Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5324
Peer-review started: June 22, 2021
First decision: August 19, 2021
Revised: August 28, 2021
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: June 6, 2022
Laparoscopic duodenojejunostomy (LDJ) has become the standard surgical procedure for superior mesenteric artery syndrome due to its sufficient outcome in terms of safety and symptom relief. However, there are only a few reports about LDJ for malignant stenosis and its indication remains uncertain.
A 77-year-old woman with a history of pancreatic cancer (PC) treated with distal pancreatectomy with en bloc resection of the transverse colon 7 mo ago was admitted for recurrent vomiting. Imaging upon admission revealed marked distention of the duodenum and a tumor around the duodenojejunal flexure. She was diagnosed with malignant stenosis caused by local recurrence of PC. LDJ was performed with an uneventful postoperative course, followed by chemotherapy which gave her 10 mo overall survival.
We think that LDJ is a valuable method for unresectable malignant stenosis around the duodenojejunal flexure as a part of multimodal therapy.
Core Tip: There are many reports on laparoscopic duodenojejunostomy (LDJ) for superior mesenteric artery syndrome, but rarely for malignant stenosis. In general, prognosis of patients with recurrent cancer is poor; however, development of new chemotherapeutic agents and new combination therapy improve their overall survival. Obstruction due to malignancy is often an obstacle for chemotherapy, and a safe and minimally invasive method would help enable a rapid induction. We think LDJ is a valuable method for patients with unresectable malignant stenosis around the duodenojejunal flexure.
- Citation: Murakami T, Matsui Y. Laparoscopic duodenojejunostomy for malignant stenosis as a part of multimodal therapy: A case report. World J Clin Cases 2022; 10(16): 5324-5330
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5324.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5324
Patients with recurrent or metastatic cancer have poor prognosis, and chemotherapy has a pivotal role in their survival[1-3], especially in highly malignant disease such as pancreatic cancer (PC). When patients with unresectable malignancies require surgery for symptom relief, selection of a minimally invasive procedure allows faster recovery and thus quicker induction of chemotherapy.
Laparoscopic duodenojejunostomy (LDJ) has become the standard surgical procedure for superior mesenteric artery syndrome (SMAS) due to its sufficient short- and long-term outcomes in terms of safety and symptom relief[4,5]. However, there are only a few reports about LDJ for malignant stenosis[6] and its indication remains uncertain.
We report a successful case of LDJ as palliative care in a patient with unresectable malignant stenosis around the duodenojejunal flexure caused by recurrent PC (rPC). The postoperative course was uneventful, and early food consumption and induction of chemotherapy were achieved. Hence, we think this method is valuable for the multimodal therapy of unresectable malignancies.
A 77-year-old woman presented with upper right abdominal distension and recurrent vomiting.
The patient had a history of PC treated with distal pancreatectomy with en bloc resection of the transverse colon 7 mo ago. She presented with upper right abdominal distension and recurrent vomiting since 1 d ago and was admitted to our institution as an emergency.
She underwent distal pancreatectomy with en bloc resection of the transverse colon for PC.
The patient had no specific family history.
Blood pressure 134/90 mmHg, heart rate 82 beats/min, respiration rate 12 breaths/min and body temperature 36.2 °C were noted upon arrival. The upper right abdomen was distended but soft and there was no abdominal pain.
Creatinine and blood urea nitrogen were elevated to 2.21 mg/dL (normal range: 0.65-1.1 mg/dL) and 28 mg/dL (normal range: 8-20 mg/dL), respectively. Tumor marker carbohydrate antigen 19-9 markedly increased to 6191 U/mL (normal range: 0-45 U/mL).
Computed tomography on admission revealed a soft tissue mass dorsal to the stomach and nearby duodenojejunal flexure. We found a dilated duodenum and collapsed jejunum (Figure 1). Upper gastrointestinal examination showed a dilated duodenum, limited extensibility of the stomach and no gastrografin passage through the duodenojejunal flexure (Figure 2A). Upper gastrointestinal endoscopy revealed stricture at the duodenojejunal flexure due to intraluminal stenosis but revealed no mucosal surface changes (Figure 2B), and a nasogastric tube was placed to decompress the stomach and duodenum (Figure 2C).
Malignant stenosis of the duodenojejunal flexure caused by local recurrence of PC.
Surgical intervention was essential for symptom relief and induction of chemotherapy. Gastrojejunal bypass was thought to be difficult due to the stiffness of the stomach, so we chose to perform DJ. A minimally invasive procedure was necessary for rapid recovery. LDJ was performed 9 d after admission. Decompression of the duodenum with nasogastric tube (Figure 2C) and correction of dehydration by total parenteral nutrition were performed preoperatively.
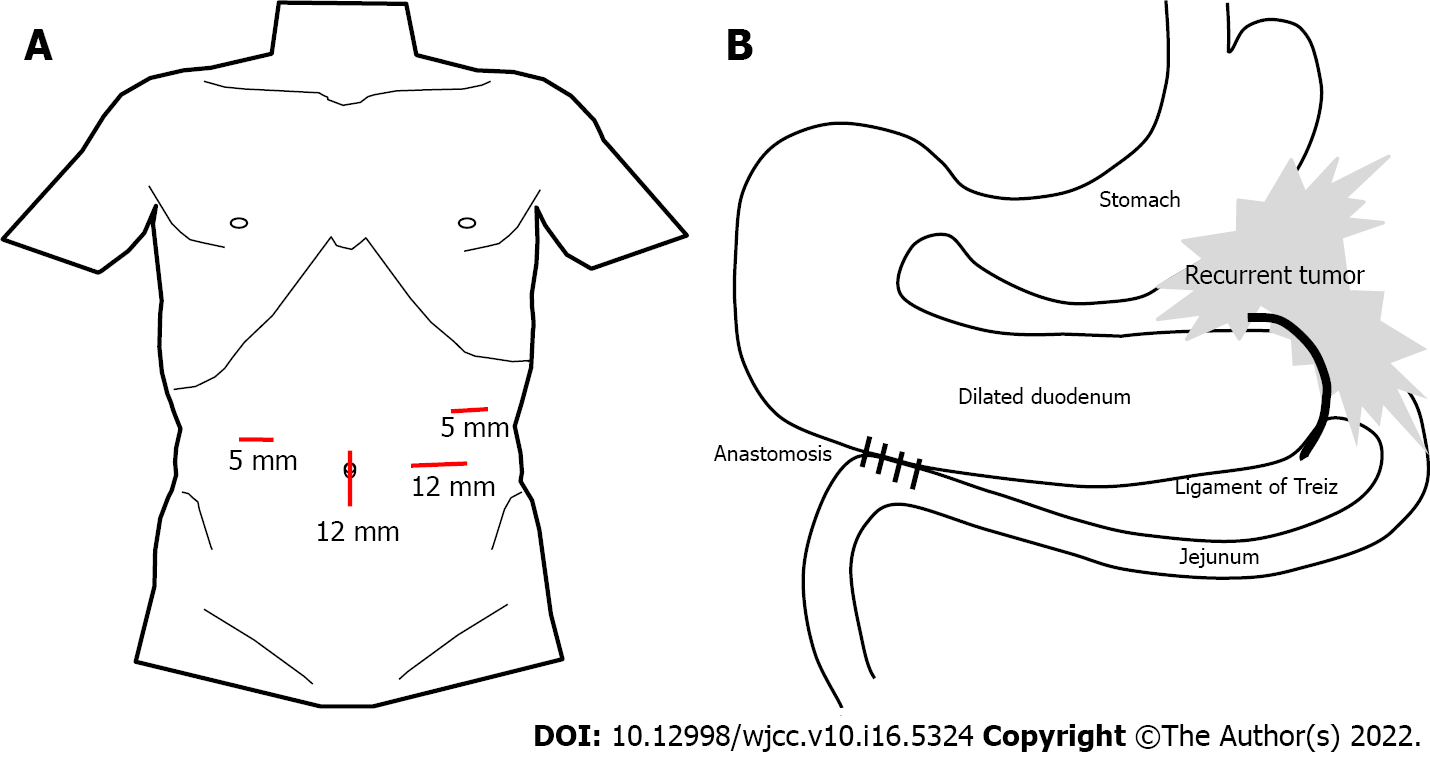
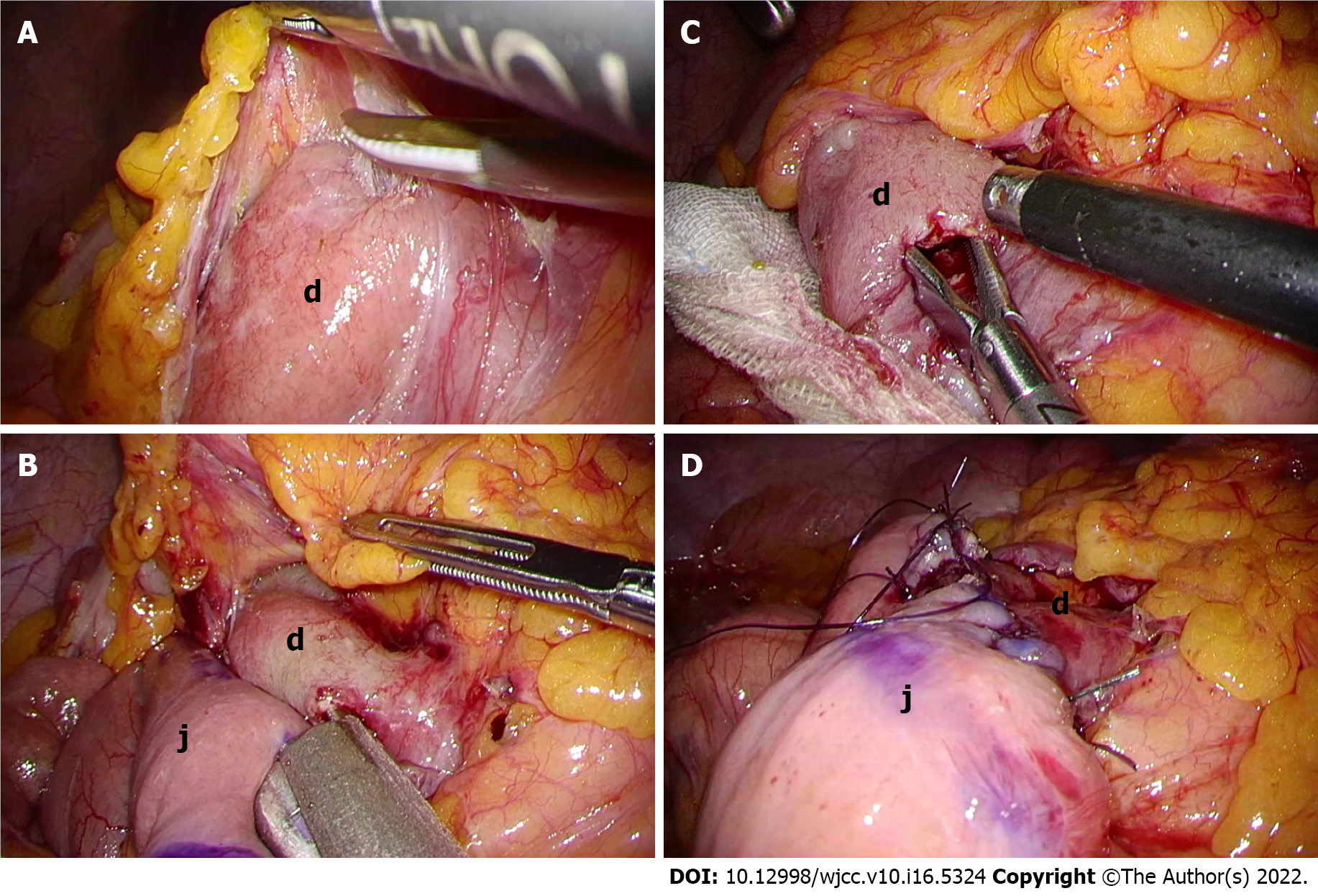
The patient was placed in the open-leg supine position and a 4-port procedure (Figure 3A) was performed with the operator on the left side of the patient. Laparoscopic findings revealed a dilated duodenum, no gastric mobility and no peritoneal metastasis. With upward traction on the transverse colon, the second and third portions of the duodenum were exposed and mobilized (Figure 4A). We chose the third portion and jejunum about 30 cm anal to the Treitz ligament for anastomosis (Figure 4B and 4C). A side-to-side DJ was performed in an antiperistaltic manner using a stapling device (Signia with 45 mm purple reload; Covidien Japan, Tokyo, Japan) (Figure 4C). The common entry hole was closed with a continuous absorbable V-Loc suture (Covidien Japan) (Figure 4D). The operating time was 90 min with trivial bleeding. No drain was placed. Intraoperative findings are summarized in Figure 3.
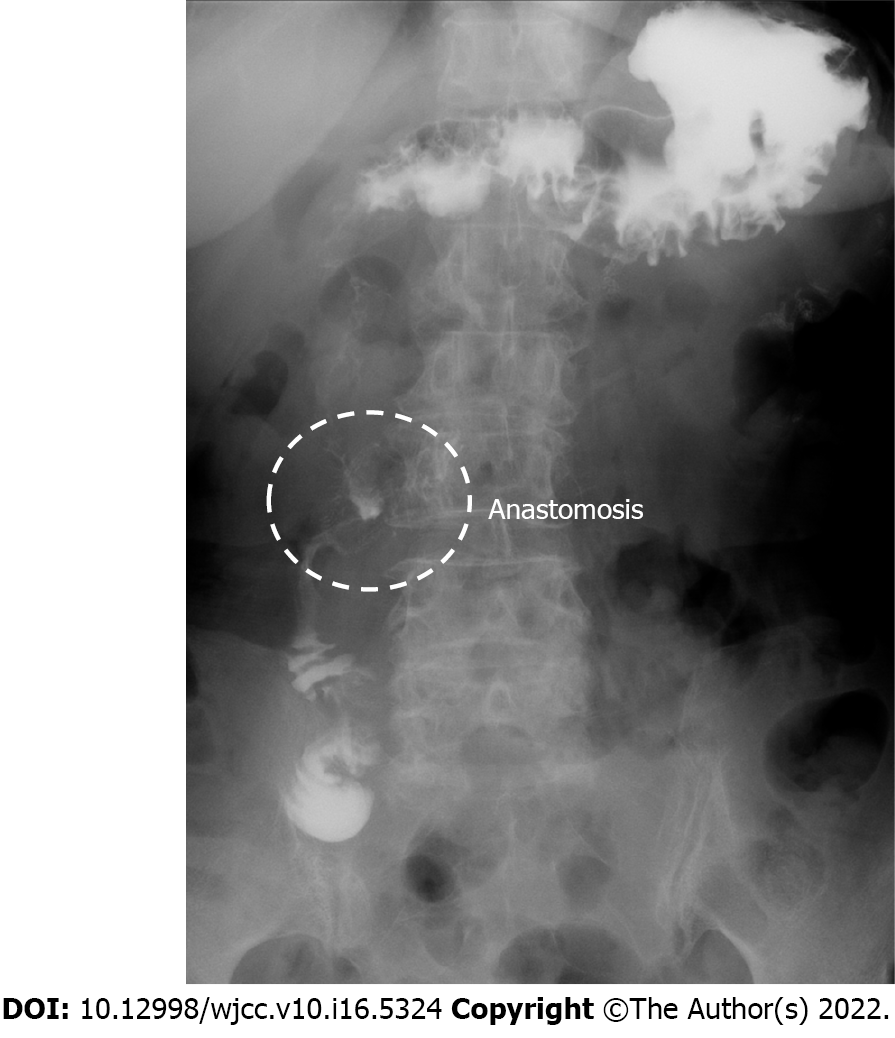
Postoperative course was uneventful. Oral fluid intake and food consumption were started on postoperative day (POD) 2 and 7, respectively. Upper gastrointestinal examination on POD 5 showed good patency of the anastomosis (Figure 5). The patient was discharged on POD 9, followed by induction of outpatient chemotherapy (nab-paclitaxel + gemcitabine) started on POD 30.
Six months later, (7 mo after the operation), chemotherapy was terminated due to disease progression and the patient’s desire for best supportive care. Although she died of PC 10 mo after the operation, she could tolerate food consumption until just before her death.
A single-center case series of LDJ, although for SMAS[4,5,7-11], showed no mortality, no anastomotic leaks, short length of stay and no recurrence of symptoms (Table 1). With such results, LDJ has been considered to be safe, efficacious and minimally invasive, and has become the standard surgical procedure for SMAS.
| Year | Author | Number of Patients | Mean operation time (min) | Mean length of stay (d) | Complication number of cases | Mortality |
| 2001 | Richardson et al[7] | 2 | 113 | 3 | None | None |
| 2003 | Kim et al[8] | 2 | 173 | 5.5 | None | None |
| 2010 | Munene et al[9] | 13 | 121 | 4.5 | Trocar site bleeding (1) | None |
| 2015 | Sun et al[10] | 14 | 119 | 5.5 | Dumping syndrome (1) | None |
| Abdominal abscess (1) | ||||||
| 2017 | Chang et al[4] | 18 | 144 | 5 | Ileus (3) | None |
| 2017 | Kirby et al[11] | 3 | N/A | 4.3 | None | None |
| 2021 | Jain et al[5] | 22 | 75 | 7.3 | Delayed gastric emptying (4) | None |
| Ileus (1) |
Gastrojejunostomy (GJS) used to be performed for SMAS. However, GJS is no longer considered to be a suitable method for SMAS since it has been associated with insufficient duodenal decompression, peptic ulcer, bile gastritis and blind loop syndrome[4,9,10]. LDJ, in contrast, provides more sufficient duodenal decompression and a more natural and physiological route for food passage.
The obstruction site in our patient resembled that of SMAS since it was located in the duodenojejunal flexure, and so we thought that LDJ could be a suitable method. As mentioned before, stiffness of the stomach makes GJS a difficult choice. Fortunately, rapid symptom relief and induction of chemotherapy was achieved, thus the selection of LDJ over GJS was an acceptable decision.
Prognosis of rPC after initial curative resection is poor and similar to that of de novo metastatic PC[1-3]. However, new anticancer agents and multiagent chemotherapy have improved overall survival (OS). The median OS for patients with rPC treated by chemotherapy is 10-14 mo compared to 3 mo without treatment[3], indicating the significant role of chemotherapy in prolonging the survival of these patients. In terms of our patient, she gained 10 mo survival, comparable to previous reports.
Improvement in quality of life (QOL) is also crucial in the multimodal therapy of cancer patients[12-15]. LDJ had a significant role in our patient by enabling oral food intake until the last few days of her life. High QOL is associated with better prognosis in patients receiving chemotherapy[12-14], although psychological distress can interfere with treatment[15]. We also believe that improving QOL is particularly important for patients with poor prognostic disease, and the fact that symptom relief and ability to eat were maintained in our patient shows that LDJ can have a significant role in palliative care of patients with obstruction around the duodenojejunal flexure due to unresectable malignant diseases such as lymphoma, PC, gastrointestinal tumor and peritoneal dissemination.
However, the indication for LDJ for unresectable malignancies remains uncertain since reports of LDJ performed on malignant stenosis are scarce[6]. LDJ is a method of palliative care, and so the absence of postoperative complications is crucial for prolonging survival of cancer patients by means of chemotherapy[16,17]. Preoperative management such as decompression of the duodenum with a nasogastric tube and correction of dehydration, electrolyte balances and nutrition are essential for avoiding complications such as anastomotic leakage. Chang et al[4] argues the importance of preoperative workup in LDJ for SMAS, and we think this can also apply for cancer patients as well.
To our knowledge, this is the first report on the role of LDJ as a part of a multimodal therapy for unresectable cancer. Many anticancer agents expected to prolong survival have been developed to date[18], and the role of minimally invasive surgery that preserve QOL will become increasingly significant. We expect more reports on cases of LDJ for malignant obstructions and hope that this procedure will be an acceptable treatment option for patients with unresectable malignant obstruction around the duodenojejunal flexure.
LDJ is thought to be a valuable method of palliative care and as a part of multimodal therapy for patients with unresectable malignant stenosis around the duodenojejunal flexure. By preserving QOL, this procedure is expected to be a bridge to chemotherapy for unresectable malignancies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Cianci P, Italy; Cianci P, Italy; Scurtu RR, Romania S-Editor: Chang KL L-Editor: Kerr C P-Editor: Chang KL
| 1. | Hajatdoost L, Sedaghat K, Walker EJ, Thomas J, Kosari S. Chemotherapy in Pancreatic Cancer: A Systematic Review. Medicina (Kaunas). 2018;54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 1223] [Article Influence: 305.8] [Reference Citation Analysis (0)] |
| 3. | Gbolahan OB, Tong Y, Sehdev A, O’Neil B, Shahda S. Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study. BMC Cancer. 2019;19:468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Chang J, Boules M, Rodriguez J, Walsh M, Rosenthal R, Kroh M. Laparoscopic duodenojejunostomy for superior mesenteric artery syndrome: intermediate follow-up results and a review of the literature. Surg Endosc. 2017;31:1180-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Jain N, Chopde A, Soni B, Sharma B, Saini S, Mishra S, Gupta R, Bhojwani R. SMA syndrome: management perspective with laparoscopic duodenojejunostomy and long-term results. Surg Endosc. 2021;35:2029-2038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Cyriac J, Klein L. A laparoscopic duodenojejunostomy for a duodenal obstruction from lymphoma. Surg Endosc. 2007;21:324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181:377-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Kim IY, Cho NC, Kim DS, Rhoe BS. Laparoscopic duodenojejunostomy for management of superior mesenteric artery syndrome: two cases report and a review of the literature. Yonsei Med J. 2003;44:526-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Munene G, Knab M, Parag B. Laparoscopic duodenojejunostomy for superior mesenteric artery syndrome. Am Surg. 2010;76:321-324. [PubMed] [Cited in This Article: ] |
| 10. | Sun Z, Rodriguez J, McMichael J, Walsh RM, Chalikonda S, Rosenthal RJ, Kroh MD, El-Hayek K. Minimally invasive duodenojejunostomy for superior mesenteric artery syndrome: a case series and review of the literature. Surg Endosc. 2015;29:1137-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kirby GC, Faulconer ER, Robinson SJ, Perry A, Downing R. Superior mesenteric artery syndrome: a single centre experience of laparoscopic duodenojejunostomy as the operation of choice. Ann R Coll Surg Engl. 2017;99:472-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Shibayama K, Kawaguchi Y, Otsuka T, Koga F, Nakashita S, Oza N, Ureshino N, Sadashima E, Kurobe K, Kosugi T, Shinchi K. Quality of Life During Chemotherapy in Japanese Patients with Unresectable Advanced Pancreatic Cancer. Asian J Human Services. 2020;19:42-54. [DOI] [Cited in This Article: ] |
| 13. | Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V, Bérille J, Conroy T. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 14. | Braun DP, Gupta D, Staren ED. Longitudinal health-related quality of life assessment implications for prognosis in stage IV pancreatic cancer. Pancreas. 2013;42:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356:1326-1327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Merkow RP, Bentrem DJ, Mulcahy MF, Chung JW, Abbott DE, Kmiecik TE, Stewart AK, Winchester DP, Ko CY, Bilimoria KY. Effect of postoperative complications on adjuvant chemotherapy use for stage III colon cancer. Ann Surg. 2013;258:847-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, Ko CY, Bentrem DJ. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 18. | Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, Yang W, Tian C, Miao Z, Wang T, Yang S. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 501] [Article Influence: 167.0] [Reference Citation Analysis (1)] |