Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5337
Peer-review started: July 17, 2021
First decision: October 3, 2021
Revised: October 20, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 6, 2022
Pneumatosis intestinalis (PI), also known as intramural gas in the small intestine, is a rare condition encountered by patients with cancer after receiving chemo
A 78-year-old man with a history of colorectal cancer developed epigastric pain and diarrhea after receiving combination chemotherapy of fluorouracil, leuco
Although chemotherapy-induced PI is rare among patients with cancer, the differential diagnosis of PI and fulminant complications (such as ischemia, infarction, and perforation of the gastrointestinal tract) should be conducted, in which case an urgent surgical intervention is required.
Core Tip: Pneumatosis intestinalis (PI) is a rare condition encountered by patients with cancer after receiving chemotherapy. The differential diagnosis of PI and chemotherapy-induced fulminant complications (such as ischemia, infarction, and perforation of the gastrointestinal tract) should be conducted, in which case an urgent surgical intervention is required.
- Citation: Liu H, Hsieh CT, Sun JM. Pneumatosis intestinalis after systemic chemotherapy for colorectal cancer: A case report. World J Clin Cases 2022; 10(16): 5337-5342
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5337.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5337
Pneumatosis intestinalis (PI), which is characterized by the presence of submucosal or subserosal gas in the intestinal wall, is an uncommon disease[1]. It can occur as an idiopathic disease, or more commonly, as a secondary condition associated with the coexistence of gastrointestinal tract disorders or respiratory system diseases such as chronic obstructive pulmonary disease, ischemic bowel disease, intestinal obstruction, necrotizing enterocolitis, immunodeficiency, bacterial infection, and viral infection[2,3]. However, chemotherapy-induced PI is relatively rare in patients with cancer. Chemotherapy agent–induced cytotoxic damage and mucosal ischemia in the gastrointestinal tract may also lead to gastrointestinal perforation, necrotizing enterocolitis, or ischemic bowel[4]. Prompt surgical intervention may be required to manage the perforation or ischemic necrosis of the gastrointestinal tract[3]. Therefore, the differential diagnosis of PI and bowel perforation in patients with cancer after chemotherapy is essential for clinical physicians to treat acute abdomen[4]. Herein, we report a rare case of a patient with colon cancer who presented with acute abdomen after systemic chemotherapy. PI was diagnosed following radiological studies and surgical intervention. The possible mechanisms of PI in patients with cancer receiving chemotherapy are discussed and reviewed.
A 78-year-old man complained of acute lower abdominal pain with cramping pain and diarrhea lasting for 3 d.
A 78-year-old man with a history of mucinous adenocarcinoma (pT3N2bcM1b, stage IVB) in the descending colon and upper rectum underwent laparoscopic radical left hemicolectomy, sigmoidectomy, and loop T-colostomy. He then underwent chemotherapy comprising fluorouracil, leucovorin, and irinotecan (FOLFIRI); intravenous injections of 400 mg/m2 fluorouracil, 200 mg/m2 leucovorin, and 120 mg/m2 irinotecan were administered every 2 wk. Starting from the second course of FOLFIRI treatment, the adjuvant chemo-agent (cetuximab) was administered at a dose of 500 mg/m2. One week after the third course of FOLFIRI combined with the second course of cetuximab chemotherapy, he complained of acute lower abdominal pain with cramping pain and diarrhea lasting for 3 days.
The patient had a history of mucinous adenocarcinoma (pT3N2bcM1b, stage IVB) in the descending colon and upper rectum and underwent laparoscopic radical left hemicolectomy, sigmoidectomy, and loop T-colostomy.
The patient presented at our emergency department with a body temperature of 36.7 °C, heart rate of 127 bpm, blood pressure of 180/108 mmHg, and respiratory rate of 18 breaths per minute. A physical examination revealed abdominal distention, tenderness in the epigastric region, and hypoactive bowel sounds. No rebounding pain was observed. The colostomy exhibited good perfusion with brown soft stool passage.
Laboratory examinations revealed a white blood cell count of 1200/μL, platelet count of 245000/μL, hemoglobin level of 10.3 mg/dL, total bilirubin of 0.6 mg/dL, lipase level of 34 mg/dL, lactate level of 1.0 mg/dL, and C-reactive protein level of 146 mg/dL.
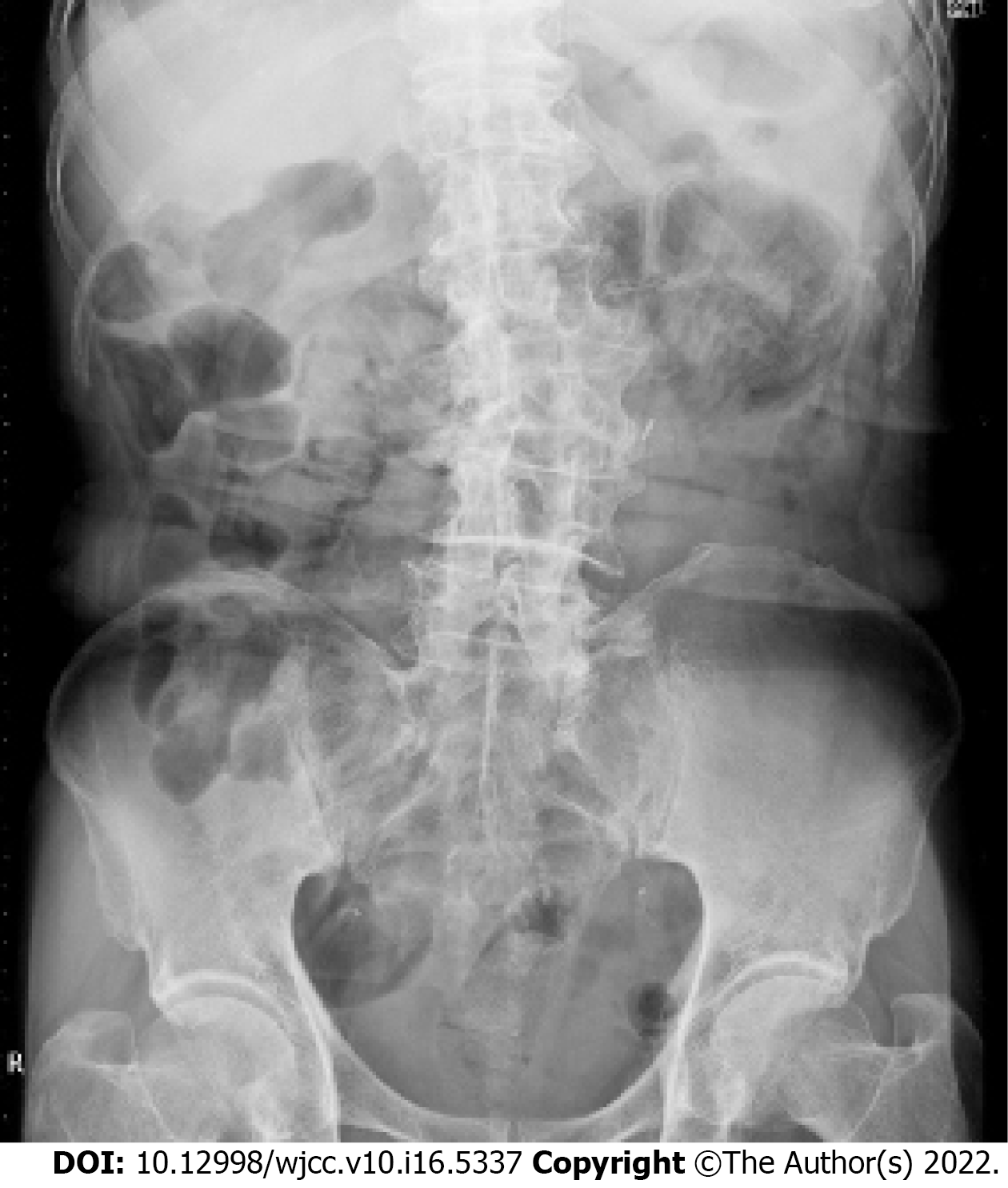
Abdominal radiograph revealed gas in the small intestinal wall (Figure 1). A computed tomography (CT) scan of the abdomen with contrast enhancement indicated intramural gas in the small intestine that expanded into the mesentery (Figure 2).
A pathological examination confirmed the diagnosis of PI with focal ischemic and ulcerative changes.
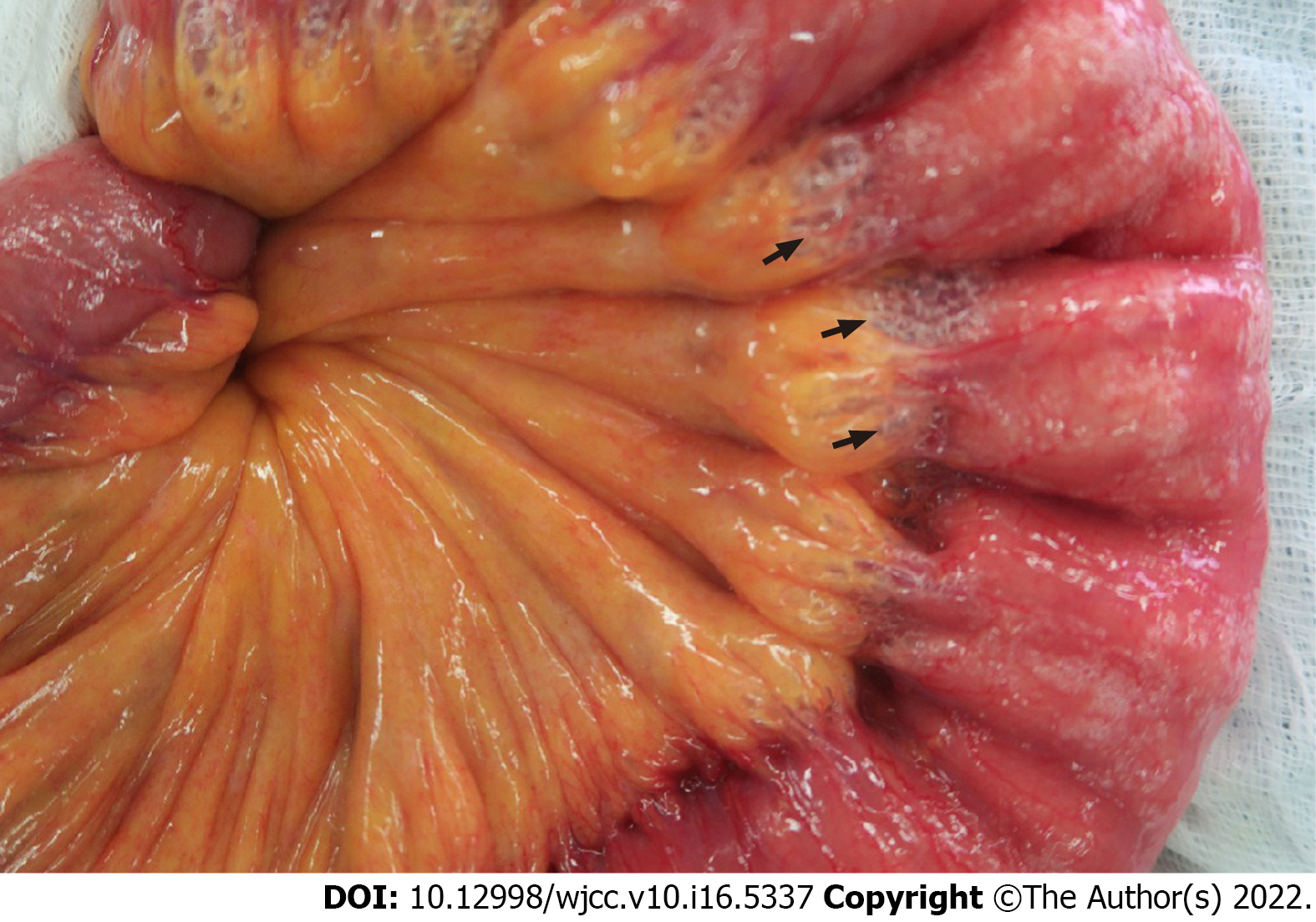
The patient underwent upper midline mini-laparotomy surgery because bowel perforation or necrotizing enterocolitis was suspected. During surgery, expanded intraluminal air spaces in the small intestine were observed (Figure 3). An approximately 50 cm length of the proximal jejunum exhibited severe edematous changes and compromised mild blood circulation without necrosis. Segmental resection of the small bowel with end-to-end anastomosis was performed.
Postoperatively, the patient’s previously observed symptoms improved after 2 wk of parenteral nutrition, antibiotic treatment, and oxygen therapy. The FOLFIRI treatment for his colorectal cancer was started 3 mo after discharge. No recurrence of PI was observed during the 9 mo follow-up.
PI was first reported radiologically in 1946 by Lerner and Gazin[5]. It is an uncommon disease characterized by the presence of gas and free air in the extraluminal spaces of the intestines from the mucosa to mesenteric vessels[3]. Several terms have been used to describe PI, including pneumatosis cystoides intestinalis, intramural gas, pneumatosis coli, pseudolipomatosis, intestinal emphysema, bullous emphysema of the intestine, and lymphopneumatosis[1]. The incidence of PI is 0.03% according to the literature; however, this is believed to be an underestimation because most patients are asymptomatic and, consequently, do not receive clinical attention[6].
PI is a radiological finding and not a diagnosis[7], and it is commonly classified as primary and secondary forms[8]. Secondary PI is found in approximately 80% to 85% of patients and is often associated with bowel ischemia, trauma, inflammatory bowel diseases, chronic obstructive pulmonary disorder, use of specific medications, immunocompromised disease, and immunosuppressive therapy[8,9]. Although the exact pathogenesis of PI remains unclear, four categories are used to describe the pathogenesis of PI, namely, bowel necrosis, mucosal disruption, increased mucosal permeability, and pulmonary disease[10]. Mechanical and bacterial theories have been proposed to explain the development of PI[6,9]. A mechanical theory suggests that a defect in mucosal integrity allows for gas to be transmitted through the dissection of submucosal or subserosal layers and that peristalsis then propagates the gas to distant sites[6]. A bacterial theory proposes that some intramural gas is normally present in the gastrointestinal tract and that the overgrowth of normal bacteria (such as Escherichia coli and Clostridium difficile) leads to bacterial invasion of the bowel wall and lymphatic system through mucosal defects and the subsequent production of excess gas.
Chemotherapy-induced PI in patients with cancer is rare[4,11-15]. Many cytotoxic chemotherapeutic agents have been reported to be associated with PI; they include cyclophosphamide, cytarabine, vincristine, doxorubicin, daunorubicin, etoposide, docetaxel, irinotecan, cisplatin, methotrexate, fluorouracil, paclitaxel, tyrosine kinase inhibitors (imatinib, sunitinib, sorafenib, and erlotinib), bevacizumab (a monoclonal body to vascular endothelial growth factor), and cetuximab (a monoclonal antibody to epidermal growth factor receptor)[11,13-16]. This may contribute to cytotoxic damage, loss of mucosal integrity, mucosal ischemia, and the development of PI. Although four chemoagents (fluorouracil, leucovorin, irinotecan, and cetuximab) might have contributed to the development of PI in our patient, no recurrence of PI was observed after subsequent FOLFIRI treatment without cetuximab. This suggests that cetuximab played an essential role in the development of PI. The perforation of the gastrointestinal tract is also a rare lift-threatening complication (due to bowel toxicity) that may occur after molecular-targeted chemotherapy[12]. Therefore, the differential diagnosis of PI and bowel perforation is crucial to the management of acute abdomen in oncologic patients receiving systemic chemotherapy, such as in the case of our patient.
The clinical presentation of patients with PI includes being asymptomatic, vomiting, weight loss, constipation, hematochezia, tenesmus, diarrhea, abdominal pain, and life-threatening peritoneal symptoms such as pneumoperitoneum, intestinal ischemia, peritonitis, and bowel obstruction[1]. The main radiological feature of PI is the presence of circular, linear, or curvilinear gas within the wall of the gastrointestinal tract on plain radiographs or CT scans[17]. CT scan is the most sensitive imaging tool for differentiating intraluminal air from the submucosal layer and identifying additional causes of PI, which include portal air, colonic tissue stranding, and dilated bowel. In a review of 37426 abdominal and pelvic CT scans, Hawn et al[18] detected PI in 108 (0.3%) patients. In another review involving 28326 abdominal CT scans, Morris et al[17] discovered that out of 104 (0.37%) patients who were diagnosed as having pneumatosis, only 23% had observable PI on their plain radiographs. Cyst-like gas collection is usually benign, whereas linear collection tends to be associated with bowel infarction[10,18]. However, the recognition of these patterns should not be the sole basis for differentiating benign and fulminant conditions.
A consensus regarding the appropriate management of PI has not been reached due to the difficulties of delineating the underlying etiologies[17]. An initial clarification to differentiate life-threatening conditions from nonurgent ones is crucial[8]. Approximately 50% of patients with PI can be successfully managed nonoperatively. Because PI indicates that necrotic tissue is allowing gas to enter the extraluminal space, ischemia or infarction of the gastrointestinal tract system should be suspected, in which case an urgent surgical intervention is required. Based on the underlying diseases leading to PI, surgical procedures such as bowel resection, lysis of adhesion, bowel biopsies, or nontherapeutic exploratory laparotomy should be performed. However, patients with concomitant PI and portal venous gas tend to have bowel ischemia, which is associated with a high mortality rate. Patients with a serum lactic acid level of more than 2 mmol/L at the time of diagnosis have an overall mortality rate of more than 80%[18]. Patients with no signs of peritonism or sepsis can usually be managed conservatively[9]. Therefore, the occurrence of PI should trigger a clinical physician to investigate the possible causes, ensure a correct diagnosis, and develop an appropriate management protocol.
PI is a radiologic sign and not a diagnosis. Although chemotherapy-induced PI is rare among patients with cancer, the differential diagnosis of PI and life-threatening conditions (such as ischemia, infarction, and perforation of the gastrointestinal tract) should be conducted for patients who present with acute abdomen.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Feng J, China; Liang P, China; Xie Y, China S-Editor: Zhang YL L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Im J, Anjum F. Pneumatosis Intestinalis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 2021. [PubMed] [Cited in This Article: ] |
| 2. | Berritto D, Crincoli R, Iacobellis F, Iasiello F, Pizza NL, Lassandro F, Musto L, Grassi R. Primary pneumatosis intestinalis of small bowel: a case of a rare disease. Case Rep Surg. 2014;2014:350312. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Medgyesy K, Trifonova R, Bevington T, Tadros M. Pneumatosis Intestinalis: To Biopsy or Not to Biopsy? Cureus. 2020;12:e12140. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Gray EJ, Darvishzadeh A, Sharma A, Ganeshan D, Faria SC, Lall C. Cancer therapy-related complications in the bowel and mesentery: an imaging perspective. Abdom Radiol (NY). 2016;41:2031-2047. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Lerner HH, Gazin AI. Pneumatosis intestinalis; its roentgenologic diagnosis. Am J Roentgenol Radium Ther. 1946;56:464-469. [PubMed] [Cited in This Article: ] |
| 6. | Kang G. Benign pneumatosis intestinalis: Dilemma for primary care clinicians. Can Fam Physician. 2017;63:766-768. [PubMed] [Cited in This Article: ] |
| 7. | Sassi C, Pasquali M, Facchini G, Bazzocchi A, Battista G. Pneumatosis intestinalis in oncologic patients: when should the radiologist not be afraid? BJR Case Rep. 2017;3:20160017. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Donovan S, Cernigliaro J, Dawson N. Pneumatosis intestinalis: a case report and approach to management. Case Rep Med. 2011;2011:571387. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Jenkins M, Courtney H, Pope E, Williamson J. A case report and approach to management in pneumatosis intestinalis. Ann Med Surg (Lond). 2017;23:25-27. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Pear BL. Pneumatosis intestinalis: a review. Radiology. 1998;207:13-19. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Mimatsu K, Oida T, Kawasaki A, Kano H, Kuboi Y, Aramaki O, Amano S. Pneumatosis cystoides intestinalis after fluorouracil chemotherapy for rectal cancer. World J Gastroenterol. 2008;14:3273-3275. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Shinagare AB, Howard SA, Krajewski KM, Zukotynski KA, Jagannathan JP, Ramaiya NH. Pneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its management. AJR Am J Roentgenol. 2012;199:1259-1265. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Yoon S, Hong YS, Park SH, Lee JL, Kim TW. Pneumatosis intestinalis after cetuximab-containing chemotherapy for colorectal cancer. Jpn J Clin Oncol. 2011;41:1225-1228. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Sherman E, Ramadas P, Lemke S. Cetuximab-Associated Pneumatosis Intestinalis. Am J Ther. 2019;26:e609-e610. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Chaudhry NS, Bi WL, Gupta S, Keraliya A, Shimizu N, Chiocca EA. Pneumatosis Intestinalis After Molecular-Targeted Therapy. World Neurosurg. 2019;125:312-315. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Vijayakanthan N, Dhamanaskar K, Stewart L, Connolly J, Leber B, Walker I, Trus M. A review of pneumatosis intestinalis in the setting of systemic cancer treatments, including tyrosine kinase inhibitors. Can Assoc Radiol J. 2012;63:312-317. [PubMed] [DOI] [Cited in This Article: ] |