Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5380
Peer-review started: September 6, 2021
First decision: December 9, 2021
Revised: December 23, 2021
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: June 6, 2022
Pulmonary lymphomatoid granulomatosis (PLG) is a lymphoproliferative disease associated with Epstein-Barr viral infection occurring mainly in adults and rarely in children. It is characterized by multiple pulmonary nodules. Its diagnosis depends on lung biopsy findings. Most patients are immunodeficient, and it commonly presents in children undergoing chemotherapy for leukemia. We report the case of a child with PLG caused by a mutation in the macrophage-expressed gene 1 (MPEG1), suggesting possible PLG occurrence in children undergoing treatment for pulmonary nodular lesions.
This study reports a case of PLG without apparent immunodeficiency, suggesting the possibility of this disease occurrence during the treatment of pulmonary nodular lesions in children. Initially, the cause was assumed to be an atypical pathogen. Following conventional anti-infective treatment, chest computed tomography findings revealed that there were still multiple nodules in the lungs. Additionally, the patient was found to be infected with the Epstein-Barr virus. Histopathological examination of the resected lung revealed lymphoproliferative lesions with necrosis. Small lymphocytes, plasma cells, and histiocytes were observed in the background, although Reed-Sternberg cells were absent. Immunohistochemical staining [CD20(+), CD30(+), and CD3(+)] and EBV-encoded small RNA1/2 in situ hybridization of small lymphocytes revealed approximately 200 cells/high-power field. Whole exon sequencing of the patient revealed a mutation in the MPEG1. The patient was eventually diagnosed with PLG and transferred to the Department of Pediatric Oncology for bone marrow transplantation.
As PLG is rare and fatal, it should be suspected in clinical settings when treatment of initial diagnosis is ineffective.
Core Tip: Pulmonary lymphomatoid granulomatosis is a rare but potentially fatal disease, especially in children. Based on the difficulty of diagnosis, the pulmonologist must have a high index of suspicion. Attention must be paid to histopathology and chest imaging findings. Furthermore, in our case, we found exome sequencing to reveal a pathogenic, pure heterozygous variant of macrophage-expressed gene 1 (NM_001039396: c.946C>T; p.P316S). No similar mutations have been reported in patients with PLG. Electronic bronchoscopy revealed many white nodules on the mucosa of the left and right main bronchi. It is necessary to consider genetic screening and the clinical application of electronic bronchoscopy.
- Citation: Yao JW, Qiu L, Liang P, Liu HM, Chen LN. Pulmonary lymphomatoid granulomatosis in a 4-year-old girl: A case report. World J Clin Cases 2022; 10(16): 5380-5386
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5380.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5380
Pulmonary lymphomatoid granulomatosis (PLG) is a rare lymphoproliferative disease associated with Epstein-Barr virus (EBV) infection. It commonly affects adults aged 30–50 years. The clinical manifestations are usually non-specific, occasionally presenting with pulmonary nodular lesions[1,2]. PLG is rarely observed in children. Herein, we present a case of a child diagnosed with PLG following a lung biopsy. This report aims to improve the clinical understanding of PLG in children.
A 4-year-old girl was hospitalized because of a 50-d history of pallor.
Four days before admission, the child developed intermittent fever (1–2 times per day; maximum temperature, 38.5 °C), accompanied by paroxysmal cough and sore throat.
The patient had no history of hemoptysis, hematemesis, hematuria, or hematochezia. She had a history of chronic diarrhea, which was unresolved despite a diet of deep hydrolyzed milk powder. Routine blood examination revealed severe anemia. Emergency infusion of leukocyte suspension (1.5 U) and mezlocillin-sulbactam antibiotics were administered.
Birth and developmental history were unremarkable.
Physical examination at admission revealed normal vital signs (temperature, 36.5 °C; pulse rate, 119 beats/min; respiratory rate, 33 cycles/min; blood pressure, 98/64 mmHg; and oxygen saturation, 98% in room air) and a body weight of 13.5 kg. She had pallor and clubbing. Rhonchi were noted in both lung fields. The liver was palpable, 5 cm below the costal margin, with blunt edges. Other physical examination findings were unremarkable.
Complete blood cell count showed normal total and differential white blood cell counts;, decreased hemoglobin level (5 g/dL), normal mean corpuscular volume (94.3 fL), mean corpuscular hemoglobin (28.4 pg) values, and increased reticulocyte count (4.98%). The platelet count and C-reactive protein levels were normal. The direct and indirect antiglobulin tests and isopropanol tests were negative. The serum glucose-6-phosphate dehydrogenase, folic acid, and vitamin B12 levels were normal. The serum ferritin and iron levels were normal. She was tested negative for thalassemia. Bone marrow aspiration showed granulocytosis and abnormal lymphocytes (3.5%).
Microbiologic examination for antigens of seven respiratory pathogens (influenza A, influenza B, respiratory syncytial virus, adenovirus, and parainfluenza 1, 2, and 3), Mycoplasma pneumoniae IgM and total antibody, and chlamydia IgM antibody were all negative. The serum fungal G, GM tests, and T-SPOT test were normal. The serum