Copyright
©The Author(s) 2022.
World J Clin Cases. Jun 26, 2022; 10(18): 6069-6081
Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6069
Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6069
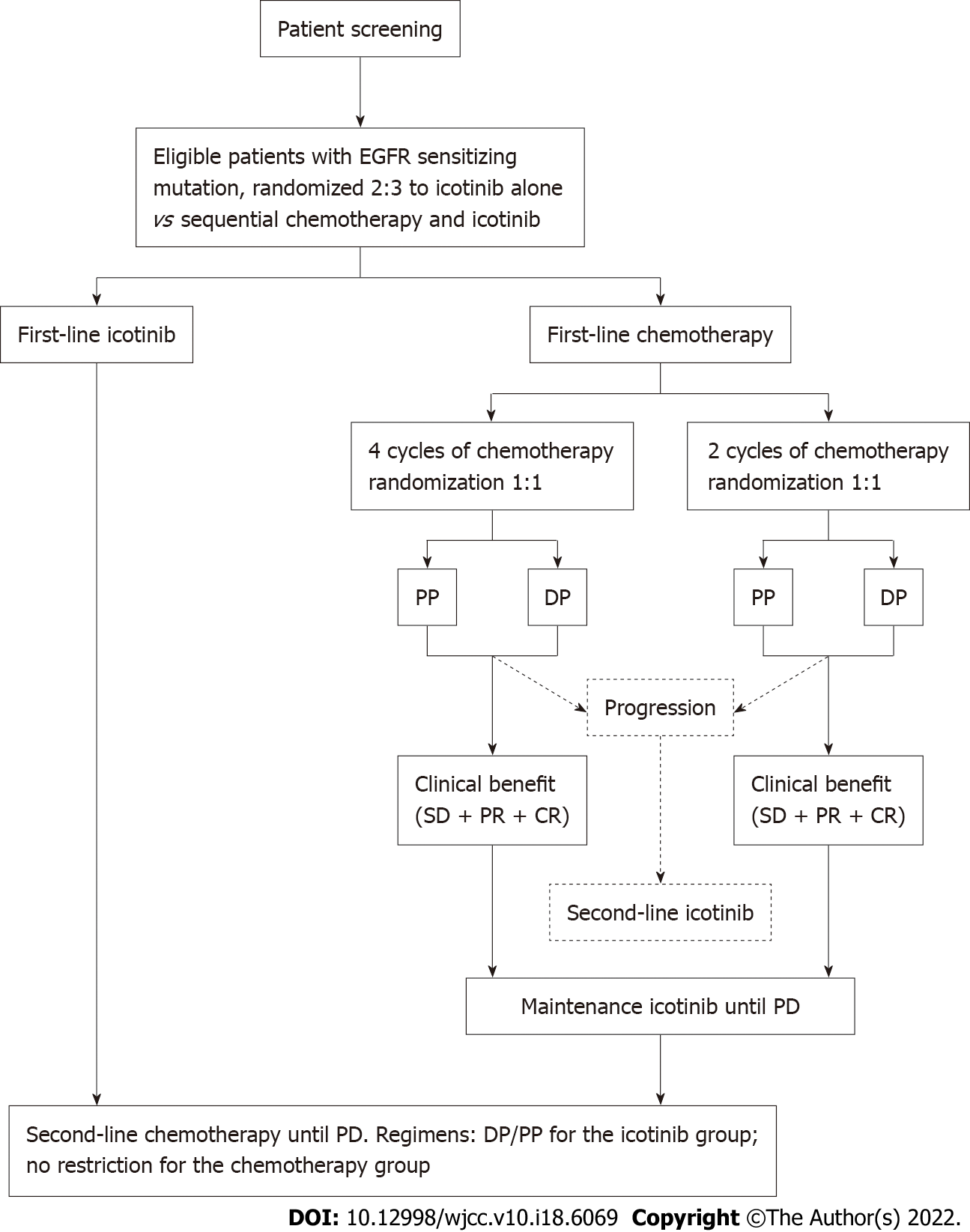
Figure 1 Study design.
DP: Docetaxel/cisplatin; EGFR: Epithelial growth factor receptor; PD: Progressive disease; PP: Pemetrexed/cisplatin; PR: Partial response; SD: Stable disease.
