Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6198
Peer-review started: November 9, 2021
First decision: December 27, 2021
Revised: January 13, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 26, 2022
Camrelizumab (SHR-1210), an immune checkpoint inhibitor, is clinically used as a therapeutic option for various types of tumors. However, reports of adverse reactions associated with camrelizumab are gradually increasing. Anaphylactic shock due to camrelizumab has not been reported previously, until now. We report here, for the first time, a case of anaphylactic shock associated with camrelizumab in a patient with esophageal squamous cell carcinoma.
An 84-year-old male esophageal cancer patient received radiotherapy and chemotherapy 11 years ago. He was diagnosed with advanced esophageal squamous cell carcinoma with liver metastasis (TxN1M1) and received the first immunotherapy (camrelizumab 200 mg/each time, once every 3 wk) dose in December 2020, with no adverse reactions. Three weeks later, a generalized rash was noted on the chest and upper limbs; palpitations and breathing difficulties with a sense of dying occurred 10 min after the patient had been administered with the second camrelizumab therapy. Electrocardiograph monitoring revealed a 70 beats/min pulse rate, 69/24 mmHg (1 mmHg = 0.133 kPa) blood pressure, 28 breaths/min respiratory rate, and 86% pulse oximetry in room air. The patient was diagnosed with anaphylactic shock and was managed with intravenous fluid, adrenaline, dexamethasone sodium phosphate, calcium glucosate, and noradrenaline. Approximately 2 h after treatment, the patient’s anaphylactic shock symptoms had been completely relieved.
Due to the widespread use of camrelizumab, attention should be paid to anti-programmed cell death 1 antibody therapy-associated hypersensitivity or anaphylactic shock.
Core Tip: Since its approval in 2019 by the National Drug Administration, camrelizumab (SHR-1210), a programmed cell death 1 inhibitor, is in wide clinical use as a therapeutic option for various tumor types. Until now, allergic reactions induced by camrelizumab have been rarely reported from various studies. For the first time, we report a case of camrelizumab-induced anaphylactic shock in a patient with esophageal squamous cell carcinoma.
- Citation: Liu K, Bao JF, Wang T, Yang H, Xu BP. Camrelizumab-induced anaphylactic shock in an esophageal squamous cell carcinoma patient: A case report and review of literature. World J Clin Cases 2022; 10(18): 6198-6204
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6198.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6198
Since its approval in 2019 by the National Drug Administration, camrelizumab (SHR-1210), an immune checkpoint inhibitor (ICI), is in wide clinical use as a therapeutic option for various tumor types[1]. However, reports of camrelizumab-associated adverse reactions are increasing gradually, with any organ or tissue being affected. Reactive cutaneous capillary endothelial proliferation is the most common adverse event that is associated with camrelizumab, with an incidence accounting for about two-thirds of all patients treated with camrelizumab[2]. It is followed by immune-related hepatitis, pneumonia, and myocarditis among other clinical complications[3,4]. Until now, allergic reactions induced by ICIs have been reported in various studies[5,6].
As a relatively new programmed cell death 1 (PD-1) inhibitor, camrelizumab-induced anaphylactic shock has not yet been reported. We report here, for the first time, a case of camrelizumab-induced anaphylactic shock in a patient being treated for esophageal squamous cell carcinoma.
An 84-year-old male patient (163 cm in height, 41 kg in weight) presenting with esophageal cancer was administered with radiotherapy and chemotherapy 11 years prior, after which he got better.
In December 2020, the patient was diagnosed with advanced esophageal squamous cell carcinoma with liver metastasis, classified as stage TxN1M1. Based on the 2020 Chinese Society of Clinical Oncology guidelines, the patient was administered the first immunotherapeutic (camrelizumab 200 mg/each time + 0.9% NS 100 mL, intravenous infusion, q3w) and did not exhibit any adverse reactions. On January 12, 2021, the patient was admitted to the hospital for the second time to be administered the same therapy. On January 19, 2021, the patient was introduced to intravenous infusions of camrelizumab. However, 10 min after initiating intravenous camrelizumab, he suddenly developed a generalized rash in the chest and upper limbs. He also experienced chest tightness without chest pain, palpitations, and breathing difficulties with a sense of dying.
The patient had a previous medical history free of allergy.
The patient had no significant personal or family history.
Electrocardiograph (ECG) monitoring revealed a pulse rate of 70 beats/min, blood pressure of 69/24 mmHg, a respiratory rate of 28 breaths/min, and a pulse oximetry of 86% in room air (no other medication was administered concomitantly). The patient presented with drowsiness and weakened cardiac sounds as well as a weak major arterial pulse.
Blood analysis revealed white blood cell count of 7.04 × 109/L, neutrophil count of 2.81 × 109/L (normal range: 2.0-7.5 × 109/L), neutrophil percentage of 39.90%, red blood cell count of 2.35 × 1012/L, hemoglobin level of 66.00 g/L (normal range: 110-160 g/L), platelet count of 219.00 × 109/L (normal range: 100-300 × 109/L), C-reactive protein level of 31.61 mg/L (normal range: < 0.5 mg/L), potassium level of 2.12 mmol/L (normal range: 3.5-5.0 mmol/L), chloride level of 117.80 mmol/L (normal range: 96-108 mmol/L), and calcium level of 1.41 mmol/L (normal range: 2.0-2.6 mmol/L). Markers of renal function and levels of cardiac enzyme and troponin were normal.
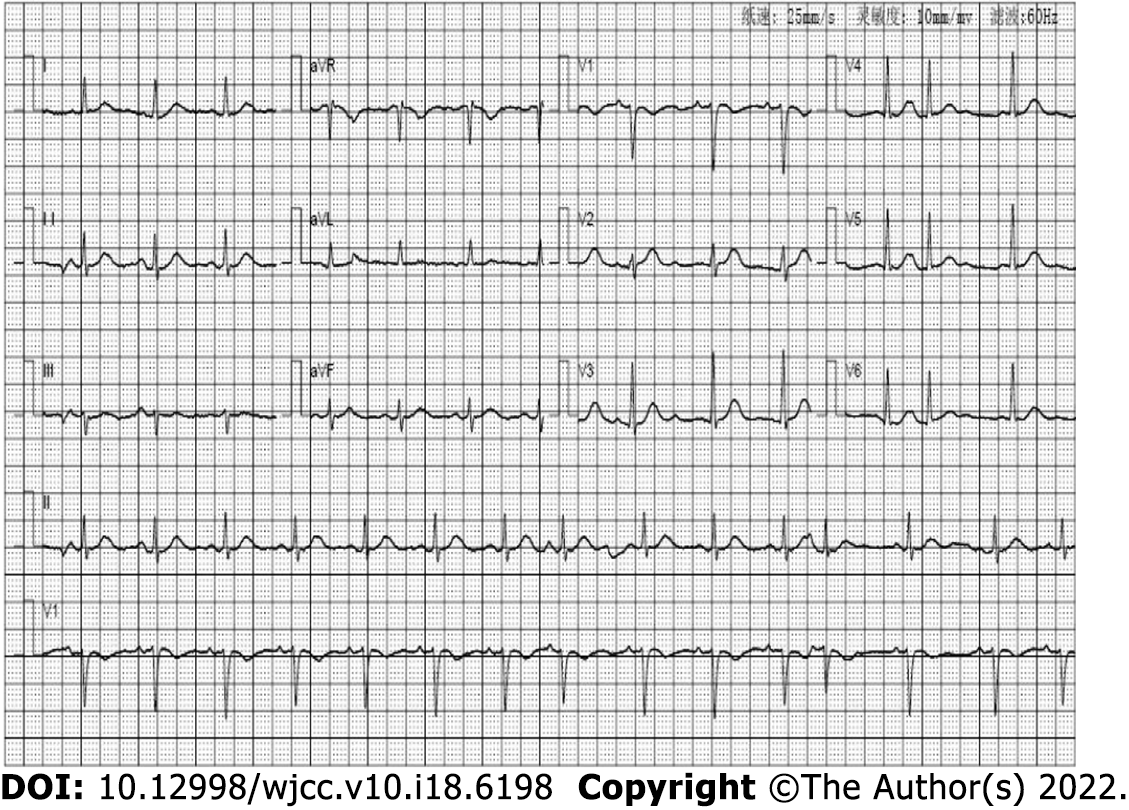
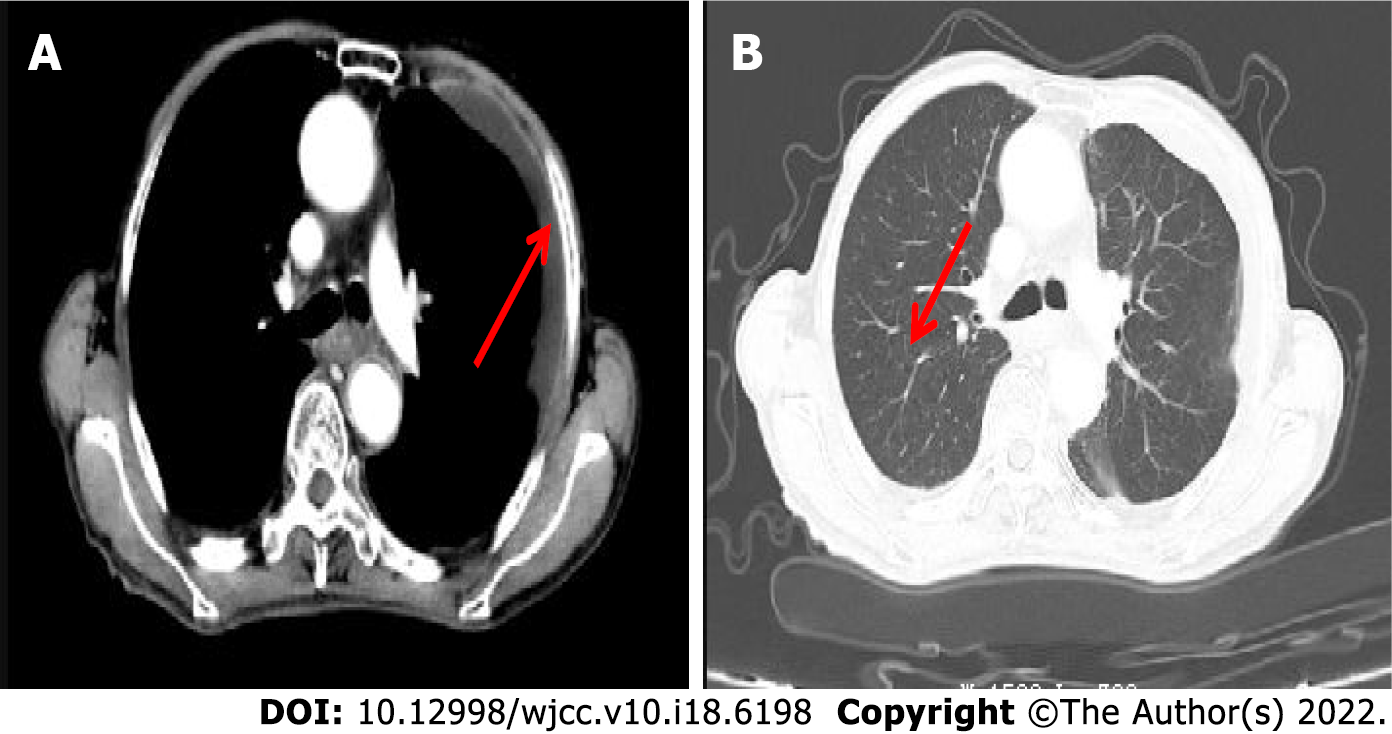
ECG (Figure 1) revealed a sinus rhythm. Enhanced computed tomography scan revealed chronic inflammation of the right lower lobe with left-side pleural slight effusion (Figure 2).
Based on the findings from the examination and investigations, we first considered the possibility of anaphylactic shock.
The intravenous camrelizumab infusion was stopped immediately. In lieu, treatment was begun with corticoids, adrenaline, norepinephrine and intravenous fluid. Continuous supplementation of intravenous potassium and calcium were also provided.
The patient reported his chest tightness to be significantly relieved. He also experienced no shortness of breath, palpitations, or discomfort. The upper limbs and chest rash subsided rapidly, at approximately 2 h after treatment. ECG monitoring revealed a pulse rate of 78 beats/min, blood pressure of 112/68 mmHg, respiratory rate of 19 breaths/min, and pulse oximetry of 99% (oxygen absorption at 2 L/min).
On January 20, 2021, biochemical examination revealed that serum potassium and calcium levels were normal. The basic treatment was continued, without repeated anaphylactic shock. It is very unfortunate, however, that the patient refused to return to use of the camrelizumab, due to his excessive fear of anaphylactic shock, even after the physician provided a sufficient explanation. As such, we consulted the published literature and found switching to another type of anti-PD-1 antibody to be a feasible alternative. Indeed, such an approach has been successfully reported, with patients exhibiting relatively good clinical effects without allergic reactions[5,6].
Another type of anti-PD-1 antibody, nivolumab, is an ICI with a similar mechanism of action that is effective for treatment. The adverse effect profile of nivolumab is similar to those of camrelizumab, so the drugs related to the prevention of allergic reactions should be administered as premedication 30 min prior to the nivolumab infusion. The drawback is that it is very expensive and, in China, it is not covered by insurance reimbursement plans. Therefore, the patient rejected the physician’s suggestion to replace the immunotherapy drugs. The patient was discharged on January 23, 2021.
Anaphylactic shock is a serious life-threatening acute systemic hypersensitivity reaction that is characterized by rapid development of life-threatening bronchospasms, or respiratory failure, or cardiovascular abnormalities. Sometimes, it is accompanied by general urticaria, erythema, and skin itch[7,8]. The symptoms associated with anaphylactic shock usually occur within minutes or less than 1 h after administration of the precipitating drug and result from activation of tissue mast cells and blood basophils, which release histamine and other inflammatory mediators[8]. Drug-induced anaphylactic shock accounts for a significantly high mortality rate among in-patients. Therefore, if not handled in time, it is often life-threatening[9].
Camrelizumab is a humanized PD-1 inhibitor that was developed by Jiangsu Hengrui Medicine Co. Ltd.[10]. It blocks the binding between programmed death ligand 1 and programmed death ligand 2 by targeting PD-1, thereby inhibiting tumor cell evasion from the immune system and ultimately causing an anti-tumor effect[11]. Camrelizumab has been clinically approved for the treatment of various tumors, including relapsed or refractory classical Hodgkins lymphoma, esophageal squamous cell carcinoma, hepatocellular carcinoma, and non-small cell lung cancer, among others[1,12]. Camrelizumab has therapeutic effects and has been shown to clinically improve various tumors, while having a manageable safety profile[13-17]. Moreover, it has exhibited potential anti-tumor effects in patients who failed chemotherapy or in those who are resistant to chemotherapy, while having an acceptable toxicity profile[18,19]. Due to the widespread application of camrelizumab, it has the potential to become a routine option for tumor immunotherapy[10]. However, camrelizumab-associated adverse events, including common reactive cutaneous capillary endothelial proliferation[2], immune-related hepatitis and pneumonia[3], immune-associated myocarditis[4], abnormal hepatic functions, anemia, and diarrhea[20], among others, have been reported. Most camrelizumab-associated adverse events are mild and can be regulated by interrupting treatment[20]. Camrelizumab-associated anaphylactic shock is rare but potentially fatal. Only two studies have reported on hypersensitivities induced by anti-programmed death ligand 1 agents[5,6]. Camrelizumab-associated allergic reactions or anaphylactic shock have never been reported previously. Therefore, the understanding of allergic reactions or anaphylactic shock caused by immune preparations such as camrelizumab is limited, which may create the potential for delays in identification and management during the early stages of hypersensitivity. This can lead to a life-threatening outcome. Here, we provide the first report of camrelizumab-associated anaphylactic shock, which should arouse the interest of clinicians.
Adverse reactions for this case were evaluated according to the national adverse drug reaction Evaluation Standard of China[21]. Our patient experienced sudden-onset of the anaphylactic shock, within 10 min after intravenous injection of the camrelizumab infusion, implying an obvious time correlation between camrelizumab administration and development of the adverse event. Although there is no description of anaphylactic shock adverse events in the instructions for camrelizumab, it has been reported that serious hypersensitivities can occur after administration of the same anti-PD-1 or anti-programmed death ligand 1 agents, such as nivolumab[5,6]. Anaphylactic shock is a special manifestation of anaphylactic reactions; therefore, based on the above evidence, it can be considered that camrelizumab may cause hypersensitivities, including anaphylactic shock. After withdrawal of camrelizumab and administration of related treatments (e.g., oxygen inhalation, anti-allergies, stable blood pressure treatment, and fluid resuscitation) were initiated, out patient’s blood pressure returned to normal, chest tightness symptoms were significantly relieved, and all his other medications were continued while he gradually improved after 3 h. Since then, the patient has not had symptoms of anaphylactic shock. In addition, the patient had no history of drug anaphylaxis, and there were no changes in the use of other drugs before and after the occurrence of anaphylactic shock. The patient did not experience anaphylactic shock again after stopping the camrelizumab treatment; this allowed us to exclude the association of anaphylactic shock for the other drugs he was taking. Since his Naranjo Adverse Drug Reaction Probability Scale score was 5[22], we concluded that the anaphylactic shock was most likely caused by the camrelizumab administration.
Infections can aggravate or induce the occurrence of severe allergic reactions. About 1.3% to 11.0% of adults with severe allergic reactions have infectious etiologies[23]. These allergic reactions may be attributed to the immunoglobulin G produced during infection[24,25]. The patient had no adverse reactions after the first camrelizumab therapy, and the second treatment plan was the same as the initial treatment. He had been admitted to the hospital due to esophageal tumor accompanied by lung infection. After anti-infection treatment, findings of routine blood tests, including white blood cell and neutrophil counts, were normal, while C-reactive protein levels decreased from 116.16 to 70.35 mg/L. Computed tomography of the patient’s lungs showed that his lesions were improved, while his lung infections had come under control. The patient suffered a sudden anaphylactic shock after second camrelizumab administration. Although the infection was controlled, the C-reactive protein levels remained elevated, implying that the inflammatory medium in the body had not been removed completely, which may have been one of the inducing factors of anaphylactic shock. Therefore, for patients with mixed infections, clinicians should be cautious in their application of camrelizumab.
Solvent mediums and drug configurations play an important role in hypersensitivity or anaphylactic shock. Camrelizumab is a powder, requiring suspension for injection. The drug manual requires that every 200 mg of camrelizumab be dissolved in 5 mL of sterilized injection water. For this, the sterile injection water is slowly added along the wall of the bottle containing the camrelizumab powder and dissolved by slow vortexing, to avoid direct sprinkling of water droplets on the surface of the powder. Then, the compound solution is extracted to make a 100 mL 0.9% sodium chloride solution or 5% glucose injection in an infusion bag dilution for intravenous administration by drip over a 30-minutes period. For our patient, the drug was prepared in strict accordance with these instructions, and the patient had no allergic reaction during the first dose. Therefore, neither the drug configuration nor solvent factors explain the patient’s anaphylactic shock.
Individual factors also lead to the occurrence of allergic diseases. Epidemiologically, most anaphylactic shock cases occur in the older population, with higher risks among those aged over 70 years[26]. The mortality rate for females is lower than that of males[9]. Our patient was an 84-year-old male with esophageal squamous cell carcinoma and liver metastases. Therefore, he was at a high risk of anaphylactic shock.
Due to its increased clinical use, camrelizumab-associated hypersensitivity or anaphylactic shock should arouse the attention of clinicians. There are limited specific treatments for anaphylactic shock in clinical practice. Therefore, early identification is very important[27]. Generally, drugs that may cause anaphylactic shock should be immediately discontinued. Open venous channels, oxygen inhalation, and ECG monitoring should be performed[28]. Epinephrine is often administered for anaphylactic shock, which can excite α receptors and constrict peripheral blood vessels[29]. Rapid intravenous fluids can restore the effective blood volume, and generally about 250-500 mL of the fluids are recommended. Vasoactive drugs, including norepinephrine and dopamine, are recommended if blood pressure cannot be maintained after fluid resuscitation[30]. Secondly, glucocorticoids and histamine receptor antagonists should be administered as anti-allergic treatments. In cases of severe dyspnea and laryngeal edema, emergency organ intubation and tracheotomy are required[28]. It has not been conclusively determined whether immunotherapy should be restarted after the occurrence of anaphylactic shock. Studies reported continuous immunotherapeutic administration after successfully trying desensitization therapy. However, re-anaphylactic shock and failure of desensitization treatment can occur during desensitization[6,31], and the safety and efficacy of desensitization therapy for patients with anaphylactic shock both need to be verified further. Switching to another immunotherapeutic drug is, thus, recommended. This approach has been applied successfully in previous studies, with the reported patients exhibiting relatively good clinical effects without allergic reactions[5,6].
Due to widespread use of camrelizumab, attention should be paid to anti-PD-1 blockade treatment-associated hypersensitivity or anaphylactic shock. We have reported herein a case of camrelizumab-induced anaphylactic shock in a patient with esophageal squamous cell carcinoma. Strengthening the monitoring of adverse drug reactions and identification of allergic reactions caused by camrelizumab treatment in the early stages should be taken into consideration by clinicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Allergy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Costa T, Portugal; Trabelsi M, Tunisia S-Editor: Guo XR L-Editor: A P-Editor: Guo XR
| 1. | Markham A, Keam SJ. Correction to: Camrelizumab: First Global Approval. Drugs. 2019;79:1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, Lin X, Chen X, Li E, Wang L, Yan P, Zou J. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 83] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 3. | Tan Y, Ye Y, Chen L. Fatal immune-related hepatitis with intrahepatic cholestasis and pneumonia associated with camrelizumab: A case report and literature review. Open Med (Wars). 2021;16:553-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Wang F, Sun X, Qin S, Hua H, Liu X, Yang L, Yang M. A retrospective study of immune checkpoint inhibitor-associated myocarditis in a single center in China. Chin Clin Oncol. 2020;9:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Choi B, McBride A, Scott AJ. Treatment with pembrolizumab after hypersensitivity reaction to nivolumab in a patient with hepatocellular carcinoma. Am J Health Syst Pharm. 2019;76:1749-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Sáenz de Santa María García M, Noguerado-Mellado B, Rojas-Pérez-Ezquerra P, Prieto-García A, Bartolomé-Zavala B, Tornero P. First case of allergy to nivolumab. J Allergy Clin Immunol Pract. 2017;5:1140-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Gouel-Cheron A, Neukirch C, Kantor E, Malinovsky JM, Tacquard C, Montravers P, Mertes PM, Longrois D. Clinical reasoning in anaphylactic shock: addressing the challenges faced by anaesthesiologists in real time: A clinical review and management algorithms. Eur J Anaesthesiol. 2021;38:1158-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Limsuwan T, Demoly P. Acute symptoms of drug hypersensitivity (urticaria, angioedema, anaphylaxis, anaphylactic shock). Med Clin North Am. 2010;94:691-710, x. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Liu FC, Chiou HJ, Kuo CF, Chung TT, Yu HP. Epidemiology of Anaphylactic Shock and its Related Mortality in Hospital Patients in Taiwan: A Nationwide Population-Based Study. Shock. 2017;48:525-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Song H, Liu X, Jiang L, Li F, Zhang R, Wang P. Current Status and Prospects of Camrelizumab, A Humanized Antibody Against Programmed Cell Death Receptor 1. Recent Pat Anticancer Drug Discov. 2021;16:312-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Su H, Yu C, Ma X, Song Q. Combined immunotherapy and targeted treatment for primary alveolar soft part sarcoma of the lung: case report and literature review. Invest New Drugs. 2021;39:1411-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wang CY, Sheng CC, Ma GL, Xu D, Liu XQ, Wang YY, Zhang L, Cui CL, Xu BH, Song YQ, Zhu J, Jiao Z. Population pharmacokinetics of the anti-PD-1 antibody camrelizumab in patients with multiple tumor types and model-informed dosing strategy. Acta Pharmacol Sin. 2021;42:1368-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Wang P, Fang X, Yin T, Tian H, Yu J, Teng F. Efficacy and Safety of Anti-PD-1 Plus Anlotinib in Patients With Advanced Non-Small-Cell Lung Cancer After Previous Systemic Treatment Failure-A Retrospective Study. Front Oncol. 2021;11:628124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Wang C, Li X, Dong L, Yang Q, Chen M, Shi F, Brock M, Liu M, Mei Q, Liu J, Nie J, Han W. Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother Cancer. 2021;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Chen X, Qin S, Gu S, Ren Z, Chen Z, Xiong J, Liu Y, Meng Z, Zhang X, Wang L, Zou J. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer. 2021;149:1944-1954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Liao R. Effectiveness of anti-PD-1 for hepatocellular carcinoma. Lancet Oncol. 2020;21:e293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, Zhao J, Er P, Zhang T, Chen X, Wang Y, Jiang Y, Wang Q, Zhang B, Qian D, Wang J, Zhou D, Ren X, Yu Z, Zhao L, Yuan Z, Wang P, Pang Q. Safety and Feasibility of Radiotherapy Plus Camrelizumab for Locally Advanced Esophageal Squamous Cell Carcinoma. Oncologist. 2021;26:e1110-e1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 18. | Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen J, Fang Y, Duan J, Zhou C, Hu Y, Yang H, Zhou J, Lin X, Wang L, Wang Z, Xu Y, Zhang T, Shi W, Zou J, Wang J. Camrelizumab Plus Apatinib in Extensive-Stage SCLC (PASSION): A Multicenter, Two-Stage, Phase 2 Trial. J Thorac Oncol. 2021;16:299-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Cong X, Chen J, Zheng W. The combination of camrelizumab and apatinib obtained ongoing partial remission for a patient with osimertinib-resistant non-small cell lung cancer: case report. Ann Palliat Med. 2021;10:3469-3474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, Chen J, Zhang H, Niu Z, Fan Q, Lin L, Gu K, Liu Y, Ba Y, Miao Z, Jiang X, Zeng M, Fu Z, Gan L, Wang J, Zhan X, Liu T, Li Z, Shen L, Shu Y, Zhang T, Yang Q, Zou J; ESCORT Study Group. Camrelizumab vs investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 317] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 21. | Li B, Gao R, Li R, Lu F, Zi MJ, Li QN, Tang XD. Causal determination of the adverse events and adverse drug reactions in drug clinical trials. Zhongguo Xinyao Zazhi. 2014;23:1465-1470. [DOI] [Cited in This Article: ] |
| 22. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7061] [Cited by in F6Publishing: 7634] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 23. | Jiang NN, Yin J. Trigger Factors and Mechanism of Anaphylaxis. Zhonghua Linchuangmianyi He Biantaifanying Zazhi 2016; 10: 269-275. [DOI] [Cited in This Article: ] |
| 24. | Mazur N, Patterson R, Perlman D. A case of idiopathic anaphylaxis associated with respiratory infections. Ann Allergy Asthma Immunol. 1997;79:546-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Custovic A, Murray C, Simpson A. Allergy and infection: understanding their relationship. Allergy. 2005;60 Suppl 79:10-13. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Park HK, Kang MG, Yang MS, Jung JW, Cho SH, Kang HR. Epidemiology of drug-induced anaphylaxis in a tertiary hospital in Korea. Allergol Int. 2017;66:557-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Takazawa T, Oshima K, Saito S. Drug-induced anaphylaxis in the emergency room. Acute Med Surg. 2017;4:235-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Müller-Werdan U, Werdan K. [Anaphylactic shock]. Anaesthesist. 1997;46:549-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Bennett MJ, Hirshman CA. Epinephrine for anaphylactic shock. JAMA. 1985;253:510-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 30. | Brown SG. The pathophysiology of shock in anaphylaxis. Immunol Allergy Clin North Am. 2007;27:165-175, v. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Kim KW, Chung S, Lee SY, Yoon SS, Kang HR. Successful Infusion of Obinutuzumab by Desensitization: A Case of Anaphylactic Shock During Desensitization. J Investig Allergol Clin Immunol. 2020;30:457-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |