INTRODUCTION
In 2002, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) instituted new guidelines that established a novel chronic kidney disease (CKD) staging paradigm[1,2]. In this model, by use of pre-specified estimated glomerular filtration rates (eGFR) ranges, CKD was characterized into five stages: I, II III IV and V[1,2]. More recent updates of the NKF KDOQI guidelines have incorporated the degree of associated proteinuria to plausibly enhance the utility, applicability, validity and clinical relevance of this CKD staging paradigm[3]. Furthermore, it must be acknowledged that the entire paradigm of the NKF K/DOQI 2002 CKD staging and the design of various CKD risk stratification scoring models and published CKD progression prediction formulas are all firmly predicated on the validity of this albeit unproven and only assumed archetype of a predictable linear and time-dependent progression of CKD through stages I, II, III, IV and V, and finally to inexorably end in end stage renal disease (ESRD) and the need for renal replacement therapy[1-3]. Thus, note worthily, this NKF KDOQI CKD staging archetype assumes that CKD patients follow this linear, predictable, smoothly progressive and time-dependent curve to advance through the increasing CKD stages before inexorably reaching ESRD and the need for renal replacement therapy (RRT). Indeed, current nephrology literature on ESRD outcomes in CKD patients, is unquestionably primarily and almost entirely predicated on this presumed theory of a smooth, continuous, predictable and time-dependent linear progressive loss of eGFR[4-8]. This presumption is so strongly held that several reports over the decades have described CKD progression to ESRD in terms of annual rates of GFR decline or as mean annualized eGFR slopes in mL/min per 1.73 m2 every year[4,6,7,9-18]. A detailed investigative analysis of these reports has been recently published in a recent review[19]. Undeniably, this generally accepted consensus classic view of CKD-ESRD progression presumes that functional nephron loss in chronic progressive kidney disease, and therefore as measured by serum creatinine, is orderly and mathematically definable[9].
However, it must be acknowledged that such a premise of predictable linear time-dependent progressive step-wise decline in kidney function, with mathematically linear falling eGFR over time, and with eGFR methodically marching through these incremental projected CKD stages I through V and then inexorably ending in symptomatic ESRD and the need for renal replacement therapy is unproven, untested and potentially flawed[20-32]. Contrary to the classic premise of a smooth predictable linear decline of renal function from CKD to ESRD described in the preceding section of this chapter, more and more data have over the years suggested that the natural pattern of progression from CKD to ESRD followed a more staccato and unpredictable course[20-32]. Moreover, and again contrary to the NKF schema of a predictable linear CKD staging referred to above, the results of new analysis of CKD databases suggest a high degree of variability of CKD staging among CKD patients, and this CKD staging variability is often unpredictable, and with significant intra-patient variability, such that our current knowledge of this very important patient group can, at best, be described as limited and incomplete[19,33-36].
A recent publication in the Kidney International journal put CKD smack back into the realms of public discourse when it was reported that 59% of Americans had a lifetime risk of developing CKD and that Americans had a 1 in 3 chance of CKD risk[37]. Whereas, it has been known for decades that eGFR declines in parallel with age[37,38], to what extent this is true and linearly predictable at the individual (older) CKD level remains unclear[19,33-36]. To further buttress the latter sentiment and concerns regarding the validity of current CKD staging paradigms, in August 2012, the authoritative United States Preventative Task Force had in a very comprehensive analysis acknowledged that we know surprisingly little about whether screening adults with no signs or symptoms of CKD improve health outcomes and that we deserve better information on CKD[39].
HOW MUCH DO PHYSICIANS AND OTHER HEALTHCARE PROVIDERS EMBRACE THE NKF K/DOQI 2002 CKD GUIDELINES?
Without a doubt, the introduction of the K/DOQI 2002 CKD guidelines has heralded a growing appreciation of the impact of CKD in health outcomes[3,40]. Undoubtedly, there has followed an exponential increase in nephrology specialty referrals and nephrology specialty office visits for purported “CKD follow up” since the introduction of the 2002 NKF KDOQI CKD clinical practice guidelines[41-49]. However, given the above limitations and constraints of the K/DOQI CKD clinical practice guidelines model, one interesting question is to what extent physicians and providers have embraced its recommendations and structure[50]. An online survey, developed by the NKF KDOQI Education Committee, was sent to 16323 healthcare providers of which 951 completed the survey[50]. Whereas 78% of all providers reported using the NKF KDOQI guidelines in their practice, the most common barrier perceived by physicians was lack of evidence supporting the KDOQI guidelines[50]. Notably, compared to physician extenders and allied health professionals, not surprising, we must admit, a larger proportion of nephrologists cited lack of evidence and too much influence from industry as barriers to the implementation of the KDOQI guidelines[50]. Surely and undoubtedly, and this fact was recognized very much earlier on, the validity of the clinical practice guidelines of the NKF K/DOQI 2002 report is limited in some areas because of the paucity of evidence-based data[40].
UTILITY OF CKD PREDICTION MODELS IN THE NEPHROLOGY LITERATURE
Tangri et al[51] in a recent 2011 publication devised and validated different new CKD prediction models using demographic, clinical, and laboratory data from two independent Canadian cohorts of patients with CKD stages III to V (estimated GFR, 10-59 mL/min per 1.73 m2) who were referred to nephrologists between April 1, 2001, and December 31, 2008[51]. The most accurate model included age, sex, estimated GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin (C statistic, 0.917; 95%CI: 0.901-0.933 in the development cohort and 0.841; 95%CI: 0.825-0.857 in the validation cohort)[51]. In the validation cohort, this model was more accurate than a simpler model that included age, sex, estimated GFR, and albuminuria (integrated discrimination improvement, 3.2%; 95%CI: 2.4%-4.2%; calibration (Nam and D’Agostino 2 statistic, 19 vs 32); and reclassification for CKD stage 3 (NRI, 8.0%; 95%CI: 2.1%-13.9%) and for CKD stage 4 (NRI, 4.1%; 95%CI: -0.5%-8.8%)[51]. The conclusion was that a model using routinely obtained laboratory tests can accurately predict progression to kidney failure in patients with CKD stages III to V[51].
In a subsequent 2013 review of CKD prediction models, 13 studies describing 23 models were analyzed[52]. Eight studies (11 models) involved kidney failure, five studies (6 models) involved all-cause mortality, and three studies (6 models) involved cardiovascular events[51-64]. Measures of eGFR or serum creatinine level were included in 10 studies (17 models), and measures of proteinuria were included in 9 studies (15 models)[51-64]. Only 2 studies (4 models) met the criteria for clinical usefulness, of which 1 study (3 models) presented reclassification indices with clinically useful risk categories[52]. A 2013 VA study of 1866 participants aged 65 and older with CKD with an eGFR less than 30 mL/min per 1.73 m2 body surface area (BSA), concluded that a new CKD prediction model using commonly available clinical measures (age, congestive heart failure, systolic blood pressure, eGFR, potassium, and albumin) showed excellent ability to predict the onset of ESRD within the next year in elderly adults[65]. Peeters et al in an analysis of 595 Canadian CKD patients in stages III-V CKD, developed an eight-variable model [including age, sex, eGFR, albuminuria, calcium, phosphate, bicarbonate, albumin] and the conclusion was that this more complex model also accurately predicted the progression to kidney failure[66]. Despite these foregoing claims and assertions regarding the utility of CKD prediction models in patient care and in individual patient CKD prognostication, from direct clinical experience, and after a critical unbiased review of currently available CKD progression prediction models, and related CKD literature, we have concluded that all currently published CKD progression prediction models remain untested, not validated, and of limited use for individual CKD patient prognostication, at any given pre-specified point in time[19-36].
LIFETIME CKD RISK MODELING AMONG THE US POPULATION–HOW ACCURATE AND RELIABLE CAN SUCH POPULATION WIDE ESTIMATES BE?
Grams et al[37], in a Markov Monte Carlo model simulation study of current United States black and white populations had concluded that from birth, the overall lifetime risks of CKD stages 3a+, stage 3b+, stage 4+, and ESRD were 59.1%, 33.6%, 11.5%, and 3.6%, respectively[37]. According to this very publicized report, the risk of CKD increased with age, with approximately one-half the CKD stage 3a cases developing after 70 years of age[37]. However, to what extent this is true and linearly predictable at the individual (older) CKD level remains unclear[67]. The clinical relevance, the validity and real life implications of these estimates remain to be more appropriately elucidated[29-32]. Moreover, as is evident from the analysis in the next paragraph, such population-based disease risk estimates can often be shown to be very significantly off-mark, more so when validated against actual reported ESRD incidence values as available in the United States Renal Data System (USRDS).
OVERESTIMATION OF ESRD INCIDENCE RATES AMONG US CKD COHORTS-REPORT OF A COMPARATIVE ANALYSIS
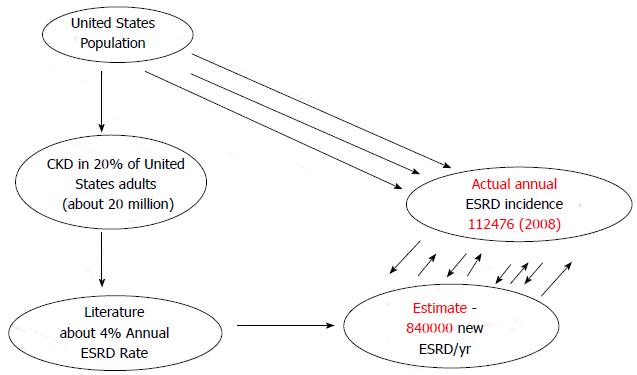
A 2007 United States of America Centers for Disease Control report revealed that 16.5% of the United States. population 20 years of age and older had CKD as defined by eGFR < 60 mL/min per 1.73 m2 BSA[68]. This CKD prevalence estimate, at the time of this report, translated that > 20 million adult Americans had CKD[68]. Besides, three United States studies published between 2004 and 2009, among different CKD patient population cohorts, had demonstrated annualized ESRD rates among CKD patients of 4%, 8.2% and 4.5%, respectively[29,69,70]. A meta-analysis of these three studies produced a weighted average annualized ESRD incidence rate of 4.2%[30-32]. This weighted average annualized ESRD incidence rate of 4.2% among the entire United States CKD population from 2007 would produce an estimated 840000 new ESRD cases/year for 2008[30-32]. This represented a gross over-estimation when compared to the actual reported USRDS incident ESRD for 2008 of 112476[30-32,71] (Figure 1). Thus, the estimated ESRD incidence for 2008 of 840000 was a whopping 650% gross overestimate over the actual recorded number of new ESRD patients in the United States for 2008 of 112476[30-32,71].
Figure 1 Gross overestimation of annual incident end stage renal disease patients for 2008 from projected estimates based on annualized end stage renal disease rates from three cited concurrent United States chronic kidney disease cohort studies.
CKD: Chronic kidney disease; ESRD: End stage renal disease.
EVIDENCE OF CKD STAGE VARIABILITY AND UNPREDICTABILITY AMONG STUDIED CKD COHORTS: THE PROBABLE IMPACT OF THE EFFECTS OF THE SYNDROME OF LATE ONSET RENAL FAILURE FROM ANGIOTENSIN BLOCKADE
In a 2011 retrospective analysis, Sikaneta et al [35], analyzed the longitudinal changes during a 1.1-year observation period of eGFR and CKD stages among 1262 patients, mean age 71.25 years, drawn from two large Canadian renal clinics. This study demonstrated CKD stage variability (defined by changes in CKD stages) and reported that CKD stage changed in 40% of the cohort (including 7.4% in whom CKD stage improved) whereas CKD stage remained static in 762 (60.4%) patients, the majority of this CKD cohort[35]. Furthermore, although CKD stage had remained static in 762 (60.4%) patients, that is to say, they did not experience a change in CKD stage during the initial observation period, 204 (40%) of 512 patients from this subgroup who were available for follow-up 2.3 years later still ended up on dialysis, suggesting subsequent acute unanticipated yet irreversible ESRD, a picture that is consistent with the newly described syndrome of rapid onset end stage renal disease which syndrome is expatiated in greater detail in a following section of this review[72]. This Canadian study demonstrated the clear unpredictability of CKD staging and prognostication at the individual patient level[35,36]. Moreover, the observation that 7.4% of this Canadian CKD cohort actually demonstrated improved CKD stage is still more dramatic[35,36]. In an editorial correspondence, we had speculated that some of these CKD patients who revealed improved CKD stages during follow up may indeed represent some CKD patients who had experienced acute worsening kidney function while on angiotensin blockade and who following withdrawal of angiotensin inhibition had exhibited improved kidney function[36]. This would only further strengthen the existence of the previously unrecognized syndrome of late onset renal failure from angiotensin blockade, a syndrome that we first described in 2005[73-77]. In another Canadian study, Levin et al studied 4231 CKD IV patients characterized by an index eGFR of less than 30 mL/min per 1.73 m2, at least 3 subsequent eGFR values available for analysis, and no less than 4 months of follow-up between January 2000 and January 2004[33]. In this CKD cohort, mean age of 67 years, median follow-up of 31 mo, during the first 2 years of follow-up, 24% started dialysis therapy, 1% received a transplant, 7% died, and 1% was lost to follow-up[33]. The conclusion from this study was that the clinical course of patients with CKD stage 4 was unpredictably variable[33].
A JUNE 2011 MAYO CLINIC LABORATORY DATABASE TWO-YEAR SNAP SHOT ANALYSIS OF CKD STAGE CHANGES IN CKD IV PATIENTS
In June 2011, we pulled by IT reporting, all stage IV CKD patients with eGFR in the 15.0-29.9 mL/min per 1.73 m2 BSA range in a Mayo Clinic Laboratory Database reported between April 19, 2009 and April 19, 2011[19,30,32,75]. All patients who had received renal replacement therapy for acute kidney injury (AKI) or ESRD were excluded from the analysis. We included for analysis, all patients with at least three recorded eGFR values, and with a minimum of 6 mo between the first and the last reported eGFR estimations[19,30,32,78]. After excluding 62 ESRD patients, and all who received renal replacement therapy for AKI, 241 patients qualified for this analysis - 102 males and 139 females. In over 95% of the patients, eGFR remained stable and did not vary by as much as 5 eGFR points (< 25% from baseline) over the two-year study period[19,30,32,78]. The conclusion following this snap-shot cross sectional analysis was that eGFR in the majority of CKD stage IV patients remained stable after two years of follow up[19,30,32,78].
EVIDENCE IN THE CKD LITERATURE OF THE EXISTENCE OF CKD“PROGRESSORS” AND CKD “NONPROGEESSORS”
Our experience studying the natural history of CKD at the Mayo Clinic Health System Renal Unit in Northwestern Wisconsin, United States, suggests that some CKD patients are able to maintain stable, albeit diminished eGFR levels over several years, the so-called “non-progressors” or “asymptomatic” CKD patients, whereas, other CKD patients, for often unclear reasons, have an apparent enhanced propensity to progressively lose eGFR over time, the so-called “progressors” or “symptomatic” CKD[31,79].
In a 2012 retrospective report from South Korea, of 347 CKD III patients, enrolled between January 1997 and December 1999, who were followed up through June 2010, a period of 10 years, 167 patients (48.1%) did not progress, 60 (17.3%) progressed to stage 4 and 120 (34.6%) progressed to stage 5, with 91 (26.2%) starting dialysis[80].
Other investigators, as reviewed below, have also documented such disparate behavior of individual patient CKD stages over time, with the additional introduction of the terms, “improvers” and “nonimprovers”, to describe this dichotomy of observed CKD nonprogression vs progression, respectively, with follow up[81-85]. In a 2-year follow-up of the MDRD study, GFR remained stable in 19% of patients and improved in 11%[81]. In the African American Study of Kidney Disease and Hypertension (AASK) trial, however, over a longer follow up period of 8.8 years and with Bayesian models, eGFR improved among only 3.3%, with a mean slope of +1.06 mL/min per 1.73 m2 per year[82]. In another study of individual GFR progression trajectories over 12 years of follow-up in participants in AASK trial, Li et al[83] demonstrated that many patients with CKD have a nonlinear GFR trajectory or a prolonged period of nonprogression. In this study, 352 (41.6%) patients showed a > 0.9 probability of having either a nonlinear trajectory or a prolonged nonprogression period; in 559 (66.1%), the probability was > 0.5. Baseline eGFR > 40 mL/min per 1.73 m2 and urine protein-creatinine ratio < 0.22 g/g were associated with a higher likelihood of a non progression period. 74 patients (8.7%) had both a substantial period of stable or increasing eGFR and a substantial period of rapid eGFR decrease[83]. In another population with mild CKD receiving primary care through a large integrated health care system between 2004 and 2009, eGFR rose over time among 41.3%[84]. In yet another retrospective study of patients before nephrology referral, eGFR did not progress among 16% of those with stages 3-5 of CKD[85].
CKD “IMPROVERS” AND “NONIMPROVERS” IN A 2013 FRENCH REPORT
Recently, French investigators examined 406 patients in the NephroTest cohort with measured glomerular filtration rates (mGFR) measured by 51Cr-EDTA clearance at least 3 times during at least 2 years of follow-up[86]. Individual examination of mGFR trajectories by 4 independent nephrologists classified patients as “improvers”, defined as those showing a sustained mGFR increase, or “nonimprovers”[86]. Twelve patients with erratic trajectories were excluded. Measured GFR improved over time in 62 patients (15.3%). Their median mGFR slope was + 1.88 (IQR, 1.38, 3.55) mL/min per year; it was 22.23 (23.9, 20.91) for the 332 “nonimprovers”. “Improvers” had various nephropathies, but not diabetic glomerulopathy or polycystic kidney disease[86]. They did not differ from “nonimprovers” for age, sex, cardiovascular history, or CKD stage, but their urinary albumin excretion rate was lower. GFR improvement is possible in CKD patients at any CKD stage through stage 4-5[86]. It is noteworthy that this GFR improvement is associated with a decrease in the number of metabolic complications over time. In conclusion, this French report showed that renal function can improve over time in a significant proportion of CKD patients, even at a severe stage of CKD[86]. Indeed, the 15.3% prevalence of GFR improvement observed in this cohort is consistent with the few reports previously published.
These observations warrant further study of CKD progression or nonprogression among stable population-based asymptomatic CKD patients to improve our knowledge of the natural history of CKD and to help optimize CKD care around the world - our soon to be introduced new IT Software Program, The CKD Express© IT Software Program, which would help accelerate the harnessing of such new information about the natural history of CKD will be discussed in a later section of this review[49,87,88].
GRAPHICAL CASE SUMMARIES OF SELECTED INDIVIDUAL PATIENT-LEVEL SERUM CREATININE TRAJECTORIES OVER SEVERAL YEARS TO DEMONSTRATE CKD STAGING UNPREDICTABILITY AS SEEN AT THE MAYO CLINIC HEALTH SYSTEM RENAL UNIT, NORTHWESTERN WISCONSIN, UNITED STATES, 2002-2014: THE CONCEPT OF “SYMPTOMATIC”VS“ASYMPTOMATIC” CKD
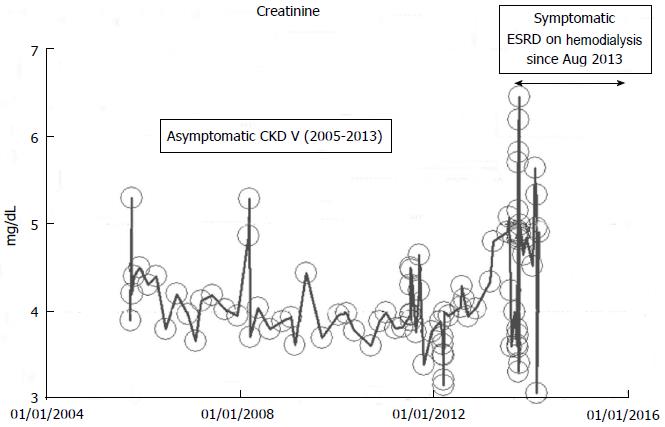
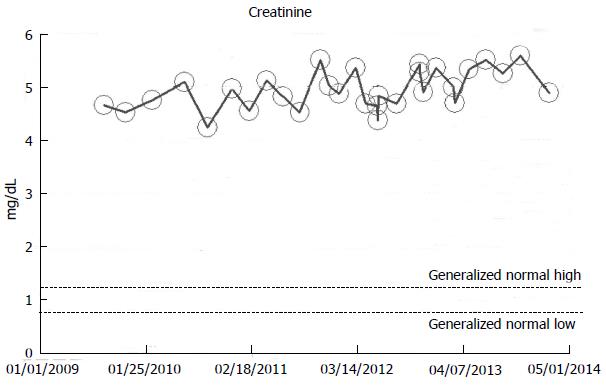
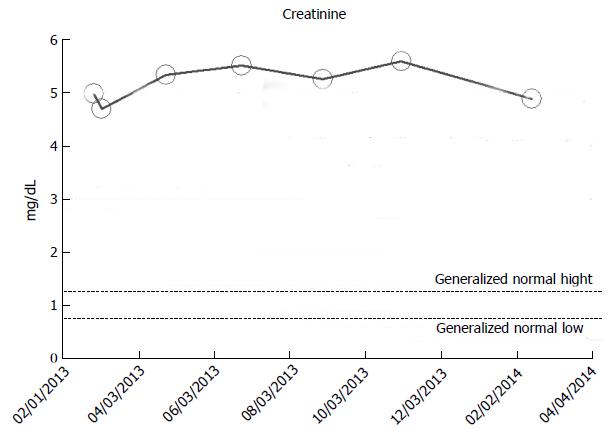
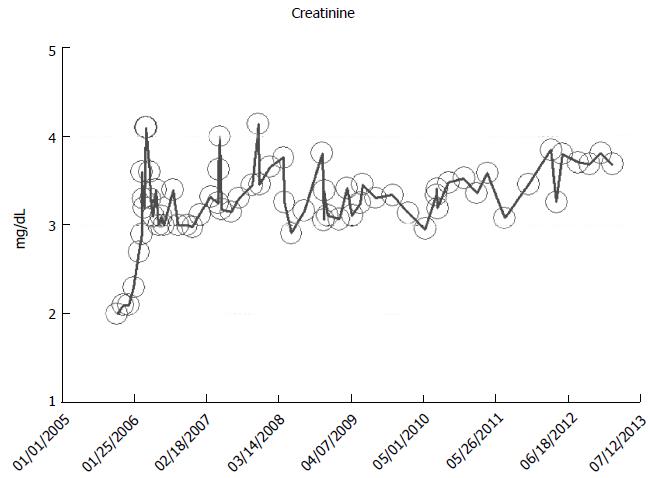
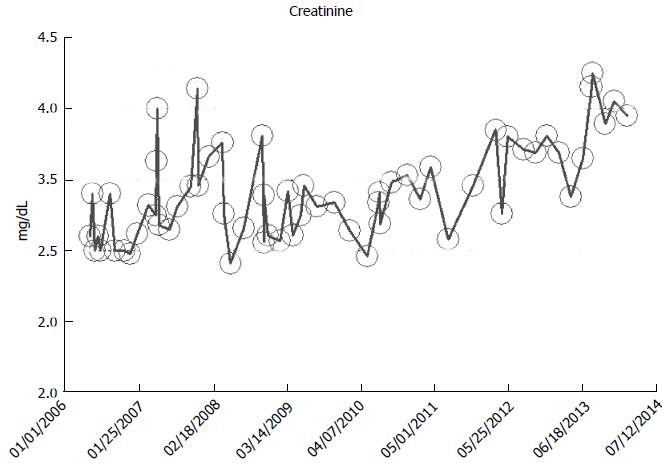
From our experience of studying the natural history of CKD at the Mayo Clinic Health System Renal Unit, Northwestern Wisconsin, United States, during the period, 2002-2014, and from a dispassionate review of the nephrology literature, it would appear that some CKD patients are able to maintain stable, albeit diminished eGFR levels over years, the so-called “non-progressors” or “asymptomatic” CKD patients, whereas, other CKD patients, for often unclear reasons, have the enhanced propensity to progressively lose eGFR over time, the so-called “progressors” or “symptomatic” CKD[32,78-86]. We will show in this section of this review, graphical representations, some selected CKD patients that we have encountered in the last 12 years in our Mayo Clinic Health System Renal Unit, Northwestern Wisconsin, United States, that demonstrate these patterns of behavior: (1) as at March 2014, a now 82-year-old white male, ex-smoker, with a history of hypertension and CKD has been on maintenance hemodialysis for symptomatic ESRD since August 2013. However, for 8 years, between 2005 and 2013, despite a stable CKD stage V with serum creatinine of 4.0-5.0 mg/dL (eGFR 8-14 mL/min per 1.73 m2 BSA), he had remained asymptomatic. He had an AVF created in June 2005 which was subsequently revised for non-maturation in January 2006. For unclear reasons, he then became symptomatic in July 2013 with nausea, vomiting and anorexia, and has been on maintenance hemodialysis since August 2013 (Figure 2); (2) as at February 2014, a now 78 year-old white male, with a history of hypertension, type II diabetes mellitus, obesity, recurrent UTI and CKD V, has over the last 7 years, between 2006 and 2013, despite a stable CKD stage V with serum creatinine of 4.5-5.5 mg/dL (eGFR 8-11 mL/min per 1.73 m2 BSA), had remained asymptomatic. He continues on alternate monthly course of prophylactic short course of oral Levofloxacin for recurrent UTI prophylaxis. (Figures 3 and 4); and (3) as at early 2014, a now 78-year-old white male, diagnosed with Wegener’s Granulomatosis with AKI on CKD in 2005. Serum creatinine had increased from 2.0 mg/dL to 3.5-4.0 mg/dL. The Wegener’s Granulomatosis was treated with standard chemotherapy (Prednisone), and he has been in remission since 2006, albeit with a new baseline serum creatinine of 3.5-4.0 mg/dL (eGFR 16-22 mL/min per 1.73 m2 BSA). He has remained otherwise an asymptomatic CKD IV patient between 2006 and early 2014 (Figures 5 and 6).
Figure 2 A now 82-year-old white male on maintenance hemodialysis August 2013-March 2014, after 8 years of asymptomatic chronic kidney disease V, as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program.
CKD: Chronic kidney disease; ESRD: End stage renal disease.
Figure 3 A now 78-year-old white male on with asymptomatic stable chronic kidney disease V for 8 years, 2006-2014, serum creatinine of 4.
5-5.5 mg/dL (eGFR 8-11 mL/min per 1.73 m2 Body Surface Area), as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program. eGFR: Glomerular filtration rates.
Figure 4 A now 78-year-old white male on with asymptomatic stable chronic kidney disease V for 8 year, 2006-2014, serum creatinine of 5.
0-5.5 mg/dL (eGFR 8-11 mL/min per 1.73 m2 Body Surface Area), in the last one year, February 2013-March 2013, as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program. eGFR: Glomerular filtration rates.
Figure 5 A now 78-year-old white male with treated Wegener’s Granulomatoisis in remission since 2006, with new stable chronic kidney disease IV 2008-2014, following initial acute kidney injury on chronic kidney disease in 2005, as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program.
Figure 6 The same patient as in Figure 5 has remained otherwise an asymptomatic chronic kidney disease IV patient between 2006 and early 2014, serum creatinine of 3.
5-4.0 mg/dL (eGFR 16-22 mL/min per 1.73 m2 Body Surface Area), as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program. eGFR: Glomerular filtration rates.
UNPREDICTABILITY OF THE IMPACT OF AKI EVENTS ON CKD PROGRESSION-A BRIEF REVIEW OF SELECTED CASES FROM THE MAYO CLINIC HEALTH SYSTEM RENAL UNIT, EAU CLAIRE, NORTHWESTERN WISCONSIN, UNITED STATES
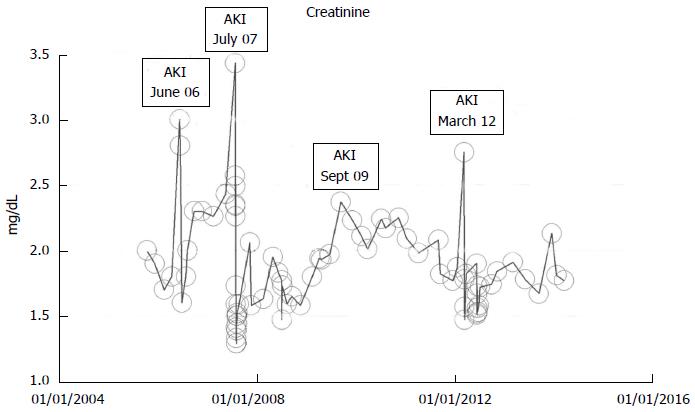
In a just published book chapter, we had extensively reviewed the multifaceted nature of renal outcomes following AKI in CKD patients[89-91]. As a result of the varied nature of these renal outcomes, we dubbed this “the rainbow syndrome of too many colors”[91]. We would briefly describe three patients with AKI on CKD and three observed different outcomes to demonstrate the array of possible renal outcomes following AKI on CKD - complete recovery of renal function back to baseline, partial recovery following AKI, and a third outcome where despite multiple repeated AKI events over several years, a 92-year-old Caucasian female was still able to maintain her baseline serum creatinine (Figure 7). In the following section, we shall describe the previously unrecognized syndrome of acute yet irreversible ESRD following unpredictably on an AKI event in a CKD patient, the syndrome of rapid onset end stage renal disease or (SORO-ESRD)[19,30,72,89].
Figure 7 Serum creatinine trajectory in a now 92-year-old Caucasian female patient who despite multiple repeated AKI episodes between 2006 and 2012, has otherwise maintained a baseline serum creatinine of 1.
7-2.0 mg/dL through March 2014, as monitored and tracked through a simulation of The chronic kidney disease Express IT Software Program. AKI: Acute kidney injury.
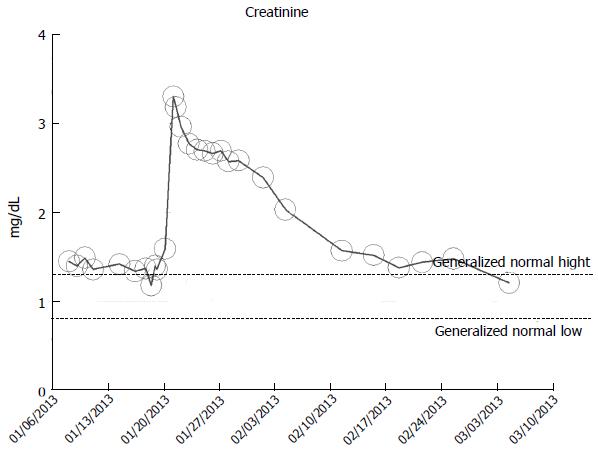
A-48 year-old obese hypertensive Caucasian male patient, with otherwise stable stage II CKD, baseline serum creatinine of 1.2 mg/dL (eGFR > 60 mL/min per 1.73 m2 BSA), in January 2013, developed AKI on CKD following fever of unknown origin (FUO) complicating methicillin-resistant Staphylococcus Aureus (MRSA) bacteremia. He at the time of admission in January 2013 was on hydrochlorothiazide 25 mg daily, Lotrel (5 mg of amlodipine + 10 mg of benazepril) daily. Lotrel was promptly discontinued on admission and Amlodipine only was continued. MRSA septicemia was treated with parenteral antibiotics, guided by antibiogram[91]. A kidney biopsy revealed acute interstitial nephritis. Peak serum serum creatinine was 3.3 mg/dL. He exhibited full recovery of kidney function as at March 2013 (Figure 8).
Figure 8 Serum creatinine trajectory in an 48-year-old obese hypertensive male patient who developed acute kidney injury from biopsy-proved acute interstitial nephritis complication methicillin-resistant Staphylococcus Aureus septicemia, with full renal recovery after one month, as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program.
AKI: Acute kidney injury.
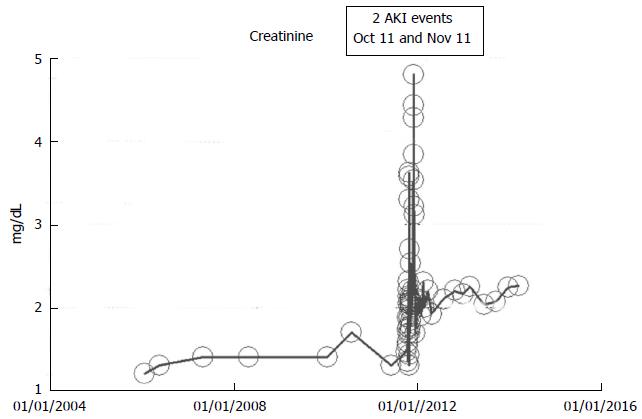
An 83-year-old white woman with hypertension and otherwise stable CKD III had undergone a right hemicolectomy procedure together with 150 cm small intestinal resection with end-to-end ileo-colic anastomosis for colon cancer in October 2011. This was further complicated by dehydration and diarrhea and the need for a second laparoscopic procedure in November 2011. She experienced AKI in October 2011 following the initial surgical procedure and another AKI episode in November 2011 from hypovolemic dehydration with peak creatinine values as shown in Figure 9. She subsequently partially recovered kidney function and since December 2011, she has maintained a serum creatinine of 1.8-2.2 mg/dL through March 2014. She otherwise remains asymptomatic, and continues to feel great at her current age of 85 years. Her baseline serum creatinine was 1.2 mg/dL in January 2006, 1.3-1.4 mg/dL in 2010-2011 (CKD stage III, eGFR approximately 36-38 mL/min per 1.73 m2 BSA), AKI intervened in October-November 2011, and she has had a new higher baseline serum creatinine of 2.0-2.6 mg/dl (eGFR 20-25 mL/min per 1.73 m2 BSA), CKD stage IV, in the last three years, between December 2011 and March 2014 (Figure 9).
Figure 9 Serum creatinine trajectory in an 85-year-old obese hypertensive female patient who developed acute kidney injury following a complicated right hemicolectomy with small intestinal resection and ileo-colic anastomosis in October 2011, with partial but stable renal recovery (chronic kidney disease IV) after one month, as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program.
A 92-year-old white woman in March 2014, with a history of multiple AKI events between 2005 and 2014, over nearly 10 years, but is still able to generally maintain an otherwise asymptomatic CKD IV status with serum creatinine of 1.7-2.0 mg/dL (CKD stage IV, eGFR 20-29 mL/min per 1.73 m2 BSA), through March 2014, the same baseline serum creatinine she had 10 years ago in 2005 (Figure 7). The repeat episodes of AKI events are shown in Figure 7 and were caused by the following causes: (1) Pyelonephritis, treated with Bactrim, June 2006, peak creatinine was 3.0 mg/dL; (2) Left lower lobe pneumonia with diarrhea, dehydration and hypotension, July 2007, peak creatinine was 3.43 mg/dL; (3) Pyelonephritis, September 2009, peak creatinine was 2.37 mg/dL; and (4) Acute gastroenteritis and dehydration, March 2012, peak creatinine was 2.75 mg/dL.
THE SYNDROME OF RAPID ONSET END STAGE RENAL DISEASE
The commonly held consensus of the propagation of CKD to ESRD is that of a predictable, linear, progressive, relentless, time-dependent and knowable decline in renal function, with predictably increasing serum creatinine or falling eGFR, leading inexorably to ESRD and the need for renal replacement therapy[1-4,6,7,9-18]. This universally accepted paradigm of CKD-ESRD progression will be referred to in this review as the “classic” pattern of CKD to ESRD progression[19,92].
What’s more, whereas 25.2% of ESRD Medicare patients all experienced antecedent AKI, for many decades now, this relationship between antecedent AKI and irreversible ESRD had been fortuitously blamed on the so-called “residual confounding”[93]. However, in 2010, we put to rest any doubts as to the direct causality between antecedent AKI and the precipitation of acute yet irreversible ESRD, when we first described the previously unrecognized syndrome of rapid onset end stage renal disease or SORO-ESRD in the journal, Renal Failure[72]. We have defined the syndrome of rapid-onset ESRD as the unpredictable, unanticipated and accelerated progression from a-priori stable CKD to irreversible ESRD, requiring permanent RRT, following a new episode of AKI precipitated by antecedent new medical/surgical events, with the interval between AKI and the need for RRT represented by a period of often less than less than two weeks, measured only in days following surgically induced AKI[19,30,72,92,94,95]. We have also described this syndrome of acute yet irreversible ESRD in renal transplant recipients[19,92,94,95]. We would briefly describe in this section, CKD patients who demonstrate features of the “classic” CKD to ESRD translation (Figures 10 and 11), and patients who on the other hand exhibit the features of the syndrome of rapid onset end stage renal disease or SORO-ESRD (Figure 12).
Figure 10 Serum creatinine trajectory in a 82-year-old white male patient with features of the predictable linear chronic kidney disease to end stage renal disease progression from 2006 through 2010 when he started hemodialysis for symptomatic uremia, as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program.
ESRD: End stage renal disease; HD: Hemodialysis.
Figure 11 Serum creatinine trajectory in a 52-year-old white male patient with features of the predictable linear chronic kidney disease to end stage renal disease progression from 2007 through 2010 when he started hemodialysis for symptomatic uremia, as monitored and tracked through a simulation of The chronic kidney disease Express© IT Software Program.
ESRD: End stage renal disease; HD: Hemodialysis.
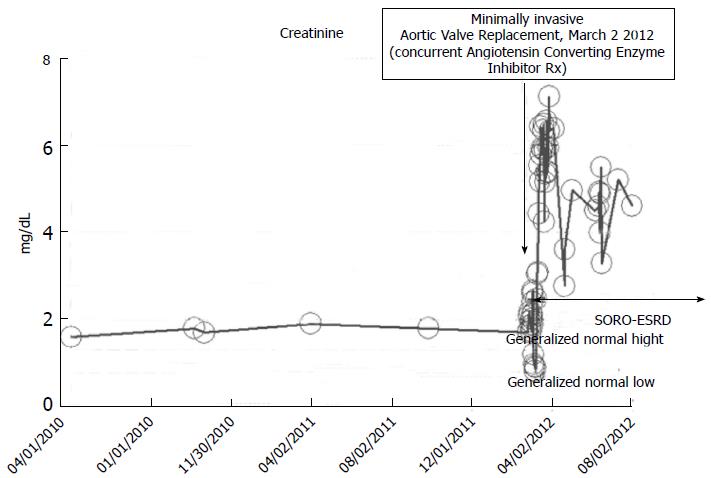
Figure 12 Serum creatinine trajectory, 2010-2012, with post-operative acute kidney injury precipitating acute yet irreversible end stage renal disease in March 2012 while on concurrent ACE inhibition, following minimally invasive aortic valve replacement in a then 73-year-old thin white woman.
SORO-ESRD: Syndrome of rapid onset end-stage renal disease.
We were the first to aptly circumscribe this syndrome of rapid onset ESRD as a distinct clinical entity[19,30,72,92,94,95]. Our findings have received significant collaborating support following the publication of several more recent reports demonstrating the not uncommon occurrence of SORO-ESRD, in incident adult ESRD populations in both the US and Canada[96-99]. In fact, a recent 2012 review in the journal Kidney International concluded that AKI can cause ESRD directly, and increase the risk of developing incident CKD and worsening of underlying CKD and even went further to posit that the distinction between AKI and CKD may be artificial[100].
“Classic” CKD to ESRD pattern
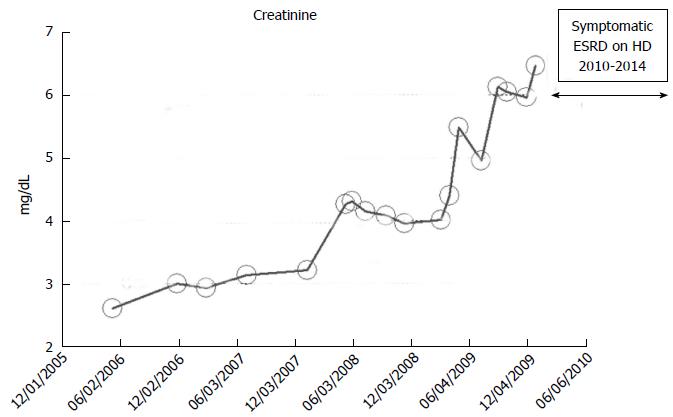
An 82-year-old while male with a history of hypertension, low ejection fraction ischemic cardiomyopathy, type II diabetes mellitus, coronary artery disease, obesity and hypothyroidism developed progressively worsening renal failure with predictable linear increase in serum creatinine from May 2006 when serum ceatinine was 2.6 mg/dL through to January 2010 when serum creatinine exceeded 6.0 mg/dL and he developed features of uremia and started renal replacement therapy in the form of in-center outpatient hemodialysis in January 2010 (Figure 10). He died in early 2014, while still on maintenance hemodialysis, from failure to thrive. No laboratory data were available for this patient before May 2006.
“Classic” CKD to ESRD pattern
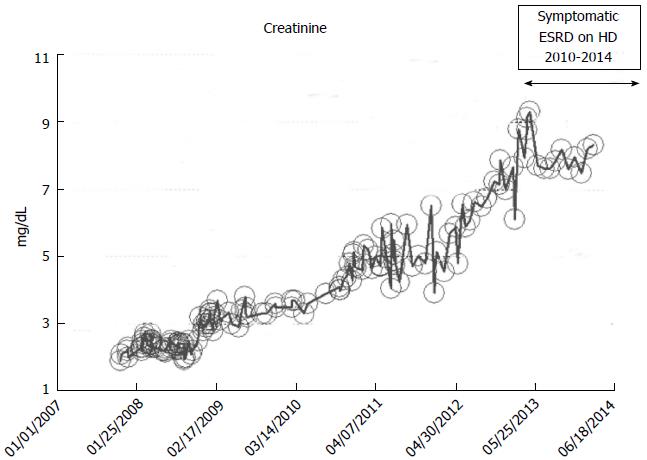
A 52-year-old while male with a history of hypertension, low ejection fraction ischemic cardiomyopathy, and hypothyroidism developed progressively worsening renal failure with predictable linear increase in serum creatinine from November 2007 when serum ceatinine was 1.9 mg/dL through to December 2010 when serum creatinine exceeded 7.0 mg/dL and he developed features of uremia and started renal replacement therapy in the form of in-center outpatient hemodialysis (Figure 11). He continues on maintenance hemodialysis as at March 2014, the date of this present review.
“SORO-ESRD” pattern in native kidneys
A 73-year-old obese male patient with a baseline serum creatinine of approximately 1.7 mg/dL, 2010-2012, with a past medical history for hypertension, type II diabetes mellitus, on concurrent ACE inhibition with Lisinopril 40 mg daily, was admitted to the coronary care unit (CCU) in February 2012 with acutely decompensating heart failure. Acute coronary syndrome was ruled out and the patient subsequently underwent minimally invasive aortic valve replacement for symptomatic aortic stenosis with a 25 mm St. Judes Epic stented tissue valve March 2 2012. He rapidly developed post-operative AKI on CKD and required hemodialysis on the 1st post-operative day (Figure 12). He has since remained on outpatient in-center maintenance hemodialysis for ESRD, from March 2012 through to March 2014, the date of present reporting.
“SORO-ESRD” pattern in a renal transplant recipient
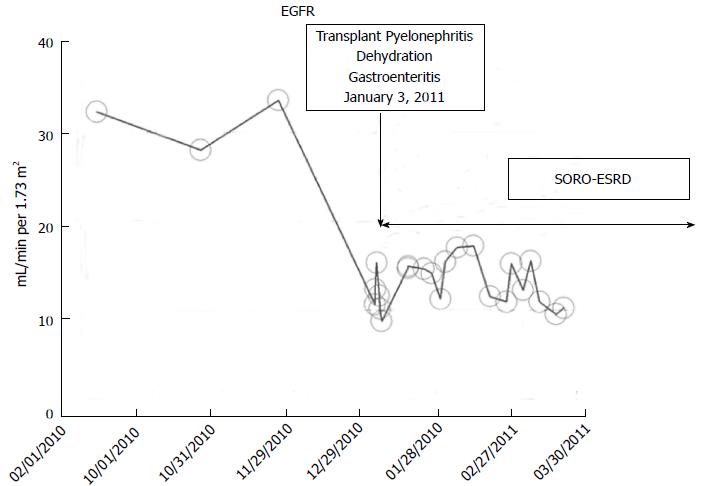
A 53-year-old Caucasian type 1 diabetic woman with simultaneous pancreas-kidney transplantation in 2000 for ESRD and type I diabetes mellitus, maintained on chronic transplant immunosuppression with tacrolimus, cellcept, and prednisone had maintained her allograft CKD stage III status throughout most of 2010 with a baseline serum creatinine of 1.6 mg/dL. In January 2011, she suffered from acute transplant pyelonephritis precipitating AKI, further complicated by dehydration from acute gastroenteritis[94]. The patient’s serum creatinine rose quickly within days to 5.16 mg/dL (Figure 13). She required hemodialysis for symptomatic renal failure. A transplant kidney biopsy revealed acute tubular necrosis with chronic transplant glomerulopathy but no rejection. She was maintained on in-center outpatient hemodialysis for oliguric irreversible ESRD until January 2012, twelve months later, when she received a new renal allograft re-transplant, a living-related kidney allograft from her then 32-year-old son at Mayo Clinic, Rochester. Additional clinical details of this renal transplant recipient are described in our recent publication[94].
Figure 13 Estimated glomerular filtration rates trajectory in a renal transplant recipient following acute kidney injury and Syndrome of Rapid Onset End-Stage Renal Disease in January 2011, was on maintenance in-center hemodialysis for 12 mo and received a second living related kidney transplant from 32-year-old son in January 2012 at Mayo Clinic, Rochester.
SORO-ESRD: Syndrome of rapid onset end-stage renal disease. EGFR: Estimated glomerular filtration rates.
CKD SCREENING REVISITED-CKD SCREENING CONTROVERSY BETWEEN ACP AND ASN: TO SCREEN OR NOT TO SCREEN?
To further emphasize on the inexactness of CKD prediction and prognostication, the August 2012 United States Preventive Services Task Force (USPSTF) report on CKD screening concluded that we know surprisingly little about whether screening adults with no signs or symptoms of CKD will improve health outcomes and that clinicians and patients deserve better information on CKD[39]. This unsettled state of affairs regarding the appropriateness or otherwise of CKD screening within the asymptomatic population came to the fore and became part of the public discourse when two very respected professional United States medical associations, the American Society of Nephrology (ASN) and the American College of Physicians (ACP) both simultaneously came down on opposite sides of the argument for and against CKD screening among asymptomatic populations, respectively[101,102]. The ASN position was that “while acknowledging the need for further and larger scale clinical research into CKD and how the disease progresses in its early stages, ASN believes current evidence strongly supports the value of early detection of, and screening for, CKD”[102]. Given the present level of evidence-base, and more so from our experience at the Mayo Clinic Health System Renal Unit, in Northwestern Wisconsin, United States, we would side with the USPSTF in cautioning against CKD screening among the otherwise asymptomatic population. Such a wide-out CKD screening model will most likely result in more unnecessary care, escalating costs of CKD care to Medicare, increased referrals to nephrologists, and all of this without any evidence of improved CKD outcomes[32,46,49,103,104]. Additionally, we could not agree more with the USPSTF on the urgent need for more research into CKD progression and prognostication.
MULTIPLE PUTATIVE MECHANISMS OF CKD INITIATION AND PROPAGATION
Clearly, from accruing evidence in the literature, there is a continuing elongating list of putative mechanisms that have been variously incriminated in the initiation and propagation of CKD progression[105-128]. We have analyzed and summarized these mechanisms in an extensively referenced recent review[105]. A critical review of current literature clearly demonstrates that culprit pathogenetic molecule(s) or mechanistic factor(s) responsible for the initiation and propagation of diabetic and/or non-diabetic nephropathy and subsequent progression of CKD to ESRD and the need for permanent renal replacement therapy, remain unverified, unconfirmed, uncertain, and possibly unknown[105-128]. Undeniably, several independent and often conflicting lines of evidence in the literature, from both human and experimental studies, propose a variety of presumed pathogenetic culprit mechanisms and factors[105-128]. Putative roles are ascribed in the literature for oxidative stress, inflammation, underlying genetic predispositions including variations of the non-muscle myosin heavy chain 9 gene (MYH9) on chromosome 22 and variants at chromosome 6q24-27 among African-Americans, advanced glycosylation end products and the interaction of these end products on the multiligand receptor of the immunoglobulin superfamily receptor for advanced glycation end products, intrarenal angiotensin II and/or renin production, lipid toxicity, podocyte injury and apoptosis, cytokine/chemokine/growth factor release causing renal injury, asymmetric dimethylarginine, and uric acid[105-128]. We posit that our current understanding of these different plausible pathways remain infantile at best, and that more studies are warranted[105]. We surmise that our better understanding of these mechanisms and processes that influence CKD initiation and CKD propagation, and our ability to decipher the relative contributions of these pathways and/or factors in CKD-ESRD translation, together with the impact of AKI on this continuum of CKD pathogenesis would implicitly help improve our overall ability to prognosticate and care for our CKD patients, in general[31,32,79,105].
THE CKD EXPRESS©-A NEWLY INTRODUCED IT SOFTWARE TO ENHANCE AND OPTIMIZE CKD CARE
From the foregoing review, it is very clear that there remains a significant knowledge gap of our understanding of the natural history of CKD, in general. As a result of these inherent deficiencies in the CKD knowledge base, in an MBA “New Product” class at the University of Wisconsin Consortium in 2011-2012, the first author together with a group of fellow MBA students devised a new IT Software Program called The CKD Express©, still currently in United States patent application[32,88,89,129].This innovative IT Software Program with its unique artificial intelligence (AI), decision support tools (DSS) and other enhancements that include algorithmic components would enable a trained Nurse Practitioner, under the supervision of a nephrologist, to remotely track and remotely manage CKD patients by tracking serum creatinine and simultaneous eGFR trajectories of individual CKD patients indefinitely over time, through established IT system networks linked with the various associated EMR systems[32,87,88,129].This way, CKD patients will carry out pre-specified blood tests including the basic metabolic profile at pre-determined time intervals as determined by the IT Software. Only targeted and identified CKD patients would then need to either be referred to see a nephrologist, or repeat a blood test, urgently be seen in an Urgent Care Center or even the Emergency department as determined by the inbuilt algorithms in The CKD Express©[32,87,88,129]. This would ensure the delivery of convenient, affordable, effective and efficient CKD care around the world[32,49,87,88,129].
CONCLUSION
CKD staging is a useful guide, but its evidence-base is shaky, at best. However, CKD care must be individualized. Clearly, despite decades of painstaking research into the dynamics, processes and mechanisms of translation from CKD to ESRD and the need for renal replacement therapy, the medical community in general, and nephrologists in particular, arguably still remain at a considerable loss in understanding the nuances of such translations. We suggest a complete reappraisal of current nephrology practices and a new push to begin to develop new models of CKD care that correctly recognize the diversity of CKD as representative of a wide spectrum of disease states[31,32]. Most appropriately, Bansal and Hsu, in a 2008 analysis of the long-term outcomes of patients with CKD had strongly echoed the observation that the apparently conflicting and disparate ESRD and/or mortality rates reported in various CKD population cohorts in the literature only emphasized the heterogeneity of different CKD populations[130]. In a recent analysis, in comparison with patients with other underlying causes of CKD, patients with APKD and IgA nephropathy had a statistically significant slower progression rate of CKD to ESRD[131]. Nephrologists must therefore not rely on CKD staging alone to direct management of or risk stratification of patients with CKD[130]. Nephrologists must always consider the etiology and rate of progression of kidney disease, patient age and a wide array of renal cardiovascular disease risk factors[130,131]. Nephrologists must recognize that CKD prediction is at best an inexact science. CKD prediction models are very limited in scope and applicability. Inter-patient and intra-patient CKD stages variability over time is immense. There are “progressors” and “nonprogressors”, “improvers” and “nonimprovers”, and sometimes the same CKD patient often then exhibits contrasting CKD progression or non-progression patterns at different time periods. The impact of AKI on CKD is multi-faceted, the so-called “rainbow syndrome”[19,30,72,92,94,95]. Moreover, the role of AKI in CKD initiation is even far less well understood[132].
The overarching need to always individualize CKD care cannot be overemphasized as CKD represents a whole wide spectrum of distinctly different clinical disease entities, with each individual patient often subject to a multitude of aggravating factors, some of which often remain unrecognized[19,30,31,72,92,94,95,132,133]. This is in fact how we practice medicine - one patient, and only one patient at a time[19,31]. A soon to be published longitudinal retrospective United Kingdom analysis of approximately 600000 patients strongly emphasized this paramount need for individualized CKD care[134]. This study identified a 5.9% incidence of CKD in the United Kingdom in 2010, and with follow up, CKD stages were static in 50% of patients, progressed in 10%-15% of patients and actually improved in 25%-30% of patients[134].
Finally, we hope that the acceptance and subsequent introduction by Medicare of our newly devised IT Software, The CKD Express© into general CKD care in the United States, will generate enough prospective patient-level serum creatinine trajectories’ data over the next few years to begin to help bridge the yawning gaps in our current knowledge of the natural history of CKD[32,87,88,129,135-137]. Every effort to reduce, if not eliminate AKI (Renoprevention) must be emphasized in both general medicine care and in nephrology specialty care, respectively[32,87,88,129,135,136]. Such reengineering protocols will reduce AKI incidence, potentially save hundreds of millions of healthcare dollars and invariably lead to less ESRD incidence[32,87,88,129,138,139]. A more forceful and pragmatic application of renoprevention strategies in the CCU that aggressively enunciate and articulate the preemptive withholding (from all CKD patients) of nephrotoxics including renin angiotensin aldosterone system blockers, the adoption of measures to aggressively prevent or treat perioperative hypotension, the avoidance of nephrotoxic exposure such as iodinated contrast and nephrotoxic antibiotics, whenever possible, would collectively lead to less AKI, and therefore potentially less SORO-ESRD[32,87,88,129,138-140]. Such paradigms of care would translate into better patient outcomes, hugely significant renal salvage, and indeed massive dollar savings[32,138,139]. Such paradigm shifts would constitute major rethinking in current nephrology practice, a form of nephrology practice reengineering[32,138-140].
ACKNOWLEDGMENTS
This work is dedicated to the memory of our very dear friends, Mr. Ikechukwu Ojoko (Idejuogwugwu), and Chigbo Eduzor MD (CC), who passed away back home in Nigeria, several years ago, after reported brief illnesses. Idejuogwugwu and CC, you are truly missed. This work is also dedicated to the memories of the 153 Nigerians who died in a fiery plane crash in Lagos, Nigeria, on June 3, 2012. May their souls rest in perfect peace. Finally, we dedicate this work to the majority millions of Nigerians who continue to suffer in poverty, destitution, deprivation amidst plenty, to Nigerians who remain continuously exposed daily to indefensible insecurity in the face of paralyzed, corrupt and weak governments and derelict governance, and we pray for better days for that country. It remains our hope and prayers that someday, we would be celebrating an effective, efficient, affordable, accessible and potent health care delivery system in Nigeria that would veritably and equitably serve all Nigerians, both rich and poor, both privileged and underprivileged. Lastly, we look forward to enhanced CKD care, together with accessible affordable ESRD care to all needy Nigerians.