NUTRITION IMBALANCE IN CHRONIC KIDNEY DISEASE
Nutritional imbalance is prevalent in children with chronic kidney disease (CKD) and may influence clinical outcomes. Wasting, defined as low weight proportion to height, is the consequence of inadequate nutrition intake, and highly prevalent in children with CKD. The term cachexia or wasting syndrome has been defined as the pathological combination of a dramatic decrease in appetite and increase in the metabolism of fat and lean body mass[1]. The International Society of Renal Nutrition and Metabolism expert panel defined the term protein energy wasting (PEW) as a state of decreased body stores of protein and energy fuels (body protein and fat masses)[2]. PEW/cachexia, a complex condition of metabolic and nutritional derangement, has been associated with not only malnutrition, but also maladaptive responses, such as anorexia, increased metabolic rate, decreased protein store, reduced body weight and muscle mass. PEW/cachexia cannot be reversed nutritionally. In contrast to PEW/cachexia, malnutrition is the consequence of insufficiency of energy intake and is accompanied by adaptive responses, including hunger, a protective decrease in energy expenditure, preferential use of fat stores for energy and preservation of lean body mass. Nutrition supplementation cannot reverse nutritional deficiency in malnourished patients. Thus, PEW/cachexia and malnutrition are not identical. In addition to PEW/cachexia, two common features of nutritional imbalance in children with CKD are obesity and growth failure[3-7]. This review focuses on these nutrition disorders and the pathophysiological role of chronic inflammation in children with CKD.
PEW and cachexia in CKD
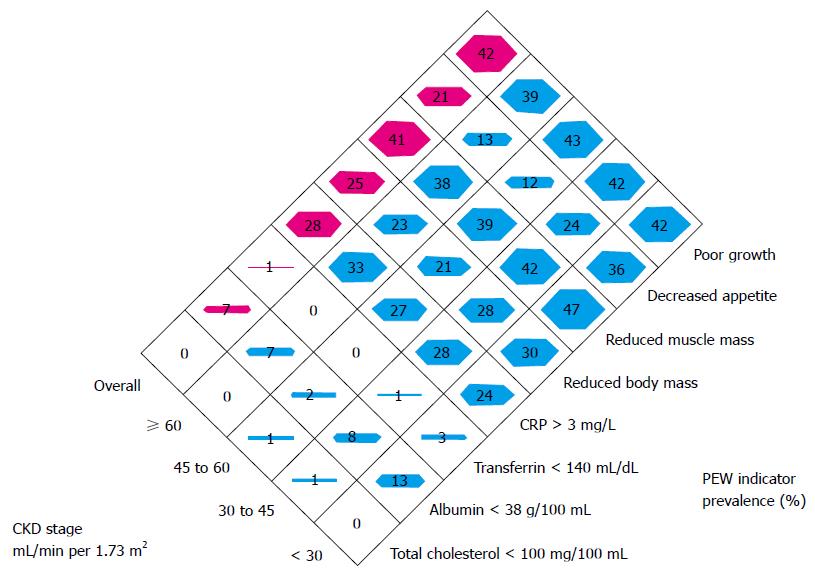
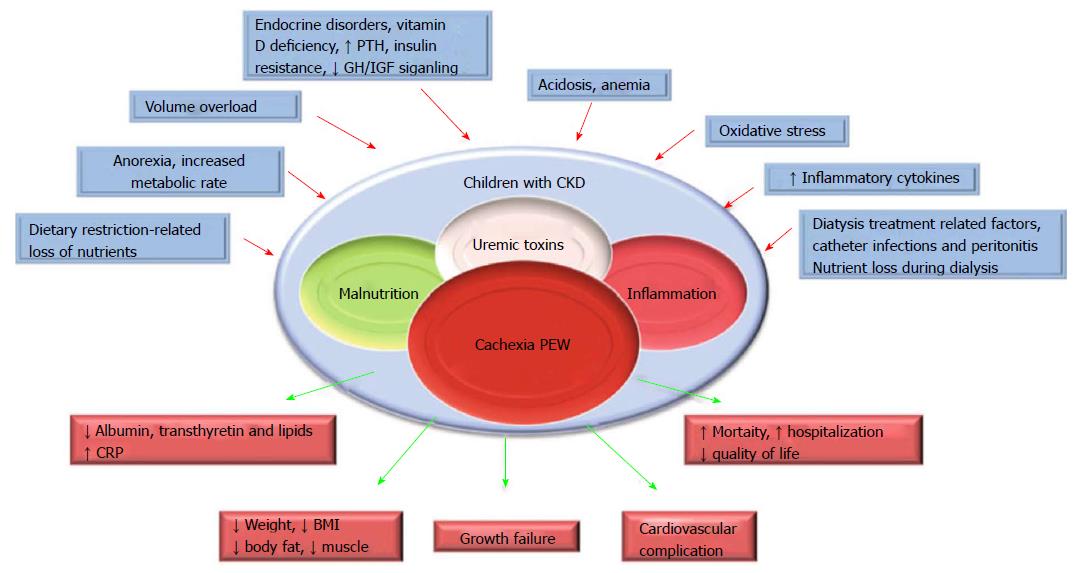
PEW and cachexia is highly prevalent in CKD patients. PEW was evident in 20% to 75% adult dialysis patients. Studies of CKD in children (CKiD), a multi-center prospective cohort study of children aged 1 to 16 in United States, revealed the prevalence of PEW estimates ranged from 6% to 65%. The wide range of prevalence of PEW in this cohort is likely due to difference in diagnostic criteria[5]. To better define PEW in children with CKD, we evaluated prevalence of PEW using different diagnostic criteria. The incidence of PEW in CKiD ranged from 7% to 20% by applying 3 different diagnostic criteria, namely, a minimal, a standard and a modified PEW definition (Figure 1). Our results suggested that only the modified PEW diagnostic criteria, which included growth retardation as a criterion, showed modest significance. Our modified PEW diagnostic criteria for children with CKD is defined as the standard ≥ 3 of the 4 criteria as described in adults PEW (biochemical parameters, body and muscle mass assessments and anorexia) with the additional incorporation of growth retardation as a diagnostic criteria. The etiology of CKD-associated PEW is complex. Common risk factors for PEW in CKD, such as poor nutrition, systemic inflammation, endocrine disorder, comorbid condition, fluid overload and metabolic acidosis have been listed (Figure 2)[1,3,5]. Of the many complications of CKD-associated PEW/cachexia, CKD patients are prone to muscle weakness and as a result, have difficulties performing their daily routine of activities. Other systemic consequences of PEW in children with CKD comprise increased risk of cardiovascular disease, infection, depression, prolonged hospitalization and mortality, and growth retardation[3,5]. The incidence rates of hospitalization were almost 2-fold higher for CKD children with PEW[3]. Mortality rate in patients with CKD is 100-200 times higher than the general population[8] and represents a major burden to health systems. Importantly, high mortality in patients with CKD has been associated with components of risk factor of PEW/cachexia as listed in Figure 2.
Figure 1 The prevalence of indicators of protein-energy wasting used to form the three definitions.
PEW: Protein energy wasting; CKD: Chronic kidney disease; CRP: C-reactive protein.
Figure 2 Schematic representation of the causes and manifestations of the protein-energy wasting syndrome in chronic kidney disease.
CKD: Chronic kidney disease; GH: Growth hormone; IGF: Insulin-like growth factor; PTH: Parathyroid hormone; BMI: Body mass index; PEW: Protein-energy wasting; CRP: C-reactive protein.
Obesity
Anorexia is prevalent and has contributed to the nutritional imbalance and growth failure in children with CKD[5]. Ironically, another nutritional disorder - over-nutrition and obesity, is also prevalent in children with CKD[9,10]. Prevalence of overweight or obesity (34%) exceeds the prevalence of PEW in CKiD cohort. Prevalence of overweight or obesity in children with glomerular and non-glomerular CKD was 46% and 32%, respectively[11]. In a large cohort of European pediatric renal replacement therapy (RRT) population, the prevalence of overweight and obesity far exceeded the prevalence of underweight (20.8%, 12.5% vs 3.5%, respectively)[12]. There was a significant increase in body mass index (BMI) after the initiation of RRT in this study cohort. Short stature and glucocorticoid treatment were further associated with an increased risk of overweight and obesity in this transplanted population. Other risk factors strongly associated with increased BMI in patients with RRT were lower initial BMI and higher age at the initiation of RRT, longer duration of dialysis as well as a longer time with a functioning graft[12].
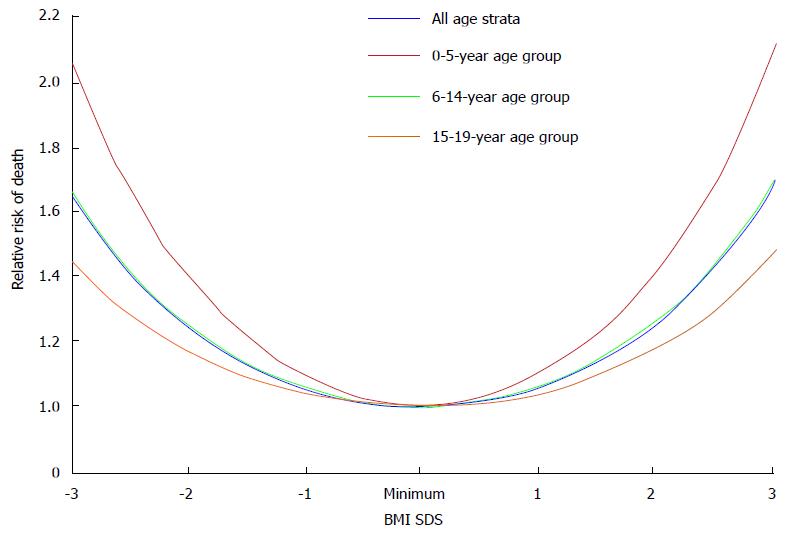
The obesity paradox or reverse epidemiology is a controversial hypothesis[13]. It proposes that obesity may, contrary to conventional wisdom, be related to decreased morbidity and mortality in some populations. This hypothesis has been reported in patients with heart failure, myocardial infarction, and acute coronary syndrome[13,14]. Nevertheless, it was not consistently supported by data in end-stage renal disease (ESRD) patients. Indeed, initial analysis of epidemiologic studies have shown a strong survival advantage of obesity in dialysis patients with the primary outcomes of all-cause and cardiovascular mortality[14]; and low BMI values are associated with increased mortality rate. However, there is a fundamental flaw in the study design as those investigators compared short-term mortality rate in dialysis patients vs long-term mortality rate in the general population. No evidence of reverse epidemiology of BMI and survival advantage was found in dialysis patients when both patients and general population were analyzed with the same time frame for outcomes, even with multivariable adjustments for age and race[15]. More recently, association of BMI values with all-cause of mortality rate and disease progression was analyzed in a large cohort of adult predialysis CKD patients. BMI showed a U-shaped relationship with clinical outcomes, with the best outcomes observed in overweight and mildly obese patients[16]. Similar findings were observed in children with ESRD, the showing of a U-shaped relationship between BMI values and the risk of all-cause mortality rate (Figure 3). Higher mortality rate was observed in obese children relative to non-obese children with CKD after renal transplantation (27% vs 17%, respectively)[17]. Furthermore, childhood and adolescent obesity have negative impacts on the cardiovascular health. Obesity in adolescence was positively associated with death rate in future decades[18]. Obesity per se is a strong and independent risk factor for the progression of CKD. Obesity hastens the deterioration of renal function among patients with IgA nephropathy and unilateral renal agenesis[19-21]. In another study, progression of CKD is increased by 1.23 fold for each standard deviation increment of BMI values[22].
Figure 3 The relative risk of death and confidence intervals for body mass index standard deviation score among children with stage 5 chronic kidney disease.
BMI: Body mass index; SDS: Standard deviation score.
Growth failure
Poor nutrition contributes to the high prevalence of growth retardation in children with CKD but growth retardation may still persist despite improvement of nutritional status in this population. Recent data from International Pediatric Peritoneal Dialysis Network registry suggested that enteral feeding by nasogastric or gastrostomy tube improved nutritional status, as indicated by an increment of BMI values in pediatric patients with stage 5 CKD. Nevertheless, nutritional supplementation did not attenuate growth failure in this population[23]. Growth failure has been associated with poor clinical outcomes of increased morbidity and mortality rate in children with CKD. About one third of children enrolled in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry in 2005 had severe short status[24]. Similar findings were observed in a recent report from the same registry in 2011[25]. Prevalence of growth retardation was 29.3% for children enrolled in the Serbian Pediatric Registry of CKD[26].
The etiology of poor growth in CKD is multifactorial and can be associated with poor nutritional status as well as other comorbidities such as metabolic acidosis, anemia, bone and mineral disorders, genetic factors and perturbations in growth hormone (GH) and insulin-like growth factor (IGF)-I axis signaling pathways. Data from NAPRTCS showed that the greatest height deficits were observed in youngest CKD patients prior to entering RRT programs. After renal transplantation, the greatest height improvement was observed for those youngest patients with the greatest height deficits prior to their RRT[9]. Growth retardation is associated with poor clinical outcomes in children with ESRD. Five year mortality rate for children on hemodialysis with severe growth failure, moderate growth failure and normal growth was 16.2%, 11.5% and 5.6%, respectively. Moreover, higher hospitalization rate was observed in ESRD children with severe and moderate growth failure relative to those with normal growth[27].
IMPACT OF INFLAMMATION ON NUTRITIONAL DISORDER IN CHILDREN WITH CKD
Levels of serum inflammatory markers such as C-reactive protein (CRP), IL-6 and TNF-α were elevated in CKD patients[28]. The etiology of CKD-associated inflammation is multifactorial. Important factors include decreased glomerular filtration rate, underlying disorders and other complications of CKD[29].
PEW/cachexia
Chronic inflammation is important for the pathogenesis of PEW/cachexia in patients with CKD through various mechanisms including leptin and melanocortin signaling modulation, inflammatory cytokines and nuclear factor kappa B (NFκB) signaling.
Aberrant leptin/melanocortin signaling and PEW/cachexia in CKD
Leptin is an anorexigenic hormone. Leptin is mainly secreted by adipose tissues and modulates energy homeostasis through melanocortin signaling. Leptin signaling in the hypothalamus nuclei is enabled by inhibiting neuropeptide Y (NPY) and agouti related peptide (AgRP) neurons and by stimulating pro-opiomelanocortin neurons, which in turn activates the release of α-melanocyte-stimulating hormone and stimulates the type 4 melanocortin receptor signaling (MC4R)[30,31]. Transgenic mice over-expressing leptin had reduced energy consumption relative to controls[32]. Leptin is degraded from the circulation in the renal tubules. Serum levels of leptin were elevated in CKD patients with the decline in renal glomerular filtration function[33,34]. We have shown that leptin/melanocortin signaling is an important mechanism underlying CKD-associated cachexia. Transgenic mice with deletion of leptin receptor (db/db) and MC4-R knockout attenuated aberrant metabolic effects of CKD-associated cachexia[35]. Administration of AgRP, a natural MC4R antagonist, normalized food intake, total weight gain, improved lean mass content as well as basal metabolic rate in CKD mice relative to control mice[36]. We also evaluated the effects of leptin receptor antagonism in CKD mice. Administration of pegylated leptin receptor antagonist (PLA) attenuated food intake, weight gain, improved lean mass and in vivo muscle function as well as normalized basal metabolic rate in mice with CKD. In addition, the administration of PLA significantly decreased expression of uncoupling proteins and corrected aberrant muscle mass signaling pathway as well as normalized muscle protein levels of IL-1α, IL-1β, IL-6, and TNF-α in CKD mice[37]. Thus, inhibition of the leptin/melanocortin signal pathway may represent a novel therapeutic approach for CKD-associated cachexia. Elevated serum levels of leptin were associated with higher prevalence of PEW/cachexia in patients. Malnourished patients had higher serum levels of leptin than those without malnutrition[38]. Increases in serum leptin levels have been associated with inflammation and a decrease in lean mass content in dialysis patients[39].
Pro-inflammatory cytokine and PEW/cachexia in CKD
Increased levels of serum inflammatory cytokines were associated with poor clinical outcomes in patients with CKD[40]. Loss of kidney function, uremia and dialysis treatment per se are important causes of inflammation in this population. In addition, gene polymorphisms of inflammatory cytokines have been implicated in CKD patients[41]. Polymorphisms of TNF-α gene predisposed malnutrition and inflammation in patients with ESRD[42]. Robust evidence supports a direct pathologic role of IL-1α, IL-6, and TNF-α in the development of PEW. Muscle wasting is a cardinal feature of CKD. Elevation of pro-inflammatory cytokines stimulates muscle catabolism. In animal models of CKD, IL-1, IL-6 and TNF-α stimulate inflammation in animal models of CKD. Increased serum levels of IL-6 correlated with increased muscle catabolism while the antagonist of IL-6 receptor attenuated CKD-associated muscle wasting[43].
PI3K-Akt signal transduction pathway mediates muscle metabolism in response to various extracellular signals. Aberrant PI3K/Akt pathway has been implicated in the etiology of muscle wasting. In skeletal muscle, Akt signaling mediates muscle fast/glycolytic fiber metabolism and muscle atrophy in CKD is associated with reduced Akt signaling in skeletal muscle tissue. In a mouse model of CKD, reduced Akt signaling was associated with skeletal muscle wasting. In contrast, skeletal muscle-specific Akt1 transgenic mice promoted skeletal muscle growth[44]. Akt1 transgenic mice attenuated renal fibrosis, apoptosis, and inflammation in unilateral ureteral obstruction-induced CKD mice. Importantly, maintenance of muscle mass is associated with favorable clinical outcomes while muscle wasting is related to deterioration of renal function in patients with CKD[45].
Pro-inflammatory cytokines signal through the central nervous system and induce anorexia[46]. A meta-analysis of 22 studies with 924 participants (anorexia nervosa = 512, health controls = 412) has shown that compared to controls, the serum level of TNF-α, IL-1β, IL-6 and TNF-receptor-II were elevated in anorexia nervosa[47]. An animal study demonstrated that anorectic effects were observed following acute administration of exogenous TNF-α and IL-1β to mice[48]. Cytokines regulates energy expenditure. Infusion of IL-1 increased resting energy expenditure in rats and administration of recombinant TNF-α increased energy expenditures in patients with disseminated cancer[49,50].
NFκB pathway and PEW/cachexia in CKD
Activation of intracellular NFκB system has been correlated with PEW/cachexia in CKD[51]. Several recent articles provide comprehensive reviews for the NFκB family of transcription factors and its regulation[52]. Cytokines induce muscle wasting via activation of NFκB while blockade of NFκB signaling attenuates muscle atrophy. Denervation-induced muscle atrophy was significantly improved in muscle specific IKK knockout mice[53]. What are the underlying mechanisms by which activation of NFκB induce significant muscle atrophy? First, ubiquitin-proteasome system (UPS) promoted muscle protein degradation and activation of NFκB stimulated expression of protein levels of several components of UPS. Second, NFκB increased the expression of several NFκB-regulated molecules, especially pro-inflammatory cytokines. This positive feedback loop resulted in the over-stimulation of NFκB and the subsequent muscle atrophy. Third, NFκB suppressed myogenic differentiation likely through the activation of transcription factor YY1[54]. And fourth, NFκB may suppress energy intake likely through the suppression of NPY. Phenylpropanolamin (PPA), a synthetic sympathomimetic amine, suppressed food intake likely via the signaling of hypothalamic NPY. Cerebral NFκB knockdown attenuated the anorexic effects in PPA-treated rats by decreasing the expression of NPY and antioxidants[55].
Obesity
Adipose tissue is an important energy reservoir and an active metabolic organ secreting numerous hormones. The adipokines are cell signaling proteins secreted by adipose tissue, including leptin, adiponectin, IL-6, TNF-α, and monocyte chemotactic protein-1[56]. Adipose tissue is an important source of inflammation in CKD patients. Adipokines mediate inflammation and accelerate the progression of vascular disease in patients with CKD[57]. Chronic inflammation may accelerate the progression of renal dysfunction in CKD patients. Elevated expression of adipokines was associated with increased numbers of infiltrated immunocompetent cells in adipose tissue in obese CKD patients[58]. Elevated serum inflammatory markers such as IL-6, TNF-α and CRP are correlated with thickness of carotid intima media and associated with high mortality rate in CKD patients. Increased expression of inflammatory cytokines in adipose tissue may accelerate atherosclerosis and induce deterioration of renal function in obese CKD patients[9,59].
Growth failure
Perturbation in the GH/IGF-I axis is an important cause of growth failure in CKD children. GH/IGF-I mediated postnatal growth, body composition and renal function. GH binds to its receptor (GHR) and subsequently regulates the expression of GH-regulated genes, including the IGF-I gene. GH insensitivity is commonly observed in growth retarded CKD children, as serum levels of GH were normal or even elevated in this population. Pharmacological or endogenous GH treatments have diminished growth-promoting effects in children with CKD. CKD caused a post-receptor defect in GH pathway via the JAK/STAT signaling which in turn, resulted in reduced expression of IGF-I[60]. GH induces the expression of suppressors of cytokine signaling (SOCS) via the JAK-STAT signaling pathway. SOCS proteins, in turn, inactivates GHR/JAK2 complex, thus establishing a feedback loop for GH activity. CKD-induced GH insensitivity was mediated by activation of GH-JAK2 via STAT transduction and the overexpression of SOCS proteins[61].
IGF-I stimulates longitudinal growth at the growth plate. Circulating IGF-I complex constitutes of IGF-I, IGF binding protein (IGFBP) and acid labile subunit. Decline in renal function in CKD patients is associated with elevated serum IGFBP1 levels and the concomitant diminished IGF-I bioactivity. Increased IGFBPs levels have been associated with decreased longitudinal growth in CKD children. A recent study further exploited the underlying mechanism of CKD-induced GH insensitivity. In CKD rats with acute inflammation, endotoxin aggregates GH resistance and reduced IGF-I gene expression, and this effect is related to the increased production of pro-inflammatory cytokines[62,63].
IMPACT OF MATERNAL NUTRITION
Maternal malnutrition negatively influences the fetal and early life development. This critical period of pre- and early postnatal development exerts long-term effects on body weight and growth. An inadequate or excess maternal nutritional environment may activate multiple fetal responses which persist postnatally and have been correlated with the development of chronic diseases, including CKD and nutritional disorders[64]. Low birth weight (LBW) was associated with impaired renal reserve (a reduction in the number of nephrons) and structure per se (smaller renal size)[65-67]. Results from animal studies strongly support the notion that maternal malnutrition caused intrauterine growth retardation and a nephron deficit[66]. LBW was correlated with increased prevalence of early-onset CKD. The odds ratio for ESRD was 1.4 in adults who were born underweight[68]. LBW was correlated with deterioration of renal function in CKD patients[69]. Low nephron numbers was a risk factor for hypertension, likely due to the effect of compensatory hypertrophy in the setting of a low nephron number.
Intrauterine and early-life environment substantially impact the development of obesity in childhood and in adulthood. Animal and human studies suggested that an adverse in utero environment such as intrauterine growth restriction (IUGR) was closely associated with postnatal development of obesity. Studies also showed that IUGR fetuses exhibited increased body fat accumulation, reduced serum levels of leptin and aberrant epigenomic properties, which subsequently promoted obesity in adult life. Food restriction during rat pregnancy produced hypoglycemic IUGR pups. Subsequently, for those IUGR pups permitted rapid catch-up growth, they exhibited aberrant metabolic responses including hypertriglyceridemia and adult obesity with insulin-resistance. The concept of developmental origins of health and disease has been generally recognized. Infants born to obese, overweight, and diabetic mothers as well as infants born to malnourished mothers are associated with a higher risk of chronic illnesses in adult life. High birth weight enhances the risk of developing obesity and CKD in adult life[64]. On the other hand, LBW accompanied by an accelerated catch-up growth has also correlated with an increased risk of obesity and CKD in adulthood. In an observational study, LBW and small gestational age in infants were associated with poor growth outcomes in children with mild to moderate CKD[70].
CONCLUSION
Nutritional disorders, including PEW, cachexia, obesity and growth failure, have major impacts on clinical outcomes in children with CKD. Chronic inflammation is important for the pathogenesis of nutritional disorders in CKD. Increased awareness of nutritional status is needed for CKD children. Further research into the pathophysiology may yield novel therapies for CKD-associated nutritional disorders.