Copyright
©The Author(s) 2022.
World J Clin Cases. Jan 21, 2022; 10(3): 790-801
Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.790
Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.790
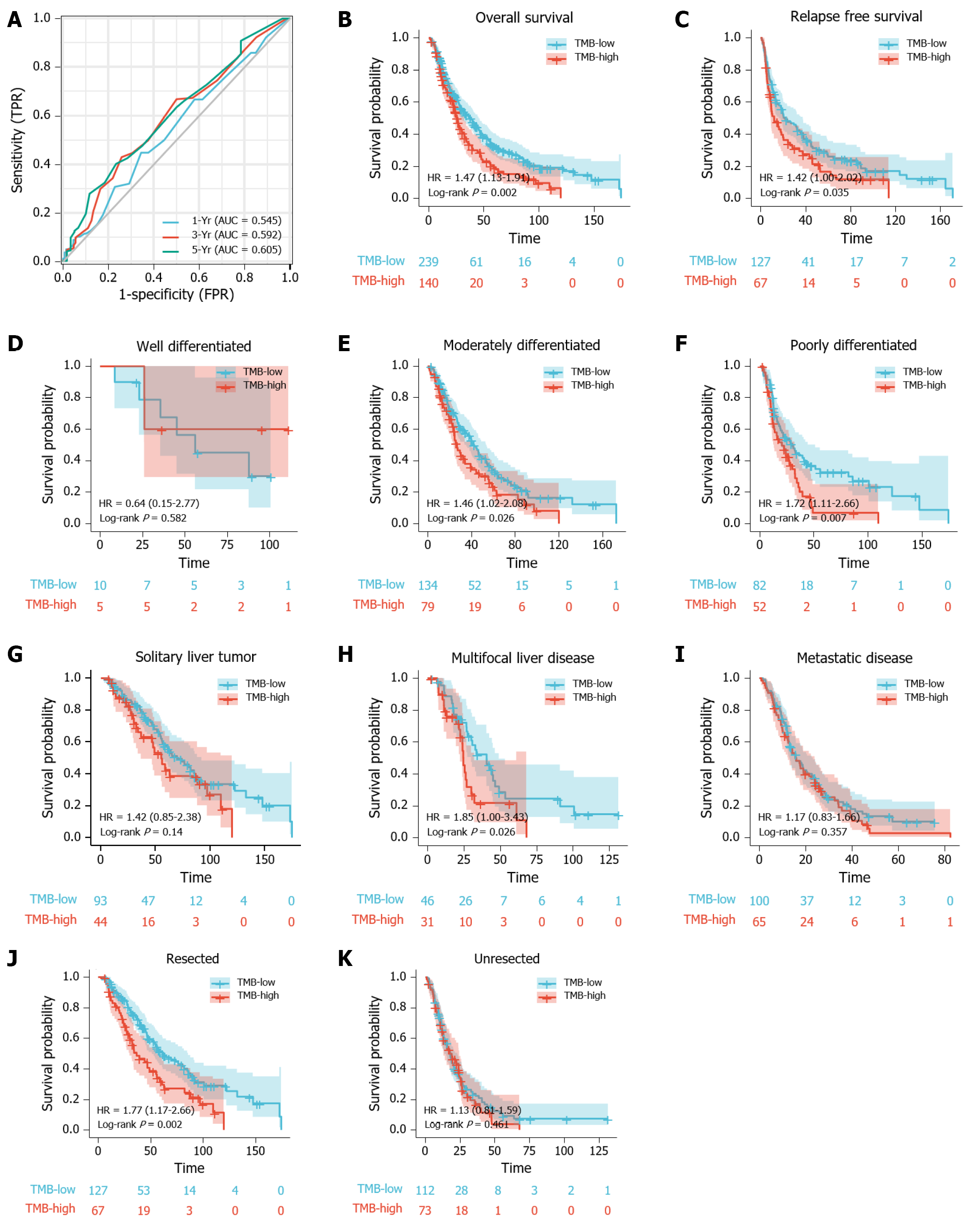
Figure 1 Prognostic ability of tumor mutation burden in predicting the prognosis of intrahepatic cholangiocarcinoma patients.
A: Time-dependent receiver operating characteristic curve analysis of tumor mutation burden (TMB) shows the area under the curve (AUC)s for 1-, 3-, and 5-year survival were 0.545, 0.592, and 0.605 respectively. The best cut-off value of TMB for all three was 3.1; B and C: Kaplan-Meier plot shows that the intrahepatic cholangiocarcinoma (ICC) patients with high-TMB had poor overall survival (OS) (HR = 1.47, P = 0.002; B) and relapse free survival (HR = 1.42, P = 0.035; C); D-F: Kaplan-Meier analysis shows the impact of TMB on the OS of ICC patients with different tumor grades, including (D) well differentiated (HR = 0.64, P = 0.582), (E) moderately differentiated (HR = 1.46, P = 0.026), and (F) poorly differentiated subsets (HR = 1.72, P = 0.007); G-I: Kaplan-Meier analysis shows the impact of TMB on the OS of ICC patients with different disease progressions, including (G) solitary liver tumor (HR = 1.42, P = 0.140), (H) multifocal liver disease (HR = 1.85, P = 0.026), and (I) metastatic disease (HR = 1.17, P = 0.357); J-K: Kaplan-Meier analysis shows the impact of TMB on the OS of ICC patients with respect to tumor resection, including patients who were (J) resected (HR = 1.77, P = 0.002) and (K) unresected (HR = 1.13, P = 0.461).
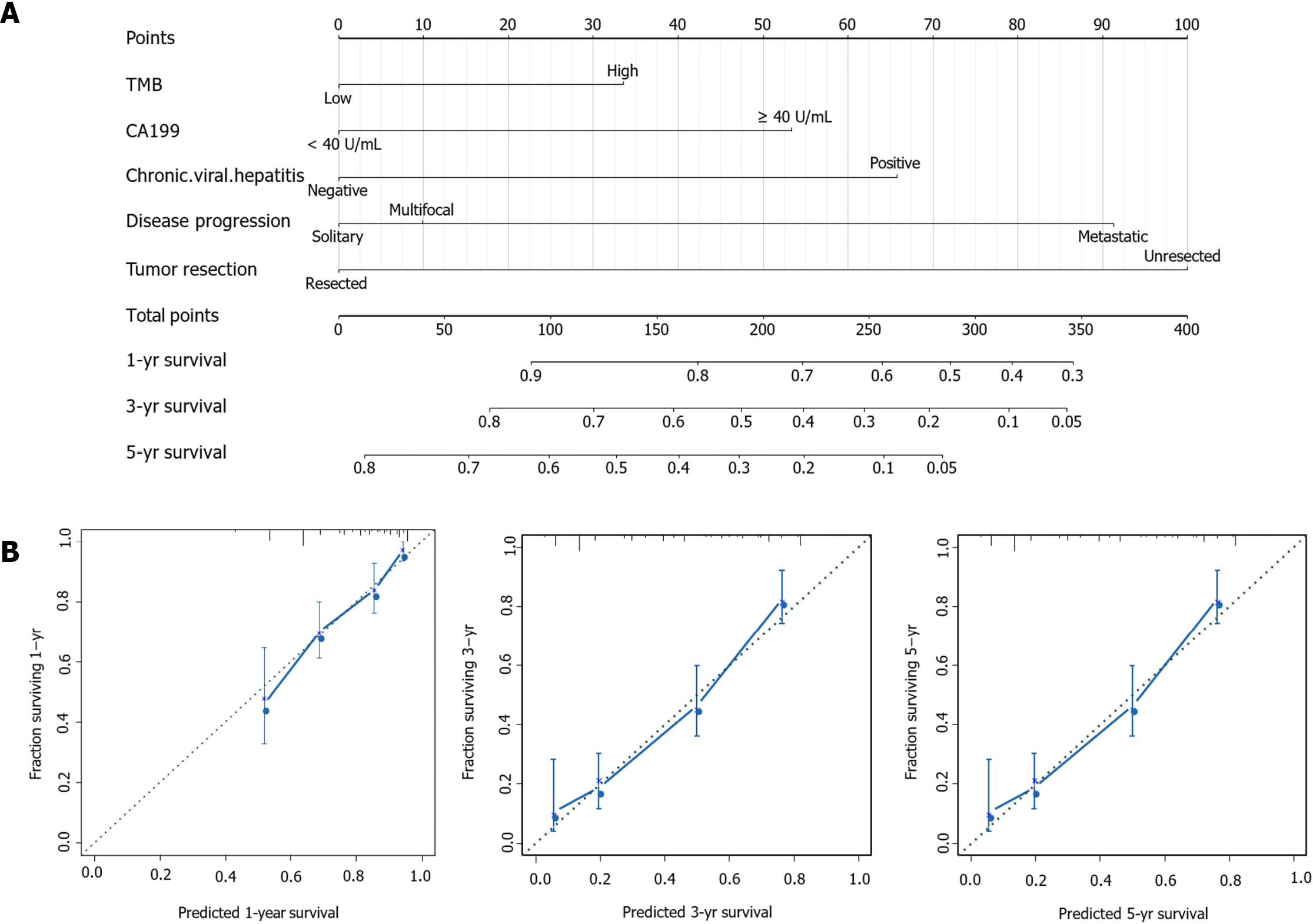
Figure 2 Construction of a prognostic nomogram for intrahepatic cholangiocarcinoma patients.
A: The predicted 1-, 3-, and 5-year survival rates in intrahepatic cholangiocarcinoma patients based on our nomogram, which included tumor mutation burden, CA19-9, chronic viral hepatitis, tumor resection and disease progression; B: Calibration plots show that the observation and prediction results of 1-, 3-, and 5-year survival rates are consistent with the actual observation and prediction.
- Citation: Song JP, Liu XZ, Chen Q, Liu YF. High tumor mutation burden indicates a poor prognosis in patients with intrahepatic cholangiocarcinoma. World J Clin Cases 2022; 10(3): 790-801
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/790.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.790