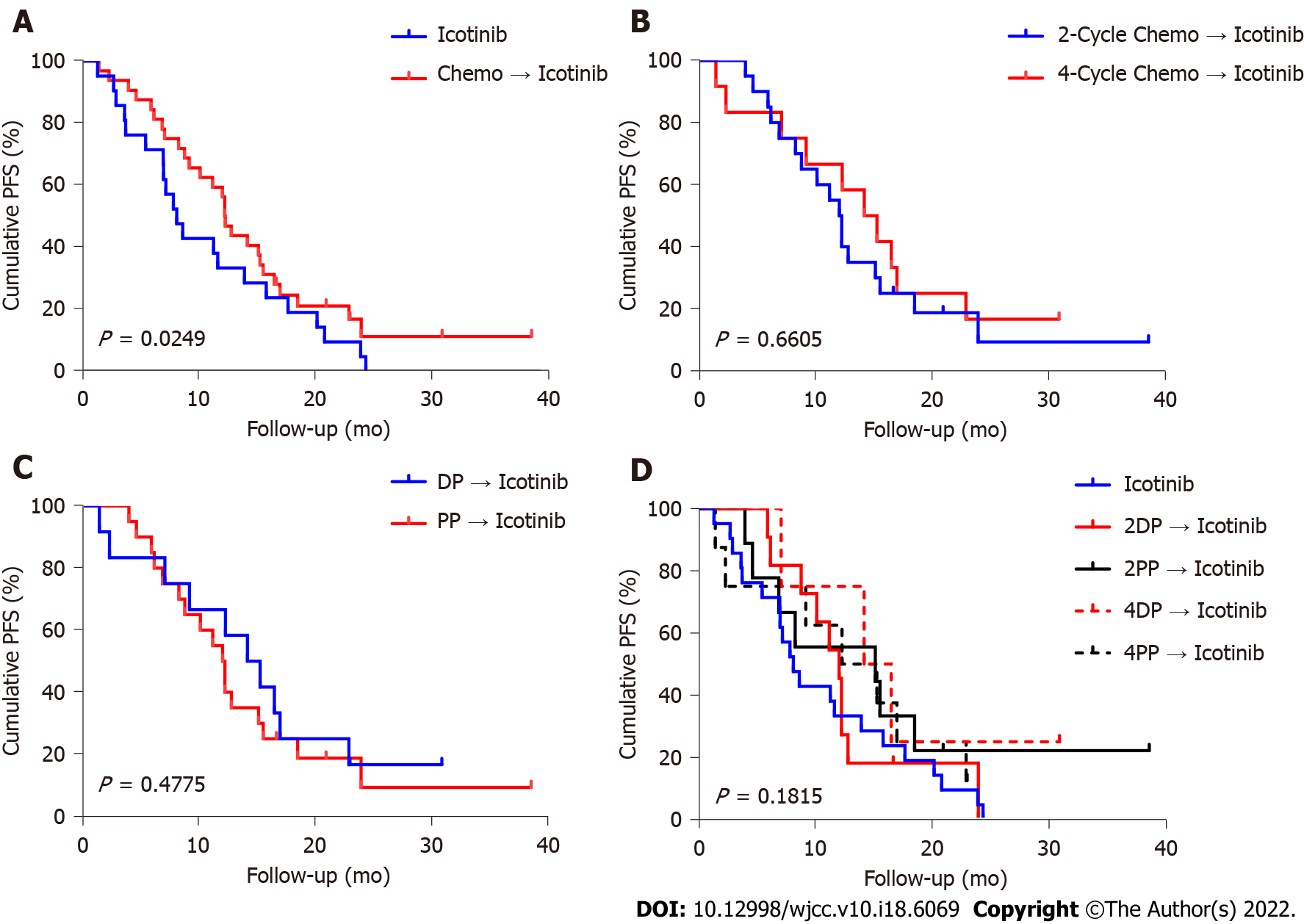
Figure 2 Progression-free survival of patients.
A: Progression-free survival (PFS) with icotinib vs chemotherapy followed by icotinib; B: PFS with two-cycle chemotherapy followed by icotinib vs four-cycle chemotherapy followed by icotinib; C: PFS with DP followed by icotinib vs PP followed by icotinib; D: PFS with icotinib vs various chemotherapy regimens followed by icotinib. DP: Docetaxel/cisplatin; PP: Pemetrexed/cisplatin.
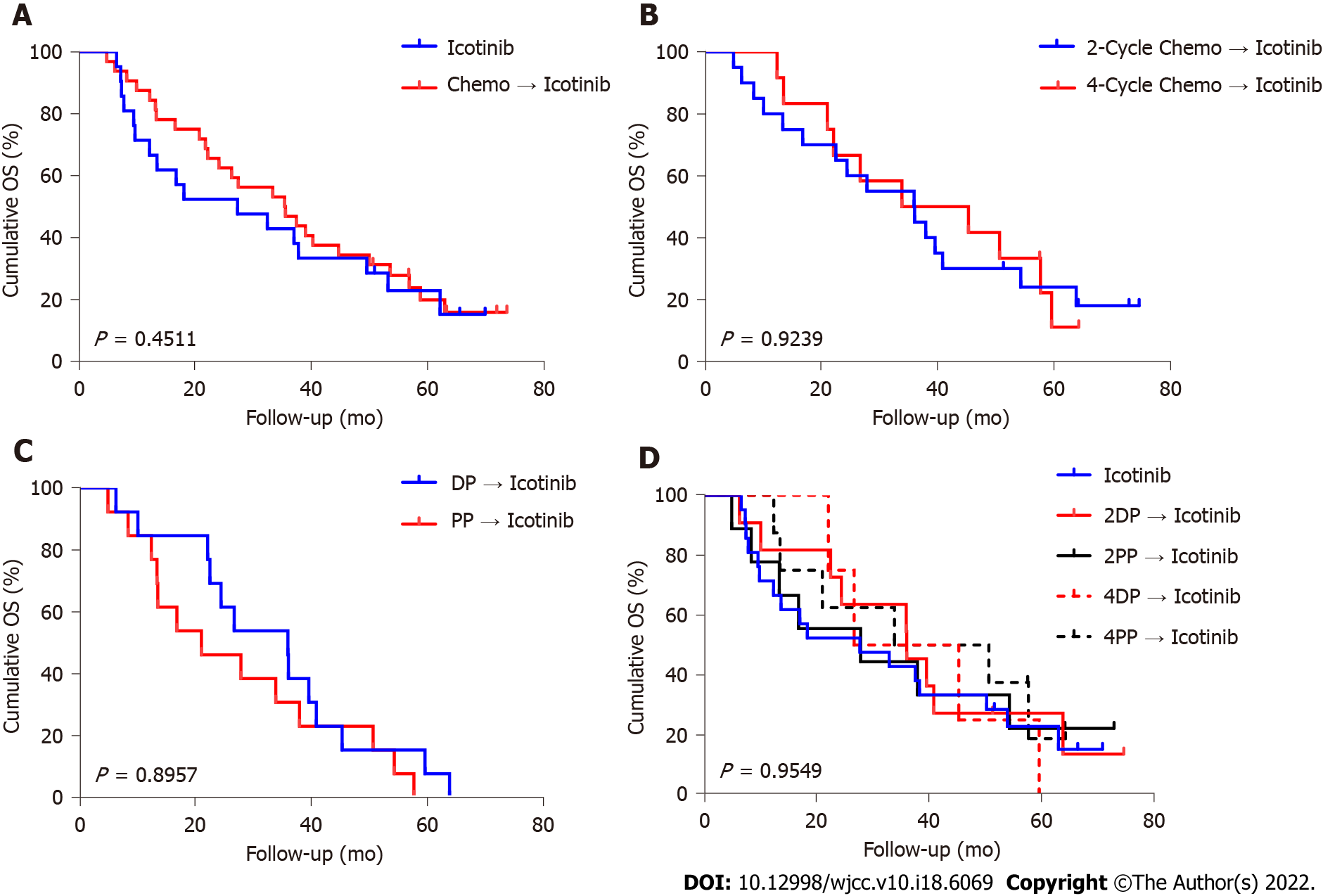
Figure 3 Overall survival of patients.
A: Overall survival (OS) with icotinib vs chemotherapy followed by icotinib; B: OS with two-cycle chemotherapy followed by icotinib vs four-cycle chemotherapy followed by icotinib; C: OS with docetaxel/cisplatin followed by icotinib vs PP followed by icotinib; D: OS with icotinib vs various chemotherapy regimens followed by icotinib. DP: Docetaxel/cisplatin; PP: Pemetrexed/cisplatin.
- Citation: Sun SJ, Han JD, Liu W, Wu ZY, Zhao X, Yan X, Jiao SC, Fang J. Sequential chemotherapy and icotinib as first-line treatment for advanced epidermal growth factor receptor-mutated non-small cell lung cancer. World J Clin Cases 2022; 10(18): 6069-6081
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6069.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6069