anti-EBV capsid antigen IgM (> 160.0 U/mL) and IgG (> 750.00 U/mL) were positive; anti-EBV early antigen IgG was also positive (> 150.00 U/mL). The quantity of EBV DNA was significantly high (5.14 × 105 copies/mL). Humoral immunity, IgG subclasses, T lymphocytes, B lymphocytes, and natural killer cells were all within the normal ranges.
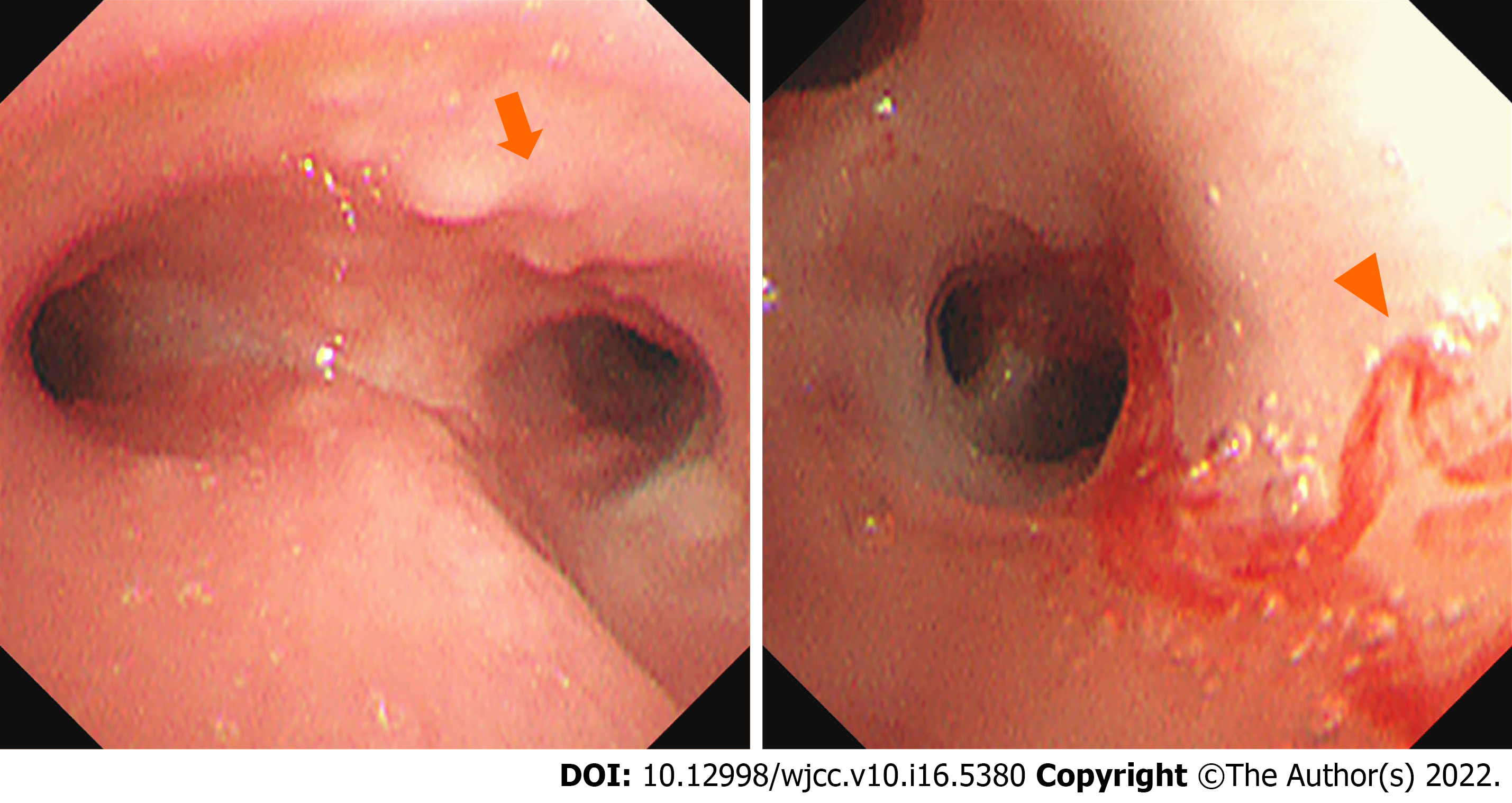
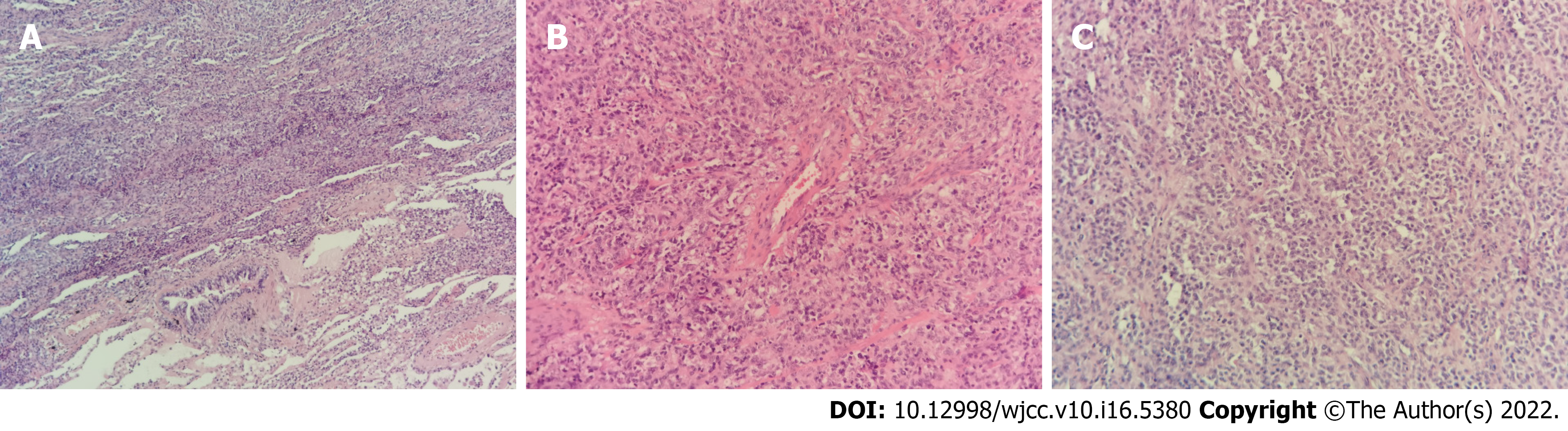
Exome sequencing revealed a pathogenic, pure heterozygous variant of macrophage-expressed gene 1 (MPEG1) (NM_001039396: c.946C>T; p.P316S). Both parents of the patient were genotypically heterozygous. Electronic bronchoscopy revealed many white nodules on the mucosa of the left and right main bronchi, left upper and lower lobe bronchi, and right upper and middle lobe bronchi, as well as mild hemorrhage in the posterior basal segment of the left lower lobe (Figure 1). Escherichia coli producing extended-spectrum β-lactamases were cultured in the bronchoalveolar lavage fluid. Histopathological examination of the resected lung revealed lymphoproliferative lesions with necrosis. The proliferative and infiltrating lymphocytes were moderately sized with round nuclei and occasional nucleoli. Mitosis and infiltration of the blood vessels by these cells were noted. Small lymphocytes, plasma cells, and histiocytes were observed in the background, although Reed-Sternberg cells were absent. Immunohistochemical staining [CD20(+), CD30(+), and CD3(+)] and EBV-encoded small RNA1/2 in situ hybridization (ISH) of small lymphocytes revealed approximately 200 cells/high-power field (Figure 2).
Chest computed tomography (CT) findings revealed multiple lower lobe nodules and shadows, multiple bilateral hilar and mediastinal lymph node enlargement, mild left pleural effusion, and bilateral pleural thickening (Figure 3A).
The patient was eventually diagnosed with PLG and transferred to the Department of Pediatric Oncology for further treatment.
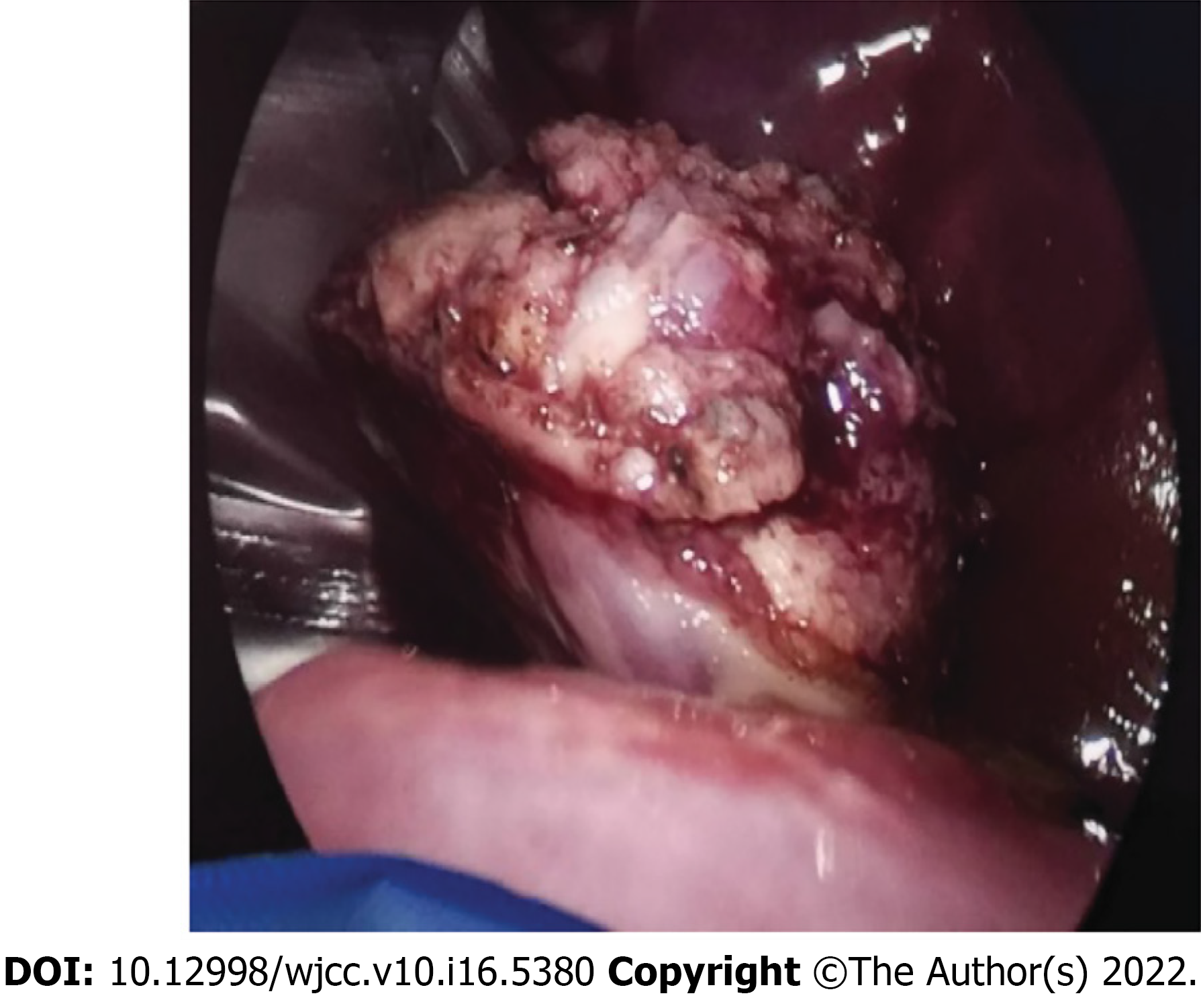
After admission, the patient was treated with imipenem-cilastatin and acyclovir antiviral therapy. However, the cough persisted, and chest CT findings showed no significant shrinkage of the pulmonary nodules even 1 mo after treatment (Figure 3B). Suspecting a possible lung tumor, we performed thoracoscopic wedge resection of the lower lobe of the left lung with pleural adhesion cauterization and lung biopsy for histopathological determination (Figure 4).
The patient was transferred to the Department of Pediatric Oncology. She was prescribed a chemotherapy regimen of prednisone, vincristine, and cyclophosphamide. Then, she received bone marrow transplantation. She remained relatively well throughout the treatment without immune deficiency or new infections.
Liebow et al[2] first identified PLG. Besides multiple lung nodules, some patients with PLG may present with cough, dyspnea, chest pain, subcutaneous nodules, weight loss, and nervous system involvement (ataxia, hearing impairment, and dysarthria). Others may present with extrapulmonary involvement, such as those of the skin and nerves. PLG was renamed as mature B-cell tumor in 2016[3,4,5].
At present, the diagnosis of PLG is primarily based on histological findings of the lung tissue showing the "triad" of pleomorphic lymphocyte infiltration with necrosis, infiltration of lymphocytes in the arterial and venous walls, and EBV-positive B cells confirmed by ISH. In this case, lung histopathology showed moderate-sized infiltrating cells, lymphoid tissue hyperplasia with necrosis, and small lymphocytes, plasma cells, and histiocytes in the background. A variable number of CD20-positive B cells existed in the background of CD3-positive small lymphocytes and were EBER1/2-ISH-positive, thus, meeting the diagnostic criteria for PLG. Histologically, PLG is graded according to the proportion of EBV-positive large B lymphocytes determined by EBER: Grade 1 Lesion is composed of scattered EBV-positive cells without necrosis; Grade 2 Lesions include an increased number of large B cells and some necrosis; and Grade 3 Lesions consist mainly of many virus-positive cells with extensive necrosis[1,3,4]. This case was classified as Grade 3 because the lesion comprised many EBV-positive cells.
EBV belongs to the γ subfamily of herpesvirus. The EBV genome is a linear double-stranded DNA molecule, a prototype virus of the genus of lymphofollicular viruses. In vitro, all γ herpesviruses can replicate in lymphoid cells, although only some can replicate lytically in epithelial cells and fibroblasts. Primate B lymphocyte infection usually leads to latent infection, characterized by the persistence of the viral genome and the expression of a series of limited latent gene products, thus, promoting the transformation process and helping to drive cell proliferation[6]. The cleavage-associated gene of EBV has a significant homology with the human genome, and some genes have significant homology with human B-cell leukemia/Lymphoma 2. This gene is involved in the apoptosis of tissue B cells and other lymphocytes. In patients with congenital or acquired immunodeficiency, B cells infected by EBV easily undergo tumorigenic transformation[7,8]. EBV is believed to play an important role in driving PLG. It is speculated that host immune deficiency leads to abnormal clearance response to EBV, consequently leading to abnormal lymphoid tissue proliferation and apoptosis inhibition. This results in a large number of leukocytes infiltrating the blood vessels, followed by injury and tissue destruction[9]. Approximately half of the PLG cases in children are found in those undergoing treatment for leukemia. Some adult PLG cases are reportedly related to the use of certain drugs (methotrexate and imatinib) that, when withdrawn, lead to the disease resolution[10].
The MPEG1 is an ancient postnatal animal protein that belongs to the pore-forming protein of the membrane attack complex/perforin (MACPF) branch of the MACPF/cholesterol-dependent cytolytic cellulose superfamily. The MACPF functions in human immunity and development. The MPEG1 facilitates the entry of numerous antimicrobial effectors into cells, including proteases, reactive oxygen and nitrogen species, and bactericidal peptides, and mediates the harsh acidic environment of phagosomes[11]. No case of MPEG1 mutations have been reported in patients with PLG. However, one study found this mutation in diffuse large B-cell lymphoma. Notably, a series of genetic mutations co-exist with MYD88, including PRDM1, all of which occur at a high frequency in the MCD subtype, as defined by Schmitz et al[12], which promote nuclear factor-κB activation in a B-cell receptor-dependent manner. These findings confirm that MYD88 alone is insufficient to drive the malignant transformation of B cells and may induce lymphoma with other genetic events.
There are few reported cases of PLG in children, including a literature review of 49 published pediatric cases of PLG[13]. The affected patients were usually immunodeficient, and only one case reported hemolytic anemia.
Mild or severe PLG on chest CT usually shows well- or ill-defined bilateral pulmonary nodules mainly located in the lower lung fields. The nodules vary in size (usually 1 cm–2 cm in diameter) and are mostly related to interstitial lung diseases. Nodules are usually distributed along with the bronchovascular bundles or interlobular septa, possibly because of the tendency of lymphocytes to infiltrate the subintimal area of blood vessels. The size of the nodules can fluctuate gradually, and some of the nodules can be matted, in consistency with our observation. Bronchoscopy also revealed many small white nodules of different sizes in the bronchial lumen. Other cases also reported pleural effusion and hilar lymph node enlargement in patients with PLG. Diffuse ground-glass degeneration is uncommon and may be caused by peri-focal hemorrhage or pneumonia[14-17], which is consistent with bronchoscopic observation of the hemorrhage in the basal segment of the lower left lung lobe in this case.
The natural course of PLG varies greatly from spontaneous regression to death. The overall prognosis of PLG is poor, with a high mortality rate (60%–90%) within 5 years. Currently, the main treatments for patients with PLG are similar to those for non-Hodgkin’s lymphoma, including the use of rituximab to eliminate B cells. In previous reports, some patients received hematopoietic stem cell transplantation, and most were also treated with corticosteroids. According to the different grades of PLG, patients in advanced stages are more likely to receive treatment combined with immunochemotherapy, while low-grade lesions can be treated with interferon-α[13,18].
In conclusion, PLG is a rare but potentially fatal disease in children. Children usually present with diffuse pulmonary nodules. Owing to the difficulty of diagnosis using clinical data and chest imaging, histopathology and chest imaging findings should be closely examined. In clinical settings, when the initial diagnosis is pneumonia and the treatment is ineffective, PLG should be considered.
The authors thank Chen ZJ and Zhang PL for their help, guidance, and valuable discussions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Biondi A, Italy; Gaman MA, Romania; Sultana N, Bangladesh S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Pittaluga S, Wilson WH, Jaffe ES. Lymphomatoid granulomatosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC, 2017: 312. [Cited in This Article: ] |
| 2. | Liebow AA, Carrington CR, Friedman PJ. Lymphomatoid granulomatosis. Hum Pathol. 1972;3:457-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 666] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Jaffe ES, Wilson WH. Lymphomatoid granulomatosis: pathogenesis, pathology and clinical implications. Cancer Surv. 1997;30:233-248. [PubMed] [Cited in This Article: ] |
| 4. | Myers JL. Lymphomatoid granulomatosis: past, present, ... future? Mayo Clin Proc. 1990;65:274-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4245] [Cited by in F6Publishing: 4858] [Article Influence: 607.3] [Reference Citation Analysis (0)] |
| 6. | Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM. Fields virology. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2005: 2603. [Cited in This Article: ] |
| 7. | Liebowitz D. Epstein-Barr virus and a cellular signaling pathway in lymphomas from immunosuppressed patients. N Engl J Med. 1998;338:1413-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 178] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Gregory CD, Dive C, Henderson S, Smith CA, Williams GT, Gordon J, Rickinson AB. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991;349:612-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 395] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Nixon CP, Sweeney JD. Facilitation of the clinical diagnosis of Mycoplasma pneumoniae by the blood bank. Transfusion. 2017;57:2564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Aiko N, Sekine A, Umeda S, Katano T, Matama G, Isomoto K, Otoshi R, Ogura T. The Spontaneous Regression of Grade 3 Methotrexate-related Lymphomatoid Granulomatosis: A Case Report and Literature Review. Intern Med. 2018;57:3163-3167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bayly-Jones C, Pang SS, Spicer BA, Whisstock JC, Dunstone MA. Ancient but Not Forgotten: New Insights Into MPEG1, a Macrophage Perforin-Like Immune Effector. Front Immunol. 2020;11:581906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Shen R, Xu PP, Wang N, Yi HM, Dong L, Fu D, Huang JY, Huang HY, Janin A, Cheng S, Wang L, Zhao WL. Influence of oncogenic mutations and tumor microenvironment alterations on extranodal invasion in diffuse large B-cell lymphoma. Clin Transl Med. 2020;10:e221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Tacke ZC, Eikelenboom MJ, Vermeulen RJ, van der Knaap MS, Euser AM, van der Valk P, Kaspers GJ. Childhood lymphomatoid granulomatosis: a report of 2 cases and review of the literature. J Pediatr Hematol Oncol. 2014;36:e416-e422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Chung JH, Wu CC, Gilman MD, Palmer EL, Hasserjian RP, Shepard JA. Lymphomatoid granulomatosis: CT and FDG-PET findings. Korean J Radiol. 2011;12:671-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Rezai P, Hart EM, Patel SK. Lymphomatoid granulomatosis. Radiology. 2011;259:604-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Sood A, Parihar AS, Malhotra P, Vaiphei K, Kumar R, Singh H, Mittal BR. Pulmonary Recurrence of Lymphomatoid Granulomatosis Diagnosed on F-18 FDG PET/CT. Indian J Nucl Med. 2020;35:167-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 17. | Handel AS, Davis J, Glass J, Hogan L, Schuval S, Beneri C. A 4-Year-Old Boy With Prolonged Cough and Fever. J Pediatric Infect Dis Soc. 2020;9:92-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Lee S, Kang MJ, Kim HJ, Kim HB, Lee J. Pulmonary lymphomatoid granulomatosis in a 4-year-old girl. Pediatr Radiol. 2015;45:1082-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |