Published online Jun 16, 2013. doi: 10.12998/wjcc.v1.i3.96
Revised: April 2, 2013
Accepted: May 7, 2013
Published online: June 16, 2013
In recent years there has been significant developments in photosensitizers (PSs), light sources and light delivery systems that have allowed decreasing the treatment time and skin phototoxicity resulting in more frequent use of photodynamic therapy (PDT) in the clinical settings. Compared to standard treatment approaches such as chemo-radiation and surgery, PDT has much reduced morbidity for head and neck malignancies and is becoming an alternative treatment option. It can be used as an adjunct therapy to other treatment modalities without any additive cumulative side effects. Surface illumination can be an option for pre-malignant and early-stage malignancies while interstitial treatment is for debulking of thick tumors in the head and neck region. PDT can achieve equivalent or greater efficacy in treating head and neck malignancies, suggesting that it may be considered as a first line therapy in the future. Despite progressive development, clinical PDT needs improvement in several topics for wider acceptance including standardization of protocols that involve the same administrated light and PS doses and establishing quantitative tools for PDT dosimetry planning and response monitoring. Quantitative measures such as optical parameters, PS concentration, tissue oxygenation and blood flow are essential for accurate PDT dosimetry as well as PDT response monitoring and assessing therapy outcome. Unlike conventional imaging modalities like magnetic resonance imaging, novel optical imaging techniques can quantify PDT-related parameters without any contrast agent administration and enable real-time assessment during PDT for providing fast feedback to clinicians. Ongoing developments in optical imaging offer the promise of optimization of PDT protocols with improved outcomes.
Core tip: Most treatment approaches including chemo-radiation and surgery can induce prolonged morbidity and functional loss resulting in severe impairment of patients’ quality of life. Photodynamic therapy (PDT) is an emerging alternative treatment option without any significant accumulative side effects due to targeted light illumination and preferential accumulation of photosensitizers (PSs). However, PDT has not found widespread applications at the clinic mainly due to variable responses that originated from unstandardized treatment protocols such as different light and PS doses. Novel optical imaging techniques can quantify PDT-dosimetry related parameters such as local light and PS dose in tissue and PDT response related parameters such as tissue oxygenation and blood flow noninvasively without any contrast agent administration, thereby providing real-time feedback about the treatment efficacy for optimizing and standardizing PDT.
- Citation: Sunar U. Monitoring photodynamic therapy of head and neck malignancies with optical spectroscopies. World J Clin Cases 2013; 1(3): 96-105
- URL: https://www.wjgnet.com/2307-8960/full/v1/i3/96.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v1.i3.96
Head and neck malignancies refer to malignancies arising from the oral cavity, pharynx, nasal cavity and sinuses[1-3]. Head and neck squamous cell carcinoma (HNSCC), constituting approximately 90% of malignancies in the head and neck region, remains the fifth most common form of cancer worldwide with an incidence of approximately 800000 new cases per year[4]. Most of these tumors may be attributed to risk factors such as tobacco and alcohol consumption. HNSCC is a heterogeneous disease with different stages ranging from benign squamous hyperplasia, dysplasia, carcinoma in situ (CIS) to invasive carcinoma[3]. Early stage diagnosis and treatment of HNSCC increases the likelihood of successful treatment and improves patients’ quality of life, lowers risk of mortality and health costs[5,6].
Substantial efforts concentrate on early detection with fair success, but still many patients present with clinically evident tumors that require effective treatment[7]. Several treatment options are available including surgery, chemotherapy, radiation therapy or combinations thereof[8]. In spite of improvements in these treatment modalities, they have their own limitations. For example, surgery may require resection of vital tissue such as part of the tongue resulting in functional loss. On the other hand, organ-preserving surgery can result in high recurrence rates. Nonsurgical management with chemo and radiation therapies to improve local-regional disease results in only modest or suboptimal improvements in survival but with significantly high cost side effects including speech and swallow function[9]. These conventional therapies may induce permanent vasculature dysfunction and necrosis, severe toxicities and irreversible injuries to non-tumor tissue such as the oral mucosa and the salivary glands, often resulting in morbidity and severe impairment of patients’ quality of life. Further, normal tissue toxicity such as mucositis, bleeding and imflammation may lead to changes in applied dose quantity, and treatment re-schedule, which may affect treatment efficacy and outcome. For these reasons, an alternative treatment modality that is effective, safe, repeatable, minimally invasive and non-surgical is desired for the management of head and neck malignancies.
Photodynamic therapy (PDT) uses light to activate a photosensitizer (PS) in the presence of oxygen for local tissue destruction, has potential in these respects and is particularly attractive due to its significant level of normal tissue preservation and its repeatability without cumulative side effects[10]. It has potential impact particularly for cases with multiple lesions and wide-spread early stage head and neck diseases (e.g., leukoplakia, invasive carcinoma) in the oral cavity[11]. However, PDT has not found widespread applications at the clinic mainly due to variable responses that originated from unstandardized treatment protocols such as different light and PS doses. Optical imaging can quantify local light and PS dose in tissue and monitor PDT; and therefore can provide feedback about the treatment efficacy. Thus, we expect optical imaging modalities will help in optimizing and standardizing PDT. Below we will detail PDT treatment and optical imaging for monitoring and ultimately predicting PDT response.
PDT is an emerging treatment option for many malignancies including head and neck. It is minimally invasive with much less side effects compared to conventional therapies. Since it does not have any significant accumulative side effects, it can be repeated many times and be applied before or after chemotherapy, radiation therapy. It can also be used as an adjuvant therapy to these therapies and surgery to eliminate residual microscopic tumor cells. PDT light can be delivered at the surface for wide and superficial malignancies and pre-malignancies such as mucosal dysplasia and CIS in the oral cavity. Interstitial light delivery is applied in treating thick and deep tumors for the aim of debulking tumors as an adjuvant to surgery.
PDT efficacy depends on three main elements: a sufficient amount of light, photosensitizing drug (also called PS) and available oxygen in tissue. The PS is activated during light illumination and the active PS reacts with molecular oxygen to produce singlet oxygen that induces direct cell killing, vascular destruction and immune response[12,13]. Most PSs are administered systematically but some can be applied topically for head and neck lesions in the oral cavity and nonmelanoma skin tumors. After a specific time, depending on the PS itself, PS accumulates specifically more in the diseased site compared to normal and surrounding periphery sites. Tumor to normal tissue contrast is generally 2-3 fold with a passive targeting mechanism, but even 10-fold contrast has been reported[14]. At the optimal time point of accumulation, a specific wavelength of light depending on the optical absorption properties of the PS is shined at a predetermined power to activate the PS to create a photodynamic reaction. Due to specific accumulation of the PS and localized light illumination, PDT is a local therapy rather than a systemic therapy like chemotherapy. The treatment volume depends on both PS and light penetration depth. For example, for the cases of Photofrin®, which is the first FDA-approved PS that was developed here at Roswell Park Cancer Institute, light illumination is at approximately 630 nm with a penetration depth of 5 mm or less. Thus, Photofrin® has been in use worldwide to treat early stage carcinomas in many organs including the head and neck.
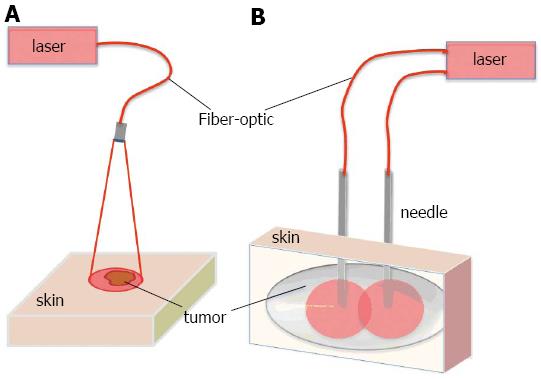
Previous studies have shown that PDT is safe and effective in the treatment of early carcinomas of the head and neck[2,10,11,13,14-35]. PDT is an excellent choice for early-stage malignancies since local treatment and limited light penetration eliminates the side effects that can occur in the sensitive areas of the oral cavity such as soft palate. Lasers are the choice for the light sources and laser light is delivered via surface illumination by using a micro-lens as shown in Figure 1A. For deeper and thicker tumors, however, superficial illumination is not suitable. In this case, light is delivered by feeding laser fibers through needles placed directly into the tumor (Figure 1B). This approach is very similar to brachytherapy or interstitial radiotherapy[36,37].
One of the main challenges of PDT is treating deeper and thicker tissues. However, this is not an issue for superficial malignancies. Pain management is a frequently reported challenge[38]. Another common side effect of PDT is the long-term skin photosensitivity, especially for the cases of systemic administration of PSs such as Photofrin® (porfimer sodium). ALA-PDT is another widely used treatment option for early stage malignancies with much reduced skin photosensitization, but with the drawback of severe pain during treatment, often necessitating anesthesia. Therefore, the development of PSs that do not induce long-term photosensitivity, produce durable results and are patient friendly is of significant clinical benefit. In this respect the second generation PSs, such as Photoclor (HPPH) used in our clinical trials, have shown clinical promise with their improved efficacy, higher penetration depths and significantly less skin photosensitivity.
Variable outcomes are the main roadblock to wider use of PDT. The lack of standardized protocols with the same light and PS type and doses, as well as imprecise dosimetry drives the variable PDT responses[36,37]. There is strong evidence that variations in clinical response are a direct result of dosimetry that does not take into account individual differences[39]. In order to bring PDT to a full realization of its potential benefits, quantitative tools are likely to play an essential role. They can provide standardization of site-specific individualized protocols by assessing light and PS doses.
Another challenge for clinical PDT of the head and neck is the difficulty in predicting the responders and non-responders[36]. Quantitative optical imaging tools can play a crucial role in filling this niche. These tools are currently in primitive stages and not widely used in clinical settings for monitoring PDT mainly because optical measurements may require extra clinical time and extra fiber replacements during PDT. The techniques are limited to pre- and post-PDT measurements but with the advent of new technologies they can be adapted for monitoring during PDT, which would have three-fold benefits: (1) reduced required clinical time, (2) no interruptions of treatment light for the optical measurements, and (3) more accurate quantification of kinetics of PDT-related parameters such as photobleaching and blood flow kinetics, which have been shown to be predictors of PDT response[36,40-50].
Tissue oxygen level is crucial for effective PDT since the PS initiates chemical reactions that result in cellular and vascular damage in targeted tissue in the presence of oxygen. Tissue oxygenation is highly affected by vascular parameters such as blood flow and blood oxygenation. During the PDT process, PS is consumed continuously. Thus, the efficacy of PDT is dependent on the vascular parameters and PS level and consumption (photobleaching)[50,51]. Vascular parameters and PS level change during PDT and these changes may be useful early markers for therapy response[36,44,52-54].
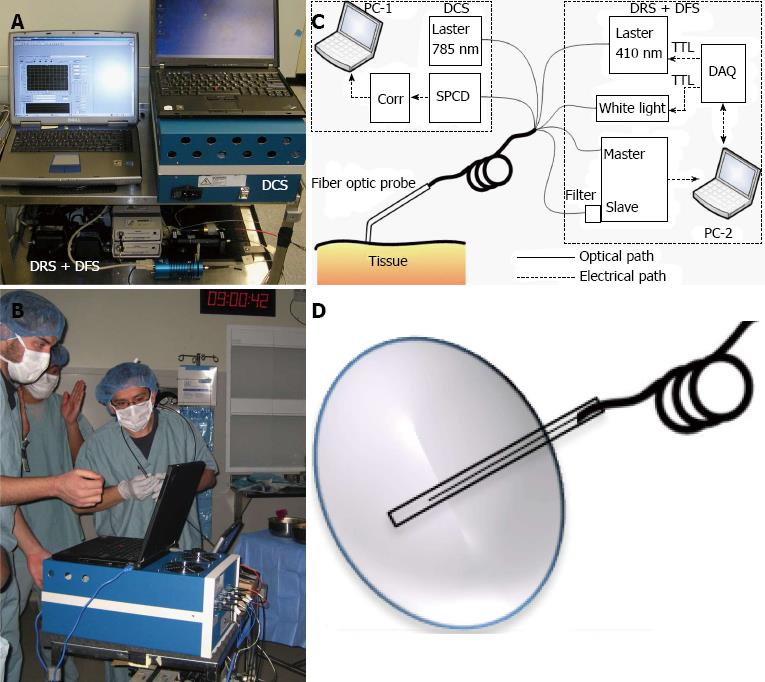
Optical imaging is a wide topic that includes many different imaging approaches. Here we will focus on a subdivision called diffuse optical spectroscopies (DOS) for probing millimeter to centimeter deep tissue[55-61]. In this context, DOS includes diffuse reflectance spectroscopy (DRS)[62-67], diffuse fluorescence spectroscopy (DFS)[40,67-70] and diffuse correlation spectroscopy (DCS)[71,72]. We have recently developed a multi-modal optical imaging technique that combines DRS, DFS and DCS in a single instrument and showed the feasibility of quantification of optical parameters (absorption and scattering), drug concentration and vascular parameters such as blood flow and oxygenation in a clinical setting[44,73].
The technical details of our multi-modal optical system can be found elsewhere[44,73], but here we briefly mention the basic working principles. The instrument performs measurements sequentially in the order of blood flow (DCS), optical parameters, blood oxygenation and volume (DRS), and fluorescence (DFS). Figure 2A and B shows the picture and schematic diagram of the instrument, respectively. The DCS instrument has a 785 nm, long coherence length laser (Crysta Laser), four single photon-counting detectors (SPCD, Perkin-Elmer), and a custom-built autocorrelator board (Correlator.com). Photodetector outputs were fed into a correlator board and intensity autocorrelation functions and photon arrival times were recorded by a computer. After blood flow measurements, the second laptop initiates fluorescence (DFS) and reflectance (DRS) data acquisition by utilizing TTL switching via a data acquisition card (DAQ, National Instruments). In absorption (DRS) mode, broadband diffuse reflectance measurements were taken by exciting the tissue with tungsten halogen lamp (Ocean Optics) and collecting the light with the Master channel of a two-channel spectrometer (Ocean Optics). In fluorescence (DFS) mode, a 410 nm laser diode (Power Technology) excites the PS in Soret band and the slave channel of the spectrometer collects the fluorescence spectra.
A hand-held “surface” probe that holds the light source and detector fibers can be used for measuring superficial malignancies by directly placing the tip of the probe on the tissue surface (Figure 2C). Although the instrument stays the same, the hand-held surface probe is ill-suited for interstitial light delivery and noninvasive measurements and the probe-tissue interface must be changed accordingly. For an “interstitial” probe, source and detector fibers are placed inside a catheter (Figure 2D).
Currently the standard PDT light dosimetry at the clinics is based on the prescribed incident dose, which does not take into account reflected and scattered light in the lesion. Head and neck malignancies can exhibit a multi-focal, wide-field nature of invasion and they may occur at diverse sites (e.g., tongue, lip, palate, etc.). Therefore, they can have different optical parameters resulting in considerable inter- and intra-patient variations in the deposited local dose[11]. It has been shown that the measured effective local dose can be more than 5-fold greater than the incident administrated dose, illustrating the need for in situ dose monitoring on an individual basis[39]. Dosimetry systems using isotropic light detectors to measure both incident and scattered light are becoming more available in clinical systems[36,37]. Multi-channel systems that can measure light dose at multiple points of interest in real time can provide on-line feedback to clinicians during treatment planning.
Tissue absorption and tissue scattering parameters modify light attenuation and thus affect the true light dose delivered to the whole three-dimensional tissue volume. Thus, direct light dose measurements may not be sufficient to quantify volumetric light distribution. Since malignancies can be highly heterogeneous, three dimensional optical parameter mapping can show heterogeneity of local light dose to the whole lesion volume. Several techniques are available for mapping of optical parameters (optical absorption and scattering) in vivo. Most of them are based on the photon diffusion equation with multi source-detector separations. Photon fluence (rate) is measured as a function of source-detector distance and measured data is fit to the diffusion model to extract optical parameters.
It has been demonstrated that PSs demonstrate significant inter- and intra-patient heterogeneity in distribution, leading to variations in the accumulated PDT dose and treatment failures[36,74,75]. It has been also suggested that the variation of the treatment outcome can be reduced by adjusting the light dose based on the pretreatment PS distribution so that PDT dose is uniform in the whole disease[36,75-78]. Although DRS can be used to quantify PS concentration by using the absorption peak of PSs, DFS is the preferred choice for this aim, since the fluorescence contrast is usually higher than the absorption contrast in vivo. However, fluorescence signal is affected by the tissue optical properties, and thus is not directly related to PS concentration. Ratiometric methods (with respect to optical attenuation and autofluorescence) may correct this signal distortion significantly[79,80]. Moreover, short source-detector separation (or single source-detector) based optical probes and empirical calibration techniques that calibrate the system with respect to reference optical phantoms may allow quantification of drug concentration. For quantifying PS concentration using DFS data, background subtracted fluorescence signal is usually normalized with the reflectance data obtained by DRS[65,66,70,81]. Fluorescence signal is assumed to be a linear combination of contributing components (i.e., PS fluorescence, tissue autofluorescence, etc.). The normalized tissue fluorescence is fit to the modeled tissue fluorescence to extract PS concentration[44,73].
Tissue oxygen is crucial for effective PDT[36,82-84]. Tissue oxygen, in turn, is affected by vascular parameters such as blood oxygenation, blood volume and blood flow[50,52]. Most PSs have significant vascular disrupting effects, and can create substantial vascular changes. All these parameters are inter-dependent to each other and can change continuously during PDT[4,36]. Blood flow changes during PDT correlated strongly with tumor growth delay, and blood oxygenation and volume changes were correlated with PDT outcome[50,52,85]. Moreover, PS photobleaching has been shown to be a surrogate marker of PDT response[40,86-90]. Therefore, continuous monitoring of these parameters could be useful for providing real-time treatment feedback, and may serve as quantitative in vivo markers for assessing treatment response[4,36,63].
For quantifying vascular parameters such as blood oxygenation and blood volume, an analytic diffuse reflectance model can be utilized to fit the diffusion model to experimental diffuse reflectance data obtained by DRS. We assume tissue absorption is composed of a linear contribution from oxy-hemoglobin and deoxyhemoglobin in blood, and PS absorption. Blood volume is related to total hemoglobin concentration and is defined as the sum of oxy-hemoglobin and dexoy-hemoglobin concentrations, and blood oxygen saturation is defined as the ratio of oxy-hemoglobin concentration to total hemoglobin concentration. Tissue scattering is usually modeled as Mie type behavior that is related to scatterer size and concentration[91]. A multi-wavelength fitting algorithm is usually used to directly extract the hemoglobin concentrations or blood oxygen saturation and blood volume[63,92,93]. Blood oxygen saturation is related to tissue oxygen and hypoxia[52,94] and blood volume is related to microvessel density[95].
Tissue blood flow is measured using a previously described and validated DCS instrument, which measures rapid light intensity temporal fluctuations in tissue and then uses the autocorrelation functions associated with these fluctuations to extract information about the speed of moving tissue scatterers, in this case red blood cells[44,49,96-101]. The decay rate of the autocorrelation function is related to blood flow[99-101]. DCS is advantageous compared to conventional imaging modalities in that it measures directly blood cell movements and does not need any contrast agent administration and pharmacokinetic models to quantify blood flow.
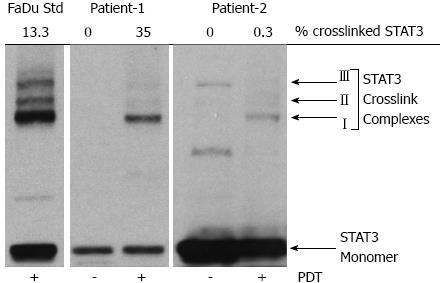
It is often desired to correlate noninvasive parameters with other techniques such as molecular biomarkers of a treatment response. We have shown previously in preclinical models and clinical biopsy samples that the cross-linking of the signal transducer and activator of transcription 3 (STAT3) correlates with the accumulated PDT dose and can be a quantitative biomarker of cellular killing[102,103]. The crosslinking is identified by immunoblot analysis for STAT3 protein in the extracts from tumor tissue sections calculated as homodimeric complex I relative to total STAT3 signal[102,103]. We compared our measured indices with the STAT3 crosslinking as showcased below.
In our previous work we demonstrated the assessment of PDT response-related multi-parameters of blood flow, oxygenation, blood volume, PS concentration in the same clinical setting of Photoclor (HPPH)-mediated PDT in head and neck lesions in the oral cavity[44]. We reported an interesting case where two patients had lesions treated with the same administered PS dose (HPPH, 4.0 mg/m2) and a similar delivered light dose (approximately 125 J/cm2), but the accumulated local doses were more than 100-fold different as determined by the STAT3 crosslinking (Table 1). The first patient had a large CIS of the hard palate on the roof of the mouth and PDT induced photoreaction with 35% STAT3 crosslinking, and the second patient had high-grade dysplasia in a papilloma of the buccal mucosa with only 0.3% STAT3 crosslinking (Figure 3). We quantified local PDT-related parameters with diffuse optical methods to investigate whether this substantial difference could be detected noninvasively since these parameters can affect accumulated local dose.
| Lesion type | STAT3 | ΔBFI | ΔBVf | ΔStO2 | ΔcHPPH | |
| P1 | CIS | 35% | 83.4% | 23% | +15.2% | 51.8% |
| P2 | Dysplasia | 0.3% | 59.2% | 7.5% | -17% | 38.6% |
As Table 1 summarizes, PDT-induced changes in the quantified optical parameters were significantly different between these lesions. Changes in PS concentration (ΔcHPPH), blood flow index (ΔBFI) and blood volume fraction (ΔBVf) were significantly higher in Patient-1 (P1) than in Patient-2 (P2), but the changes in blood oxygen saturation were similar for both patients, though the trend was different: P1 had an increase and P2 showed a decrease trend.
We further investigated whether this difference could be observed before therapy by quantifying pre-PDT contrasts (mean ± SE) by noninvasive methods. All parameters except blood volume fraction were significantly different between the lesions (Table 2). The lesion of P1 had more favorable properties related to accumulated local PDT dose, since its PS content as well as blood flow, blood volume and blood oxygen saturation were higher than P2.
| Lesion type | BFI (a.u.) | BVf (%) | StO2 (%) | cHPPH (μmol/L) | |
| P1 | CIS | 6.7 ± 2.8 | 2.5 ± 0.7 | 74 ± 2 | 0.34 ± 0.02 |
| P2 | Dysplasia | 1.8 ± 0.5 | 1.3 ± 0.2 | 64 ± 3 | 0.10 ± 0.03 |
Our results indicated that parameters quantified with DOS at pre-PDT as well as PDT-induced changes may be indicative of local PDT reaction and may be in vivo predictors of PDT outcome. Since each parameter showed different contrast and therapy-induced changes, one parameter alone may not be a strong indicator of PDT response and multi-parameters assessed by optical methods may provide accurate measure of PDT response[44].
In summary, PDT is regarded as an emerging treatment option for the head and neck malignancies. PDT can be applied repetitively if the previous treatment fails. With the advent of newly developed PSs, specificity and penetration depth can be improved. The simplicity of the PDT treatment and reduced cost of technology such as light sources and light delivery devices can help wide usage at the clinical settings. Moreover, there is a need for standardization of clinical protocols by using the same light and drug types and doses. Novel optical methods can provide PDT-dose related parameters such as optical parameters and PS concentration in the whole lesion, as well as can quantify blood flow, oxygenation and PS photobleaching for assessing the PDT response and providing feedback to clinicians for optimization and standardization of PDT in clinics.
This review is dedicated to late Britton Chance for his excellent mentoring. The author is grateful to Dr. Arjun G. Yodh at University of Pennsylvania for his continuous support and for the training in diffuse optical imaging during the Ph.D. studies. Further special thanks go to Shoko Nioka for providing initial support in the head and neck research.
P- Reviewer Andrzej MB S- Editor Song XX L- Editor A E- Editor Ma S
| 1. | Sunar U, Quon H, Durduran T, Zhang J, Du J, Zhou C, Yu G, Choe R, Kilger A, Lustig R. Noninvasive diffuse optical measurement of blood flow and blood oxygenation for monitoring radiation therapy in patients with head and neck tumors: a pilot study. J Biomed Opt. 2006;11:064021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Biel M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg Med. 2006;38:349-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890-1900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 974] [Cited by in F6Publishing: 1009] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 4. | Robinson DJ, Karakullukcu MB, Kruijt B, Kanick SC, van Veen RPL, Amelink A, Sterenborg HJCM, Witjes MJH, and Tan IB, Optical Spectroscopy to Guide Photodynamic Therapy of Head and Neck Tumors. IEEE J Sel Top Quantum Electron. 2010;16:854-862. [DOI] [Cited in This Article: ] |
| 5. | Menzin J, Lines LM, Manning LN. The economics of squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2007;15:68-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Hopper C, Niziol C, Sidhu M. The cost-effectiveness of Foscan mediated photodynamic therapy (Foscan-PDT) compared with extensive palliative surgery and palliative chemotherapy for patients with advanced head and neck cancer in the UK. Oral Oncol. 2004;40:372-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Upile T, Jerjes W, Sterenborg HJ, El-Naggar AK, Sandison A, Witjes MJ, Biel MA, Bigio I, Wong BJ, Gillenwater A. Head & amp; neck optical diagnostics: vision of the future of surgery. Head Neck Oncol. 2009;1:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Feldmann HJ, Molls M, Vaupel P. Blood flow and oxygenation status of human tumors. Clinical investigations. Strahlenther Onkol. 1999;175:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Quon H, Grossman CE, Finlay JC, Zhu TC, Clemmens CS, Malloy KM, Busch TM. Photodynamic therapy in the management of pre-malignant head and neck mucosal dysplasia and microinvasive carcinoma. Photodiagnosis Photodyn Ther. 2011;8:75-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem Photobiol. 2007;83:1063-1068. [PubMed] [Cited in This Article: ] |
| 11. | Grant WE, Hopper C, Speight PM, Macrobert AJ, Bown SG. Photodynamic therapy of malignant and premalignant lesions in patients with ‘field cancerization’ of the oral cavity. J Laryngol Otol. 1993;107:1140-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Henderson BW, Dougherty TJ. How does photodynamic therapy work. Photochem Photobiol. 1992;55:145-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1827] [Cited by in F6Publishing: 1694] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 13. | Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 363] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 14. | Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011;71:6040-6050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Lou PJ, Jones L, Hopper C. Clinical outcomes of photodynamic therapy for head-and-neck cancer. Technol Cancer Res Treat. 2003;2:311-317. [PubMed] [Cited in This Article: ] |
| 16. | Green B, Cobb AR, Hopper C. Photodynamic therapy in the management of lesions of the head and neck. Br J Oral Maxillofac Surg. 2013;51:283-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Sharwani A, Jerjes W, Hopper C, Lewis MP, El-Maaytah M, Khalil HS, Macrobert AJ, Upile T, Salih V. Photodynamic therapy down-regulates the invasion promoting factors in human oral cancer. Arch Oral Biol. 2006;51:1104-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Jerjes W, Upile T, Hamdoon Z, Mosse CA, Akram S, Hopper C. Photodynamic therapy outcome for oral dysplasia. Lasers Surg Med. 2011;43:192-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Jerjes W, Upile T, Hamdoon Z, Alexander Mosse C, Morcos M, Hopper C. Photodynamic therapy outcome for T1/T2 N0 oral squamous cell carcinoma. Lasers Surg Med. 2011;43:463-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Jerjes W, Hamdoon Z, Hopper C. Photodynamic therapy in the management of potentially malignant and malignant oral disorders. Head Neck Oncol. 2012;4:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Hopper C, Kübler A, Lewis H, Tan IB, Putnam G. mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int J Cancer. 2004;111:138-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Hopper C. The role of photodynamic therapy in the management of oral cancer and precancer. Eur J Cancer B Oral Oncol. 1996;32B:71-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Grant WE, Speight PM, Hopper C, Bown SG. Photodynamic therapy: an effective, but non-selective treatment for superficial cancers of the oral cavity. Int J Cancer. 1997;71:937-942. [PubMed] [Cited in This Article: ] |
| 24. | Grant WE, Hopper C, MacRobert AJ, Speight PM, Bown SG. Photodynamic therapy of oral cancer: photosensitisation with systemic aminolaevulinic acid. Lancet. 1993;342:147-148. [PubMed] [Cited in This Article: ] |
| 25. | Fan KF, Hopper C, Speight PM, Buonaccorsi GA, Bown SG. Photodynamic therapy using mTHPC for malignant disease in the oral cavity. Int J Cancer. 1997;73:25-32. [PubMed] [Cited in This Article: ] |
| 26. | Fan KF, Hopper C, Speight PM, Buonaccorsi G, MacRobert AJ, Bown SG. Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer. 1996;78:1374-1383. [PubMed] [Cited in This Article: ] |
| 27. | Kulapaditharom B, Boonkitticharoen V. Photodynamic therapy in management of head and neck cancers and precancerous lesions. J Med Assoc Thai. 2000;83:249-258. [PubMed] [Cited in This Article: ] |
| 28. | Biel MA. Photodynamic therapy and the treatment of head and neck neoplasia. Laryngoscope. 1998;108:1259-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Schweitzer VG. PHOTOFRIN-mediated photodynamic therapy for treatment of early stage oral cavity and laryngeal malignancies. Lasers Surg Med. 2001;29:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Zhao SP, Tao ZD, Xiao JY, Peng YY, Yang YH, Zeng QS, Liu ZW. Clinical use of hematoporphyrin derivative and photoradiation therapy in nasopharyngeal carcinoma. Chin Med J (Engl). 1988;101:86-91. [PubMed] [Cited in This Article: ] |
| 31. | Freche C, De Corbiere S. Use of photodynamic therapy in the treatment of vocal cord carcinoma. J Photochem Photobiol B. 1990;6:291-296. [PubMed] [Cited in This Article: ] |
| 32. | Gluckman JL. Hematoporphyrin photodynamic therapy: is there truly a future in head and neck oncology Reflections on a 5-year experience. Laryngoscope. 1991;101:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Gluckman JL. Photodynamic therapy for head and neck neoplasms. Otolaryngol Clin North Am. 1991;24:1559-1567. [PubMed] [Cited in This Article: ] |
| 34. | Feyh J, Goetz A, Müller W, Königsberger R, Kastenbauer E. Photodynamic therapy in head and neck surgery. J Photochem Photobiol B. 1990;7:353-358. [PubMed] [Cited in This Article: ] |
| 35. | Wenig BL, Kurtzman DM, Grossweiner LI, Mafee MF, Harris DM, Lobraico RV, Prycz RA, Appelbaum EL. Photodynamic therapy in the treatment of squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1990;116:1267-1270. [PubMed] [Cited in This Article: ] |
| 36. | Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53:R61-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 574] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 37. | Zhu TC, Finlay JC. The role of photodynamic therapy (PDT) physics. Med Phys. 2008;35:3127-3136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Bredell MG, Besic E, Maake C, Walt H. The application and challenges of clinical PD-PDT in the head and neck region: a short review. J Photochem Photobiol B. 2010;101:185-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Tan IB, Oppelaar H, Ruevekamp MC, Veenhuizen RB, Timmers A, Stewart FA. The importance of in situ light dosimetry for photodynamic therapy of oral cavity tumors. Head Neck. 1999;21:434-441. [PubMed] [Cited in This Article: ] |
| 40. | Wilson BC, Patterson MS, Lilge L. Implicit and explicit dosimetry in photodynamic therapy: a New paradigm. Lasers Med Sci. 1997;12:182-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 259] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Boere IA, Robinson DJ, de Bruijn HS, Kluin J, Tilanus HW, Sterenborg HJ, de Bruin RW. Protoporphyrin IX fluorescence photobleaching and the response of rat Barrett’s esophagus following 5-aminolevulinic acid photodynamic therapy. Photochem Photobiol. 2006;82:1638-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Boere IA, Robinson DJ, de Bruijn HS, van den Boogert J, Tilanus HW, Sterenborg HJ, de Bruin RW. Monitoring in situ dosimetry and protoporphyrin IX fluorescence photobleaching in the normal rat esophagus during 5-aminolevulinic acid photodynamic therapy. Photochem Photobiol. 2003;78:271-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Robinson DJ, de Bruijn HS, van der Veen N, Stringer MR, Brown SB, Star WM. Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: the effect of light dose and irradiance and the resulting biological effect. Photochem Photobiol. 1998;67:140-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 195] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Sunar U, Rohrbach D, Rigual N, Tracy E, Keymel K, Cooper MT, Baumann H, Henderson BH. Monitoring photobleaching and hemodynamic responses to HPPH-mediated photodynamic therapy of head and neck cancer: a case report. Opt Express. 2010;18:14969-14978. [PubMed] [Cited in This Article: ] |
| 45. | Khurana M, Moriyama EH, Mariampillai A, Wilson BC. Intravital high-resolution optical imaging of individual vessel response to photodynamic treatment. J Biomed Opt. 2008;13:040502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Mariampillai A, Standish BA, Moriyama EH, Khurana M, Munce NR, Leung MK, Jiang J, Cable A, Wilson BC, Vitkin IA. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett. 2008;33:1530-1532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 410] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 47. | Li H, Standish BA, Mariampillai A, Munce NR, Mao Y, Chiu S, Marcon NE, Wilson BC, Vitkin A, Yang VX. Feasibility of interstitial Doppler optical coherence tomography for in vivo detection of microvascular changes during photodynamic therapy. Lasers Surg Med. 2006;38:754-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Yang VX, Mao YX, Munce N, Standish B, Kucharczyk W, Marcon NE, Wilson BC, Vitkin IA. Interstitial Doppler optical coherence tomography. Opt Lett. 2005;30:1791-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Yu G. Near-infrared diffuse correlation spectroscopy in cancer diagnosis and therapy monitoring. J Biomed Opt. 2012;17:010901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Yu G, Durduran T, Zhou C, Wang HW, Putt ME, Saunders HM, Sehgal CM, Glatstein E, Yodh AG, Busch TM. Noninvasive monitoring of murine tumor blood flow during and after photodynamic therapy provides early assessment of therapeutic efficacy. Clin Cancer Res. 2005;11:3543-3552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 51. | Georgakoudi I, Foster TH. Singlet oxygen- versus nonsinglet oxygen-mediated mechanisms of sensitizer photobleaching and their effects on photodynamic dosimetry. Photochem Photobiol. 1998;67:612-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Wang HW, Putt ME, Emanuele MJ, Shin DB, Glatstein E, Yodh AG, Busch TM. Treatment-induced changes in tumor oxygenation predict photodynamic therapy outcome. Cancer Res. 2004;64:7553-7561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Yu G, Durduran T, Zhou C, Zhu TC, Finlay JC, Busch TM, Malkowicz SB, Hahn SM, Yodh AG. Real-time in situ monitoring of human prostate photodynamic therapy with diffuse light. Photochem Photobiol. 2006;82:1279-1284. [PubMed] [Cited in This Article: ] |
| 54. | Becker TL, Paquette AD, Keymel KR, Henderson BW, Sunar U. Monitoring blood flow responses during topical ALA-PDT. Biomed Opt Express. 2010;2:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia. 2000;2:26-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 511] [Cited by in F6Publishing: 388] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 56. | Pham TH, Hornung R, Berns MW, Tadir Y, Tromberg BJ. Monitoring tumor response during photodynamic therapy using near-infrared photon-migration spectroscopy. Photochem Photobiol. 2001;73:669-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Kukreti S, Cerussi AE, Tanamai W, Hsiang D, Tromberg BJ, Gratton E. Characterization of metabolic differences between benign and malignant tumors: high-spectral-resolution diffuse optical spectroscopy. Radiology. 2010;254:277-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Chen Y, Intes X, Tailor DR, Regatte RR, Ma H, Ntziachristos V, Leigh JS, Reddy R, Chance B. Probing rat brain oxygenation with near-infrared spectroscopy (NIRS) and magnetic resonance imaging (MRI). Adv Exp Med Biol. 2003;510:199-204. [PubMed] [Cited in This Article: ] |
| 59. | Chen Y, Tailor DR, Intes X, Chance B. Correlation between near-infrared spectroscopy and magnetic resonance imaging of rat brain oxygenation modulation. Phys Med Biol. 2003;48:417-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Matcher SJ, Cope M, Delpy DT. Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy. Phys Med Biol. 1994;39:177-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 261] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Carpenter CM, Pogue BW, Jiang S, Dehghani H, Wang X, Paulsen KD, Wells WA, Forero J, Kogel C, Weaver JB. Image-guided optical spectroscopy provides molecular-specific information in vivo: MRI-guided spectroscopy of breast cancer hemoglobin, water, and scatterer size. Opt Lett. 2007;32:933-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Amelink A, van der Ploeg van den Heuvel A, de Wolf WJ, Robinson DJ, Sterenborg HJ. Monitoring PDT by means of superficial reflectance spectroscopy. J Photochem Photobiol B. 2005;79:243-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Bargo PR, Prahl SA, Goodell TT, Sleven RA, Koval G, Blair G, Jacques SL. In vivo determination of optical properties of normal and tumor tissue with white light reflectance and an empirical light transport model during endoscopy. J Biomed Opt. 2005;10:034018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Cottrell WJ, Paquette AD, Keymel KR, Foster TH, Oseroff AR. Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas. Clin Cancer Res. 2008;14:4475-4483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Kienle A, Patterson MS. Improved solutions of the steady-state and the time-resolved diffusion equations for reflectance from a semi-infinite turbid medium. J Opt Soc Am A Opt Image Sci Vis. 1997;14:246-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 230] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 66. | Farrell TJ, Patterson MS, Wilson B. A diffusion theory model of spatially resolved, steady-state diffuse reflectance for the noninvasive determination of tissue optical properties in vivo. Med Phys. 1992;19:879-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1021] [Cited by in F6Publishing: 680] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 67. | Brown JQ, Wilke LG, Geradts J, Kennedy SA, Palmer GM, Ramanujam N. Quantitative optical spectroscopy: a robust tool for direct measurement of breast cancer vascular oxygenation and total hemoglobin content in vivo. Cancer Res. 2009;69:2919-2926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 68. | Wagnières GA, Star WM, Wilson BC. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem Photobiol. 1998;68:603-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 498] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 69. | Cottrell WJ, Oseroff A, Foster TH. Portable instrument that integrates irradiation with fluorescence and reflectance spectroscopies during PDT of cutaneous disease. Rev Sci Instrum. 2006;77:064302. [DOI] [Cited in This Article: ] |
| 70. | Wu J, Feld MS, Rava RP. Analytical model for extracting intrinsic fluorescence in turbid media. Appl Opt. 1993;32:3585-3595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 148] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Pine DJ, Weitz DA, Chaikin PM, Herbolzheimer E. Diffusing wave spectroscopy. Phys Rev Lett. 1988;60:1134-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 844] [Cited by in F6Publishing: 491] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 72. | Berne BJ, Pecora R. Dynamic Light Scattering. New York: Wiley 1976; . [Cited in This Article: ] |
| 73. | Rohrbach DJ, Rigual N, Tracy E, Kowalczewski A, Keymel KL, Cooper MT, Mo W, Baumann H, Henderson BW, Sunar U. Interlesion differences in the local photodynamic therapy response of oral cavity lesions assessed by diffuse optical spectroscopies. Biomed Opt Express. 2012;3:2142-2153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Busch TM, Hahn SM, Wileyto EP, Koch CJ, Fraker DL, Zhang P, Putt M, Gleason K, Shin DB, Emanuele MJ. Hypoxia and Photofrin uptake in the intraperitoneal carcinomatosis and sarcomatosis of photodynamic therapy patients. Clin Cancer Res. 2004;10:4630-4638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Zhou X, Pogue BW, Chen B, Demidenko E, Joshi R, Hoopes J, Hasan T. Pretreatment photosensitizer dosimetry reduces variation in tumor response. Int J Radiat Oncol Biol Phys. 2006;64:1211-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Glanzmann T, Hadjur C, Zellweger M, Grosiean P, Forrer M, Ballini JP, Monnier P, van den Bergh H, Lim CK, Wagnières G. Pharmacokinetics of tetra(m-hydroxyphenyl)chlorin in human plasma and individualized light dosimetry in photodynamic therapy. Photochem Photobiol. 1998;67:596-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 77. | Axelsson J, Swartling J, Andersson-Engels S. In vivo photosensitizer tomography inside the human prostate. Opt Lett. 2009;34:232-234. [PubMed] [Cited in This Article: ] |
| 78. | Dimofte A, Zhu TC, Hahn SM, Lustig RA. In vivo light dosimetry for motexafin lutetium-mediated PDT of recurrent breast cancer. Lasers Surg Med. 2002;31:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Bogaards A, Sterenborg HJ, Trachtenberg J, Wilson BC, Lilge L. In vivo quantification of fluorescent molecular markers in real-time by ratio imaging for diagnostic screening and image-guided surgery. Lasers Surg Med. 2007;39:605-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Moriyama EH, Kim A, Bogaards A, Lilge L, Wilson B. A ratiometric fluorescence imaging system for surgical guidance. Adv Opt Tech. 2008;1-10. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Yodh A, Chance B. Spectroscopy and Imaging with Diffusing Light. Physics Today. 1995;48:34-40. [DOI] [Cited in This Article: ] |
| 82. | Tromberg BJ, Orenstein A, Kimel S, Barker SJ, Hyatt J, Nelson JS, Berns MW. In vivo tumor oxygen tension measurements for the evaluation of the efficiency of photodynamic therapy. Photochem Photobiol. 1990;52:375-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 120] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Tromberg BJ, Kimel S, Orenstein A, Barker SJ, Hyatt J, Nelson JS, Roberts WG, Berns MW. Tumor oxygen tension during photodynamic therapy. J Photochem Photobiol B. 1990;5:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res. 1991;126:296-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 266] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 85. | Chen B, Pogue BW, Goodwin IA, O’Hara JA, Wilmot CM, Hutchins JE, Hoopes PJ, Hasan T. Blood flow dynamics after photodynamic therapy with verteporfin in the RIF-1 tumor. Radiat Res. 2003;160:452-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Georgakoudi I, Nichols MG, Foster TH. The mechanism of Photofrin photobleaching and its consequences for photodynamic dosimetry. Photochem Photobiol. 1997;65:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 180] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 87. | Sheng C, Hoopes PJ, Hasan T, Pogue BW. Photobleaching-based dosimetry predicts deposited dose in ALA-PpIX PDT of rodent esophagus. Photochem Photobiol. 2007;83:738-748. [PubMed] [Cited in This Article: ] |
| 88. | Ericson MB, Wennberg AM, Larkö O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther Clin Risk Manag. 2008;4:1-9. [PubMed] [Cited in This Article: ] |
| 89. | Sheng C, Pogue BW, Wang E, Hutchins JE, Hoopes PJ. Assessment of photosensitizer dosimetry and tissue damage assay for photodynamic therapy in advanced-stage tumors. Photochem Photobiol. 2004;79:520-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Finlay JC, Conover DL, Hull EL, Foster TH. Porphyrin bleaching and PDT-induced spectral changes are irradiance dependent in ALA-sensitized normal rat skin in vivo. Photochem Photobiol. 2001;73:54-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 91. | Mourant JR, Fuselier T, Boyer J, Johnson TM, Bigio IJ. Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl Opt. 1997;36:949-957. [PubMed] [Cited in This Article: ] |
| 92. | Wang HW, Zhu TC, Putt ME, Solonenko M, Metz J, Dimofte A, Miles J, Fraker DL, Glatstein E, Hahn SM. Broadband reflectance measurements of light penetration, blood oxygenation, hemoglobin concentration, and drug concentration in human intraperitoneal tissues before and after photodynamic therapy. J Biomed Opt. 2005;10:14004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Bargo PR. Optical measurements for quality control in photodynamic therapy. Portland: Oregon Health and Science University 2003; . [Cited in This Article: ] |
| 94. | Sunar U, Makonnen S, Zhou C, Durduran T, Yu G, Wang HW, Lee WM, Yodh AG. Hemodynamic responses to antivascular therapy and ionizing radiation assessed by diffuse optical spectroscopies. Opt Express. 2007;15:15507-15516. [PubMed] [DOI] [Cited in This Article: ] |
| 95. | Pakalniskis MG, Wells WA, Schwab MC, Froehlich HM, Jiang S, Li Z, Tosteson TD, Poplack SP, Kaufman PA, Pogue BW. Tumor angiogenesis change estimated by using diffuse optical spectroscopic tomography: demonstrated correlation in women undergoing neoadjuvant chemotherapy for invasive breast cancer. Radiology. 2011;259:365-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Binzoni T, Leung TS, Rüfenacht D, Delpy DT. Absorption and scattering coefficient dependence of laser-Doppler flowmetry models for large tissue volumes. Phys Med Biol. 2006;51:311-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Boas DA, Campbell LE, Yodh AG. Scattering and Imaging with Diffusing Temporal Field Correlations. Phys Rev Lett. 1995;75:1855-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 269] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 98. | Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. JOSA A. 1997;14:192-215. [DOI] [Cited in This Article: ] |
| 99. | Cheung C, Culver JP, Takahashi K, Greenberg JH, Yodh AG. In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys Med Biol. 2001;46:2053-2065. [PubMed] [Cited in This Article: ] |
| 100. | Culver JP, Durduran T, Furuya D, Cheung C, Greenberg JH, Yodh AG. Diffuse optical tomography of cerebral blood flow, oxygenation, and metabolism in rat during focal ischemia. J Cereb Blood Flow Metab. 2003;23:911-924. [PubMed] [Cited in This Article: ] |
| 101. | Mesquita RC, Durduran T, Yu G, Buckley EM, Kim MN, Zhou C, Choe R, Sunar U, Yodh AG. Direct measurement of tissue blood flow and metabolism with diffuse optics. Philos Trans A Math Phys Eng Sci. 2011;369:4390-4406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 102. | Henderson BW, Daroqui C, Tracy E, Vaughan LA, Loewen GM, Cooper MT, Baumann H. Cross-linking of signal transducer and activator of transcription 3--a molecular marker for the photodynamic reaction in cells and tumors. Clin Cancer Res. 2007;13:3156-3163. [PubMed] [Cited in This Article: ] |
| 103. | Liu W, Oseroff AR, Baumann H. Photodynamic therapy causes cross-linking of signal transducer and activator of transcription proteins and attenuation of interleukin-6 cytokine responsiveness in epithelial cells. Cancer Res. 2004;64:6579-6587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |