Copyright
©The Author(s) 2022.
World J Clin Cases. May 6, 2022; 10(13): 4084-4096
Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4084
Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4084
Figure 1 Baseline plasma CD40 ligand level of control subjects and colorectal cancer patients.
Crude P value: P = 0.2946; the red dot and thick line represent mean and median values, respectively. CRC: Colorectal cancer.
Figure 2 Baseline plasma CD40 ligand, interleukin-6, and thrombopoietin level and platelet count of study participants (mean ± SD).
A: Baseline plasma CD40 ligand; B: Platelet count; C: Interleukin-6 level; D: Thrombopoietin level. CD40 ligand level (Kruskal-Wallis test: P = 0.0275) and platelet count (Kruskal-Wallis test: P = 0.0004) was the highest in Stage IV colorectal cancer (CRC) patients. Interleukin-6 (Kruskal-Wallis test: P < 0.0001) and thrombopoietin (Kruskal-Wallis test: P = 0.0002) levels of the CRC patient groups, except those in Stage II in the latter, were significantly higher than those of healthy control subjects. The red dot and thick line represent mean and median values, respectively. AJCC: American Joint Committee on Cancer.
Figure 3 Change in plasma CD40 ligand level and platelet count with the course of colorectal cancer within those patients who had all three laboratory measurements (mean ± SD).
A: CD40 ligand level; B: Platelet count. n = 30. For the platelet count, a significant decrease could be observed (P < 0.0001), while the CD40 ligand level of patients did not change with the course of the disease (P = 0.6813). The red dot and thick line represent mean and median values, respectively. CRC: Colorectal cancer.
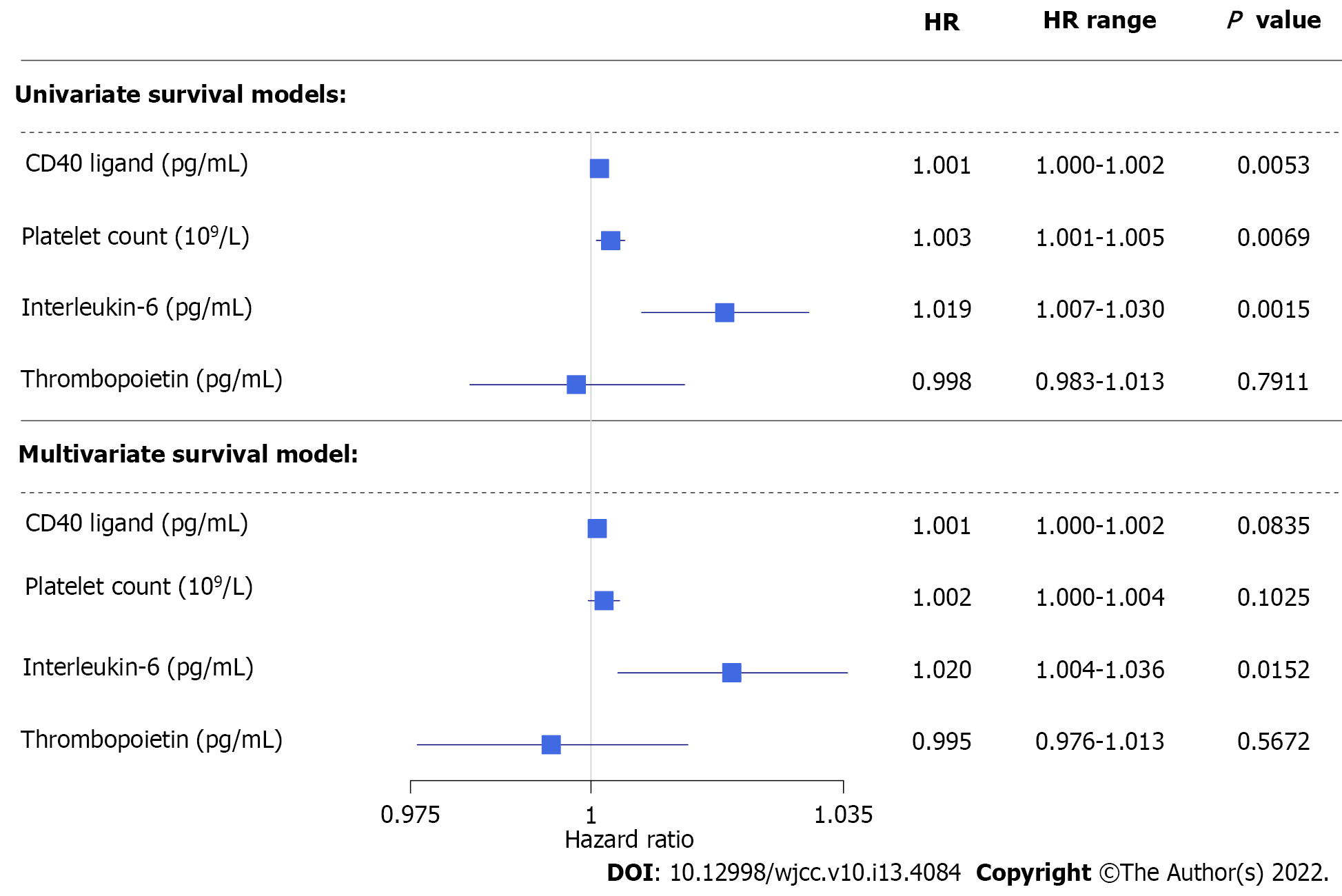
Figure 4 Results of univariate and multivariate analysis on disease-specific survival of colorectal cancer patients concerning preoperative laboratory results.
HR: Hazard ratio.
Figure 5 Results of univariate and multivariate analysis on disease-specific survival of colorectal cancer patients concerning postoperative laboratory results.
HR: Hazard ratio.
- Citation: Herold Z, Herold M, Herczeg G, Fodor A, Szasz AM, Dank M, Somogyi A. High plasma CD40 ligand level is associated with more advanced stages and worse prognosis in colorectal cancer. World J Clin Cases 2022; 10(13): 4084-4096
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4084.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4084