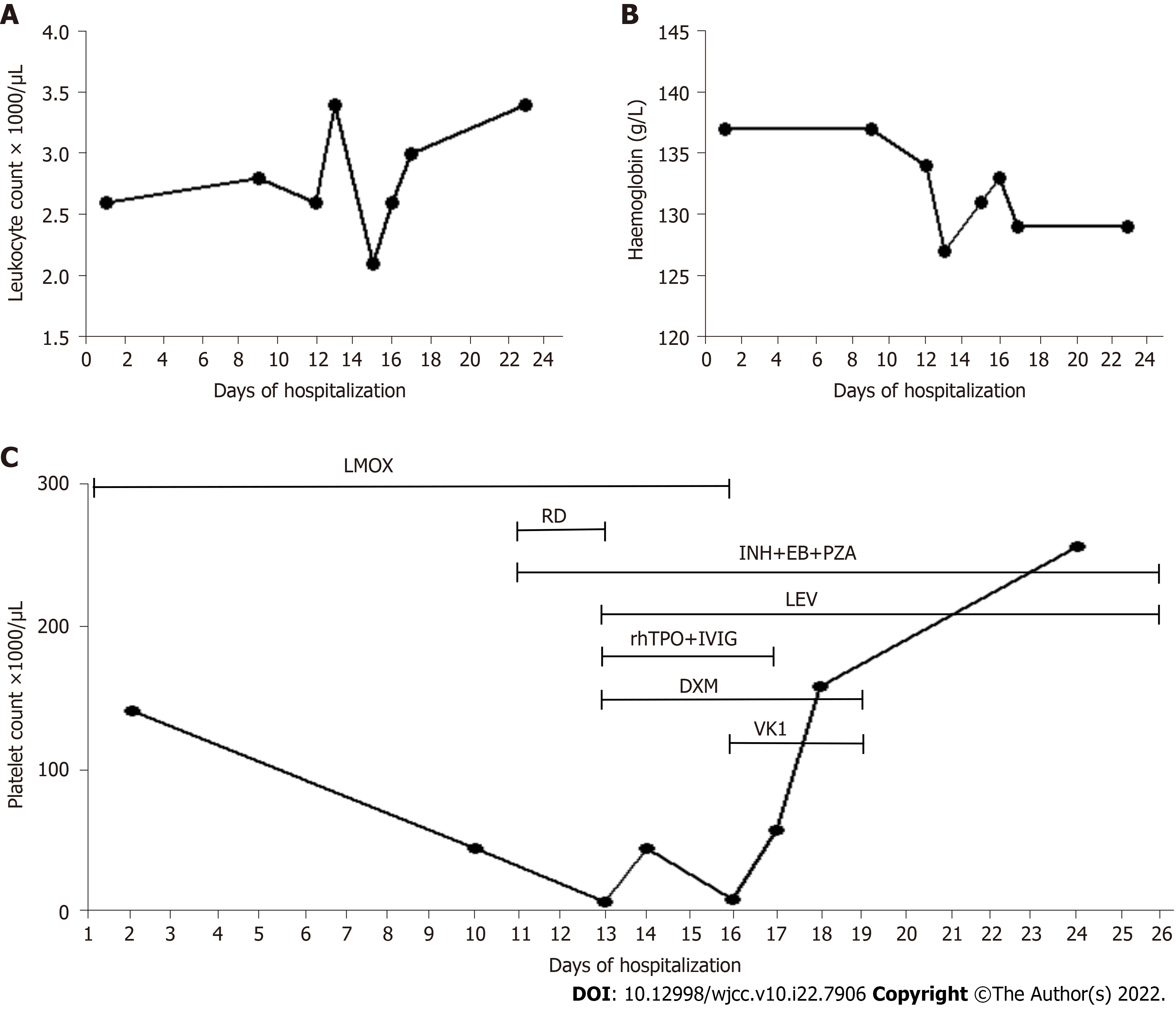
White blood cell (WBC) count was below the normal range, while hemoglobin (HGB) and platelet count were at normal levels (Figure 1). Other test indicators were in the normal ranges.
A chest computed tomogram suggested scattered mottled, high-density shadows in both lungs, mediastinal and hilar lymph node enlargement, and several nodules in the spleen.
Secondary tuberculosis with sputum smear-negative, initial treatment; Cervical lymphatic tuberculosis; Splenic tuberculosis; Crohn's disease; Thrombocytopenia; Leukopenia.
Because the diagnosis of tuberculosis was not clear initially, we considered it a bacterial infection. The patient first received anti-infective therapy with latamoxef (2.0 g) intravenously every 12 h and leucogen tablets (20.0 mg) three times per day for leukocytopenia. The timeline of the overall treatment process is presented in Table 1.
| Time | Symptom | Platelet counts | Treatment |
| Day 1 | Bloody sputum, scattered mottled, high-density shadows in both lungs | 140000/μL | Latamoxef (dose: 2.0 g) intravenously Q12H |
| Day 9 | Chills and fever to 38.2 ℃ (18:00) | 44000/μL (8:00 am) | - |
| Day 10 | Positive T SPOT-TB testing results | - | Added isoniazid tablets 0.3 g QD, rifampicin capsules 0.6 g QD |
| Day 11 | Secondary pulmonary tuberculosis, cervical lymph node tuberculosis, and splenic tuberculosis were confirmed | - | Continued adding pyrazinamide 0.5 g TID, ethambutol 1.0 g QD |
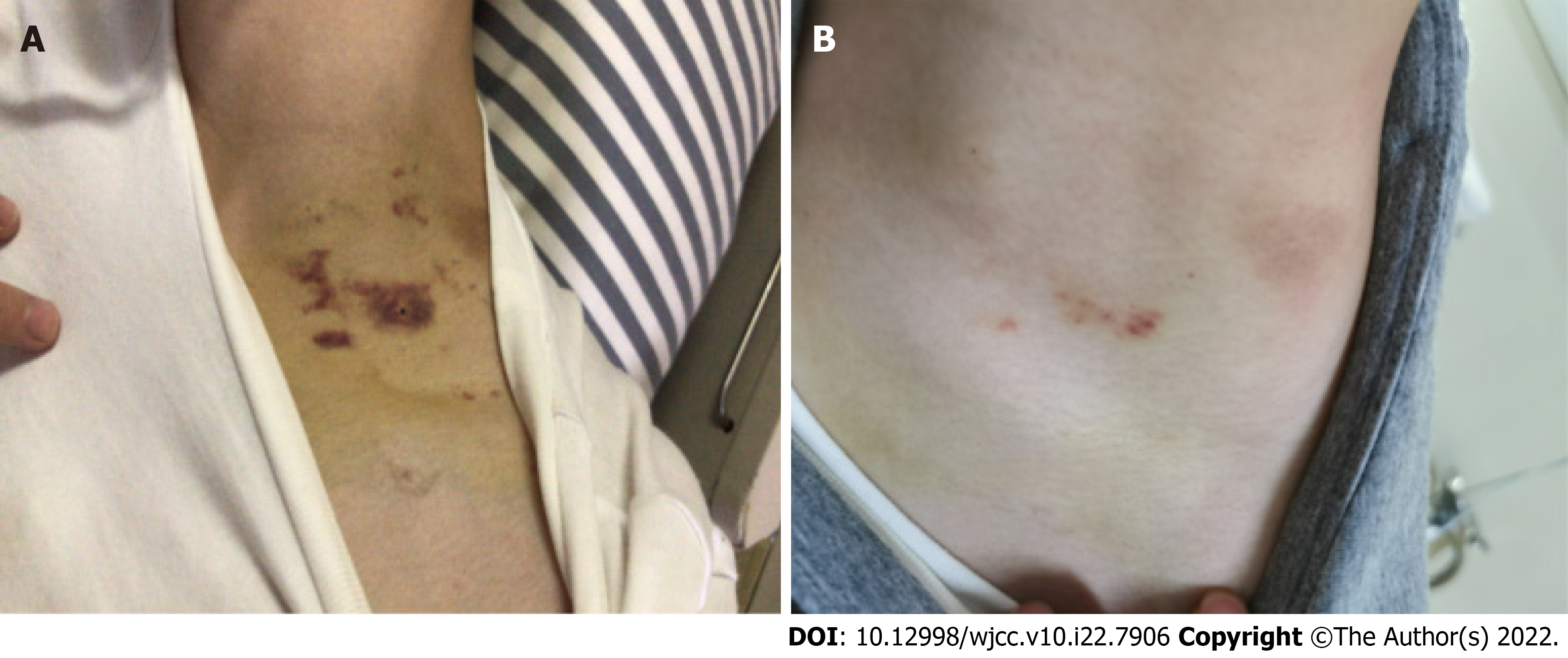
| Day 12 | Body temperature returned to normal but scattered purpura and ecchymosis appeared on his limbs and trunk's skin | 7000/μL | Replaced rifampicin with levofloxacin; Added recombinant human thrombopoietin (15000 units/d), human immunoglobulin (20.0 g/d), 15 units platelets, and 5 mg dexamethasone |
| Day 13 | Hemoptysis | 44000/μL | Continued adding tranexamic acid sodium chloride (0.5 g/d), etamsylate (2.0 g/d) and spearhead agkistrodon hemocoagulase (2.0 U/d) for hemostasis |
| Day 15 | Critical state | 9000/μL | Discontinued latamoxef 2.0 g Q12H and added vitamin K1 (10 mg/d) |
| Day 16 | - | 57000/μL | Discontinued the human immunoglobulin injection and recombinant human thrombopoietin |
| Day 17 | - | 157000/μL | Discontinued vitamin K1 and dexamethasone |
| Day 23 | - | 255000/μL | - |
| Day 24 | Discharged | - | Took isoniazid, ethambutol, pyrazinamide, and levofloxacin for tuberculosis treatment |
| The 1, 3, 5 wk, and 15-mo after discharge | - | Normal | Took isoniazid, ethambutol, pyrazinamide, and levofloxacin for tuberculosis treatment |
On day 9 (18:00 h) after initiation of latamoxef treatment, the patient developed chills and fever to 38.2 ℃ without shivering and cough with a small amount of sputum. On day 10, after initiation of latamoxef treatment, the patient received isoniazid tablets (0.3 g/d) and rifampicin capsules 0.6 g daily, considering his history of immunosuppressive agents and positive T SPOT-TB testing results, and latent infection with Mycobacterium tuberculosis was evident. On day 11, cervical lymph node aspirate fluid grew Mycobacterium tuberculosis complex sensitive to rifampicin. Pathological examination of a biopsy specimen from a left cervical lymph node revealed chronic granulomatous lymphadenitis with coagulative necrosis. Considering the presence of secondary pulmonary tuberculosis, cervical lymph node tuberculosis, and splenic tuberculosis, we added pyrazinamide 0.5 g three times per day and ethambutol 1.0 g daily in combination with isoniazid and rifampicin.
On the 12th day, the patient’s body temperature returned to normal but scattered purpura and ecchymosis appeared on his limbs and trunk. The platelet count decreased to 7000/μL. Considering that this might be thrombocytopenia induced by rifampicin, we replaced rifampicin with levofloxacin sodium chloride injection, 0.5 g intravenous drip once a day. Following consultation with hematology, we added subcutaneous injection of recombinant human thrombopoietin at 15000 units per day and intravenous infusion of human immunoglobulin (20.0 g/d), 15 units of platelets, and 5 mg of dexamethasone. The patient developed hemoptysis on day 13, and we added intravenous infusion of tranexamic acid sodium chloride (0.5 g/d), etamsylate (2.0 g/d) and spearhead agkistrodon hemocoagulase (2.0 U/d) for hemostasis.
On the 15th day, the platelet count decreased to 9000/μL, suggesting that the patient was in a critical state. Because the patient could not afford the medications, pharmacists simplified the prescriptions. We recommended discontinuing latamoxef 2.0 g Q12H and adding an intravenous injection of 10 mg of vitamin K1 once a day, and the clinicians agreed. On the 16th day, the platelet count increased to 57000/μL. We discontinued the human immunoglobulin injection and recombinant human throm
The patient was followed up at the first, third, and fifth week and monthly after discharge. The platelet counts and the HGB concentrations remained stable and in the normal range. Prothrombin and activated partial thromboplastin were normal from admission to platelet recovery. No thrombocytopenia was found at follow-up 15 mo after discharge.
Our patient's thrombocytopenia induced by latamoxef was unique. To our best knowledge, this is the first documented case in a young Chinese patient. Vayne et al[9] reported that drug-mediated immune thrombocytopenia often gave rise to a higher risk of bleeding. Generally, thrombocytopenia occurs after 5 to 10 d of drug exposure, and the median platelet count is usually less than 20000/μL. Platelet counts usually begin to recover at four to five half-lives or within two to three days after discontinuation[8,9]. The literature suggested that rifampicin had a strong tendency to cause thrombocytopenia with an incidence of between 1% and 10%[10]. A systematic evaluation of 153 drugs conducted by Arnold et al[11] found that the most drugs contributing to drug-induced immune thrombocytopenia were rifampicin, quinine, vancomycin, and ceftriaxone.
The patient started oral rifampicin on the 10th day and stopped on the 12th day. We excluded rifampicin-induced immune thrombocytopenia based on the following criteria: (1) The time of occurrence was not in line with expectations. Before taking rifampicin, the patient received latamoxef alone. At that time, the platelet count decreased significantly from 140000/μL to 44000/μL (by 68.57%); (2) The exposure time of rifampicin was short (only two days), far less than the exposure time of five to ten days; this exposure was not sufficient to cause a decline in the platelet count[8,9,12]; and (3) The elimination half-life of rifampicin is three to five hours, and the patient had been off rifampicin for three days before the recurrence of thrombocytopenia; this time-course was inconsistent with the reported recovery of platelet counts after four to five half-lives. We transfused 15 units of platelets and administered human immunoglobulin, glucocorticoid after discontinuation of rifampicin to retard platelet clearance; however, the patient’s platelet count remained at 9000/μL on the 4th day after discontinuation of rifampicin. These results suggest that rifampicin was not the primary cause of drug-induced immune thrombocytopenia.
According to an approach proposed by Arnold et al[13], the diagnosis of drug-induced immune thrombocytopenia is based on the following four criteria: (1) Severity of thrombocytopenia: platelet count nadir below 20000/μL; (2) Clinical signs: Any bleeding; (3) Time to onset: Platelet counts fall 5-10 d after initiation of a new drug or exposure to a drug previously taken; and (4) Use of drugs already identified as responsible for drug-induced immune thrombocytopenia (with clinical and laboratory tests), with the drug previously associating with drug-induced immune thrombocytopenia by clinical and laboratory criteria[13]. The first three criteria matched our patient’s presentation. Because of our hospital's limited laboratory conditions, we could not directly measure drug-dependent platelet antibodies using immunoassay or flow cytometry. Therefore, the fourth criterion could not be confirmed.
We excluded possible causes of thrombocytopenia such as tuberculosis of the spleen, pseudothrombocytopenia, primary immune thrombocytopenia, other drug-induced immune thrombocytopenia, food and beverages, infections, hypersplenism due to chronic liver disease, excessive alcohol intake, nutritional deficiencies, rheumatologic diseases, thrombotic microangiopathy, myelodysplasia, cancer with disseminated intravascular, coagulation, cancer with bone marrow infiltration or suppression, and post-transfusion purpura. On the Naranjo scale, our patient scored six, placing him in the category of potential drug-related toxicity[14]. We could not rechallenge the patient with latamoxef for apparent reasons. According to our findings, latamoxef was the cause of the drug-induced immune thrombocytopenia.
The original instructions for latamoxef did not mention thrombocytopenia or coagulation dysfunction. Some studies mentioned that the N-methyl tetrazolium side-chain in latamoxef could lead to prothrombin deficiency, thrombocytopenia, platelet dysfunction, and bleeding. In such cases, one should supplement with vitamin K to reduce adverse reactions such as coagulation dysfunction and bleeding[2,15]. We searched PubMed, Embase, CNKI, Wan-Fang, and VIP database, and located four articles related to thrombocytopenia caused by latamoxef[5-7,16]. Although several studies reported that latamoxef could cause thrombocytopenia, thrombocytopenia induced by latamoxef in the Chinese population has never been reported previously. The literature suggests that one should use latamoxef cautiously in elderly patients with hepatic and renal dysfunction, history of ulcers, long-term use of broad-spectrum antibiotics, poor coagulation function, bleeding tendency, or use of anticoagulant and antiplatelet drugs[5,6,16]. The patient in our case had none of these risk factors; however, he had recurrent fevers for more than one month. Fever leads to high metabolic rates, and disseminated tuberculosis is a consumptive disease that reduces immunity. He also had Crohn’s disease for more than five years and was treated with adalimumab as immunosuppressive therapy. Overall, the patient’s tolerance to drug-induced thrombocytopenia was lower than that of healthy adults.
Therefore, we suggested that latamoxef should be discontinued immediately when patients with thrombocytopenia suspected to be caused by latamoxef, the platelet count is less than 20000/μL and complicated by bleeding or blood loss anemia. Moreover, first-line drug treatment such as corticosteroid, human immunoglobulin, platelet-raising drugs, and transfusion of platelets or coagulation factor should be considered to alleviate the symptoms as soon as possible. We also recommend that thrombocytopenia be included among the adverse effects in the Chinese instructions for latamoxef.
This is the first case of severe thrombocytopenia induced by latamoxef in a young Chinese patient. For patients treated with latamoxef, platelet counts should be carefully monitored, and clinicians should be vigilant for the appearance of scattered ecchymoses. Clinicians should discontinue latamoxef imme
We would like to thank all medical staff who provided data and supported the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gaman MA, Romania; Socea B, Romania S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Wang F, Zhang YY. Guidelines for clinical application of antibiotics. 3rd ed. People’s Medical Publishing House 2020.. [Cited in This Article: ] |
| 2. | State Pharmacopoeia Committee. Pharmacopoeia of the people’s Republic of China, instructions for clinical use: chemical and biological products volume. 2015 edition. Zhongguo Yixue Keji Chubanshe. 2017;. [Cited in This Article: ] |
| 3. | Ma MH, Yu ZC, Xue DM, Tian WW, Liu X, Wang F. Analysis of risk factors of coagulation dysfunction induced by latamoxef sodium. Shijie Linchuang Yaowu Zazhi. 2020;41:719-722. [Cited in This Article: ] |
| 4. | Socea B, Diaconu C, Bratu OG, Pantea Stoian A, Constantin VD. Splenectomy in Immune Thrombocytopenia: When, Why and How? J Palliat Care. 2019;12:16-19. [Cited in This Article: ] |
| 5. | Au JP, Geiger GS. Thrombocytopenia associated with moxalactam administration. Drug Intell Clin Pharm. 1984;18:140-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Gabryelewicz A, Prokopowicz J, Wołosowicz N, Czajkowski A, Dabrowska M. Changes in some hemostatic parameters in patients with infections treated with ceftazidime and latamoxef. Folia Haematol Int Mag Klin Morphol Blutforsch. 1987;114:398-407. [PubMed] [Cited in This Article: ] |
| 7. | Fekety FR. Safety of parenteral third-generation cephalosporins. Am J Med. 1990;88:38S-44S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. |
|
| 9. | Vayne C, Guéry EA, Rollin J, Baglo T, Petermann R, Gruel Y. Pathophysiology and Diagnosis of Drug-Induced Immune Thrombocytopenia. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Maurício J, Flor-de-Lima B, Pacheco P. Severe rifampicin-induced thrombocytopenia in a patient with miliary tuberculosis. Pulmonology. 2020;26:247-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Arnold DM, Kukaswadia S, Nazi I, Esmail A, Dewar L, Smith JW, Warkentin TE, Kelton JG. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost. 2013;11:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Sun P. Study on the mechanism of rifampicin-induced immune thrombocytopenia. Liaoning Province: Jinzhou Medical University, 2016. [Cited in This Article: ] |
| 13. | Arnold DM, Nazi I, Warkentin TE, Smith JW, Toltl LJ, George JN, Kelton JG. Approach to the diagnosis and management of drug-induced immune thrombocytopenia. Transfus Med Rev. 2013;27:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7061] [Cited by in F6Publishing: 7634] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 15. | Working group on revision of guiding principles for clinical application of antibiotics. Guiding principles of clinical application of antibiotics 2015 edition. Beijing, People’s Medical Publishing House, 2015. [Cited in This Article: ] |
| 16. | Ye SY, Zeng CL. A case of severe thrombocytopenia caused by latamoxef. J Pract Med. 2013;29:3786. [Cited in This Article: ] |