Published online Nov 6, 2013. doi: 10.5527/wjn.v2.i4.129
Revised: August 2, 2013
Accepted: August 28, 2013
Published online: November 6, 2013
AIM: To assess the efficacy of combined Aliskiren and Losartan vs high dose Losartan and Aliskiren alone in chronic kidney disease (CKD).
METHODS: This is a retrospective study of 143 patients with non-diabetic CKD comparing combined Aliskiren (150 mg/d) with Losartan (100 mg/d) therapy vs High dose Angiotensin receptor blockers (ARB) (Losartan 200 mg/d) and the third group Aliskiren (150 mg/d) alone. This study involved only patient medical records. Entry criteria included those patients who had been treated with the above drugs for at least 36 mo within the 5 years period; other criteria included proteinuria of 1 g or more and or CKD Stage 3 at the start of the 36 mo period. The study utilised primary renal end points of estimated Glomerular Filtration Rate (eGFR) < 15 mL/min or end stage renal failure.
RESULTS: Patients treated with high dose ARB compared to the other two treatment groups had significantly less proteinuria at the end of 36 mo (P < 0.007). All 3 groups had significant reduction of proteinuria (P < 0.043, P < 0.001). Total urinary protein was significantly different between the 3 groups over the 3-year study period (P = 0.008), but not eGFR. The changes in eGFR from baseline to each year were not significantly different between the 3 therapeutic groups (P < 0.119). There were no significant differences in the systolic and diastolic blood pressure between the 3 drug groups throughout the 3 years. The incidence of hyperkalemia (> 5.5 mmol/L) was 14.2% (7/49) in the Combined Aliskiren and ARB group, 8.7% (4/46) in the Aliskiren alone group and 6.3% (3/48) in the High dose ARB group (P < 0.001).
CONCLUSION: This study in non-diabetic CKD patients showed that Combination therapy with Aliskiren and ARB was effective but was not safe as it was associated with a high prevalence of hyperkalaemia.
Core tip: The Aliskiren trial in type 2 diabetes using Cardio-Renal Endpoints (ALTITUDE) study was able to unmask serious adverse events like ischemic heart disease and strokes because it had included Cardio-Renal Endpoints among its primary end points. It may be advisable to require future trials on drugs which could impact on the kidneys, heart and brain to have similar Cardio-Renal Endpoints or Cardio-Neuro-Renal End points to further ensure therapeutic safety of the trial drug. Our modest study compared to the magnitude of the ALTITUDE study still managed to detect the problem of hyperkalaemia in the group treated with Combination therapy with Aliskiren and ARB. Based on our study it would appear that the findings of the ALTITUDE study would also apply to non-diabetic Chronic Kidney Disease patients.
- Citation: Woo KT, Choong HL, Wong KS, Tan HK, Foo M, Stephanie FC, Lee EJ, Anantharaman V, Lee GS, Chan CM. A retrospective Aliskiren and Losartan study in non-diabetic chronic kidney disease. World J Nephrol 2013; 2(4): 129-135
- URL: https://www.wjgnet.com/2220-6124/full/v2/i4/129.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i4.129
One of the important strategies in the treatment of chronic kidney disease (CKD) is the use of Angiotensin converting enzyme inhibitors Angiotensin-converting enzyme inhibitors (ACEI) and Angiotensin receptor blockers (ARBs) to reduce proteinuria as well as to retard the progression to end stage renal failure[1,2]. ACEI and ARBs compete with the receptor for angiotensin and therefore inhibit the action of angiotensin. However one of the concerns in the use of these agents is the long term side effects of progressive renal fibrosis with worsening of estimated Glomerular Filtration Rate (eGFR)[3]. ACEI and ARBs indirectly cause renal fibrosis as they also promote the increase of aldosterone which causes renal fibrosis[4]. Because renin is not converted to angiotensin, there is a build- up of renin in patients on ACEI and ARB.
Aliskiren is a new renal protective agent that inhibits renin, the rate limiting step in the renin angiotensin aldosterone system[5]. In both healthy volunteers and disease states, aliskiren reduces angiotensin II levels and plasma renin activity (PRA), without stimulating compensatory increases in PRA, angiotensin I and angiotensin II as seen with ACEI and ARB. Aliskiren allows for total blockade of the renin angiotensin system and its beneficial effect is independent of blood pressure (Bp) control[6].
In our recent paper on the beneficial effects of long term high dose ARB therapy in patients with IgA nephritis over a period of 6 years[7] , we showed that high dose ARB is more efficacious in reducing proteinuria and preserving renal function in terms of earlier and more effective improvement in eGFR in those treated with high dose ARB compared to those on normal dose ARB and ACEI. We believe that high dose ARB does so by causing regression of glomerular sclerosis[8]. Busch et al[9] have also reported the beneficial effects of high dose Irbesartan in patients with diabetic nephropathy.
The present study examines the effects of ARB (Losartan 100 mg/d) combined with Aliskiren (150 mg/d), Aliskiren (150 mg/d) alone and High dose ARB (Losartan 200 mg/d) in patients with Chronic Kidney Disease (Chronic Glomerulonephritis). We compared the therapeutic efficacy between High dose ARB (Losartan 200 mg/d) versus Combined Aliskiren (150 mg/d) with Losartan (100 mg/d) and the third group Aliskiren alone (150 mg/d to determine whether a Combined dose of Aliskiren and Losartan is as effective as High dose Losartan therapy and whether it confers additional renoprotective effects.
In a database comprising 312 patients with Chronic Kidney Disease, 143 patients with CKD due to Chronic Glomerulonephritis and not due to diabetic nephropathy, hypertensive nephrosclerosis, lupus nephritis or Henoch Schonlein nephritis were recruited for the study. Data of these 312 patients from 2007 to 2012 were examined for the purpose of a retrospective study. Non biopsied CKD patients formed the bulk of our clinical practice and were more readily recruited. For purposes of standardisation of the study, we decided to recruit only non-biopsied patients into the study. In this new database for the purpose of this study, the database of 143 patients were selected, among which 49 patients were treated with combination therapy using Aliskiren and Losartan, 46 patients were treated with Aliskiren alone and the remaining 48 patients were treated with High Dose Losartan alone. This was a retrospective study involving only patient medical records. Entry criteria included those patients who had been treated on the above drugs for at least 36 mo within the 5 years period; other criteria included proteinuria of 1 gram or more and or CKD Stage 3 at the start of the 36 mo period. There were no significant differences in the various parameters between the 3 groups on entry into the study (Table 1). All selected patients had adequate control of Bp control which was achieved with addition of atenolol, amlodipine or nifedipine.
| Combined Aliskerin and ARB | Aliskerin | High dose ARB | 1P value | |
| n= 49 | n= 46 | n= 48 | ||
| Sex (F : M) | 16:33 | 22:24 | 23:25 | NS |
| Age at biopsy (yr) | 58 ± 12 | 57 ± 12 | 60 ± 11 | NS |
| Duration of Trial (mo) | 37 ± 1 | 36 ± 1 | 36 ± 1 | NS |
| Hypertension (Yes : No) | 16:15 | 15:31 | 17:31 | NS |
| Serum Creatinine (µmol/L) | ||||
| Baseline | 142 ± 39 | 142 ± 40 | 139 ± 32 | NS |
| Year 3 | 159 ± 53 (P = 0.001) | 161 ± 54 (P < 0.001) | 150 ± 47 (P = 0.015) | NS |
| eGFR (mL/min) | ||||
| Baseline | 49 ± 19 | 48 ± 14 | 48 ± 14 | NS |
| Year 3 | 44 ± 18 (P < 0.001) | 42 ± 15 (P < 0.001) | 45 ± 15 (P = 0.002) | NS |
| Decrease in eGFR (mL/min per year) | 1.8 ± 2.5 | 2.0 ± 3.0 | 1.0 ± 2.1 | P = 0.119 |
| Urinary Protein (gm/d) | ||||
| Baseline | 0.7 ± 0.6 | 0.8 ± 0.9 | 0.6 ± 0.7 | NS |
| Year 3 | 0.5 ± 0.8 (P = 0.002) | 0.6 ± 0.7 (P = 0.043) | 0.3 ± 0.3 (P < 0.001) | P = 0.007 |
| Blood Pressure (mmHg) | ||||
| Systolic before | 132 ± 12 | 135 ± 10 | 132 ± 12 | NS |
| Systolic after | 127 ± 10 (P < 0.001) | 130 ± 9 (P < 0.001) | 128 ± 11 (P < 0.001) | NS |
| Diastolic before | 84 ± 7 | 85 ± 6 | 84 ± 7 | NS |
| Diastolic after | 82 ± 5 P = 0.040 | 83 ± 6 P = 0.084 | 83 ± 6 P = 0.361 | NS |
| Distribution of CKD at baseline | ||||
| CKD 1 | 1 | 0 | 0 | 0.465 |
| CKD 2 | 8 | 5 | 4 | |
| CKD 3 | 40 | 41 | 44 | |
| Distribution of CKD at year 3 | 0.606 | |||
| CKD 1 | 1 | 0 | 0 | |
| CKD 2 | 8 | 7 | 5 | |
| CKD 3 | 28 | 26 | 34 | |
| CKD 4 | 12 | 13 | 9 | |
| Non-ESRF | 49 | 46 | 48 | NS |
| ESRF | 0 | 0 | 0 | |
| Improvement in eGFR | ||||
| Yes | 12 | 15 | 15 | 0.645 |
| No | 37 | 31 | 33 | |
All 143 patients in the database had the following investigations documented at six monthly intervals: serum creatinine, eGFR and total urinary protein (TUP). Serum creatinine was quantitated with alkaline picrate and TUP was quantitated by biuret agent. Glomerular Filtration Rate was estimated using the Cockcroft Gault formula for eGFR[10]. Decrease in eGFR was expressed as mililiter of eGFR loss per year over the 3 year duration from time of entry to exit of the trial. Improvement in eGFR was taken as the positive difference between the entry eGFR and the exit eGFR over the study period. End stage renal failure was equated with decline of eGFR to CKD stage 5 with eGFR less than 15 mL/min per year. The primary end points were stage 5 CKD or end stage renal failure. The secondary end points were reduction of proteinuria by 50% and change in eGFR. Of the 143 patients, 49 patients were treated with combination therapy using Aliskiren and Losartan, 46 patients were treated with Aliskiren alone and the remaining 48 patients were treated with High dose ARB (Losartan alone). Entry criteria for the study included proteinuria of 1 gram or more and or CKD Stage 3. There were no significant differences in the various parameters between the 3 groups on entry into the study. The records showed that additional Bp control was achieved with atenolol, amlodipine and nifedipine
Sample size calculation was based on the proportion of patients achieving 30% decrease in TUP. A second sample size calculation was done to compare the rate of 30% TUP decrease between High dose ARB and Aliskiren alone. Assuming that the rate of TUP decrease to be 30% in the Normal dose ARB and Normal dose Aliskiren and 60% in the High dose ARB, the number of patients required in each group was 49 for a 2-sided test with α = 0.05 and power of 80%. As we expected High dose ARB to be even more efficacious, 50% reduction of TUP was chosen. We expected the effects of combination dose of ARB plus Aliskiren to be about the same as that of High dose ARB.
SPSS 10.1 for Windows was used for all analysis. Results were expressed as mean ± SD or median (range) or count (%). For univariate analysis, Pearson’s χ2 test was used for comparing categorical data and analysis of variance (ANOVA) for comparing numeric data between the 3 treatment arms. ANOVA was followed by multiple comparison with Student-Newman-Keuls (SNK) range test whenever statistical significance was found between the 3 arms. Next, Multivariate Analysis of Variance (MANOVA) with repeated measures was used to test the effect of drug treatment on both eGFR and total urine proteinuria (TUP). The dependent variables were eGFR and TUP measured at 4 time points, namely baseline and thereafter every year of the 3 years of the study. The between-subject factor was treatment group with 3 levels corresponding to Combination dose of ARB and Aliskiren, Aliskiren alone and high dose ARB. Adjustment was made for the covariates of average systolic Bp and average diastolic Bp. Average blood pressures were calculated by taking the mean of all blood pressures while on medication (mean of blood pressures from 1-3 year). Within MANOVA, the effect of high dose ARB on the outcomes of eGFR and TUP was compared with each of the other drug dosage groups by simple contrast comparison testing. Similarly, repeated contrast testing was done to obtain and compare the loss in eGFR in each year between the various drug groups.
Plots of mean values of eGFR and TUP adjusted for covariates of systolic Bp and diastolic Bp were presented. So were the contrast estimates, their corresponding 95%CI and P values for the comparison of eGFR and TUP between the levels of interest of the treatment group. Results of 2 types of comparison, change in eGFR and TUP from baseline to each time point compared between the 3 drug groups and loss in eGFR compared between the 3 drug groups were presented in a table with the F statistics and P values.
Table 1 compares the eGFR, proteinuria and decrease in eGFR between Combination dose of Aliskiren and ARB, Aliskiren alone, and high dose ARB before and after the trial. High dose ARB had significantly higher eGFR and less proteinuria at the end of the trial compared to the other 2 treatment groups by post-hoc Student-Neuman-Keuls range test. However, there was no significance difference in these parameters between the 2 other groups by the same SNK range test. The decrease in eGFR was 0.98 mL/min per year in the high dose ARB group compared to 1.77 and 2.02 mL/min per year in the Combined Aliskiren and ARB group and the group on Aliskiren alone respectively (P < 0.119). There were 15 patients out of 48 (31.3%) who had improved eGFR at the end of the study in the high dose ARB group compared to 12 out of 49 (24.4%) in Combined Aliskiren and ARB group and 15 out of 46 patients (32.6%) in the group on Aliskiren alone (P < 0.645, χ2 = 645 ). There were no patients with ESRF at the end of the study in all the 3 groups. There tended to be fewer patients with CKD4 in the high Dose ARB compared to the other 2 treatment arms at the end of the study (High dose ARB vs Combined Aliskiren and ARB vs Aliskiren alone: 18.8% vs 24.5% vs 28.3%, P =0.55, χ2 = 1.199, Table 1).
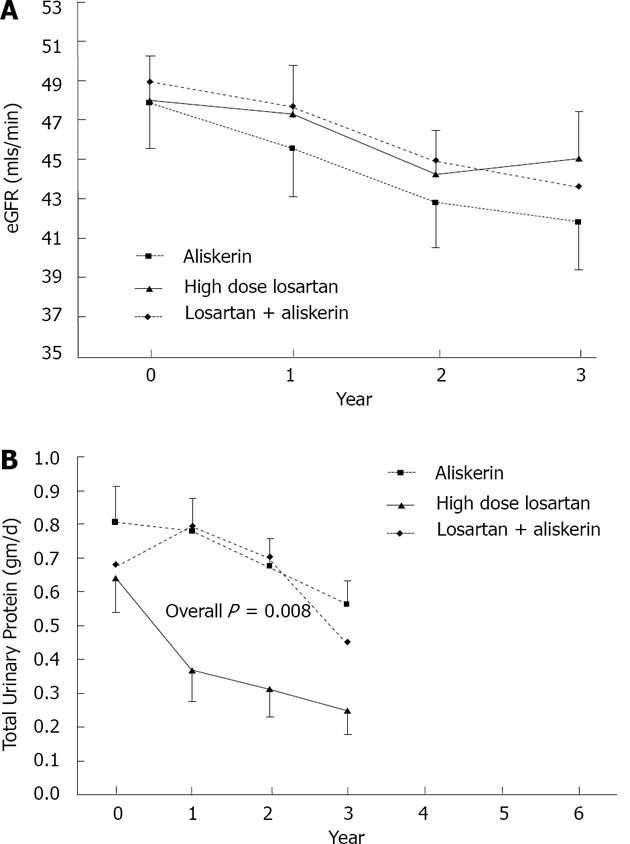
From the results of the multivariate ANOVA with repeated measures, TUP (F2140 = 5.041, P = 0.008, Figure 1A) was significantly different between the 3 groups over the 3-year study period, but not eGFR (F2140 = 0.194, P = 0.824, Figure1B). Over the whole study period, TUP for combined Aliskiren and ARB group (contrast estimate = 0.26, 95%CI = 0.06, 0.47, P = 0.013) and Aliskiren alone group (contrast estimate = 0.31, 95%CI = 0.10, 0.52, P = 0.004) were significantly higher than for high dose ARB (Table 2).
| Variable | Measure | Year | F | P value | |
| Change from baseline | Year1 treatment | eGFR | year 1 vs baseline1 | 0.63 | 0.530 |
| year 2 vs baseline | 0.46 | 0.632 | |||
| year 3 vs baseline | 2.16 | 0.119 | |||
| TUP | year 1 vs baseline | 6.00 | 0.003 | ||
| year 2 vs baseline | 4.16 | 0.018 | |||
| year 3 vs baseline | 0.90 | 0.409 | |||
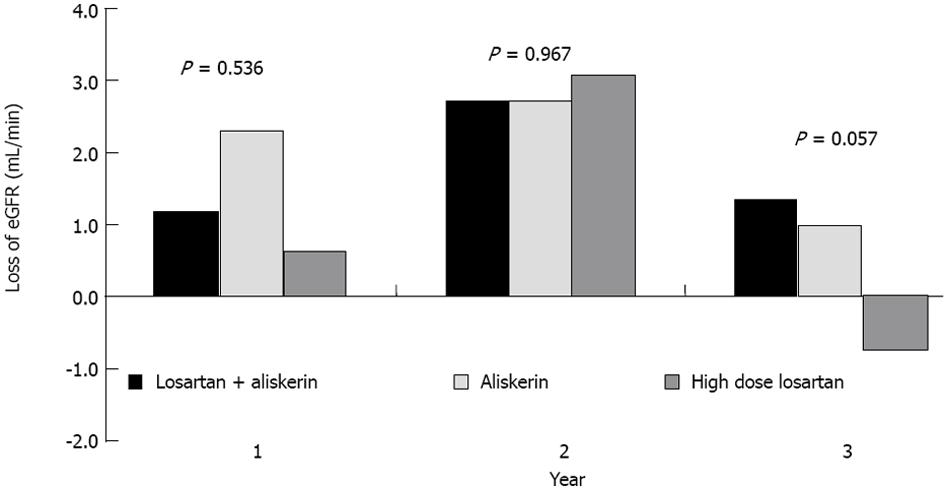
| Change from previous year | Year1 treatment | eGFR | year 1 vs baseline1 | 0.63 | 0.536 |
| year 2 vs year 1 | 0.03 | 0.967 | |||
| year 3 vs year 2 | 2.93 | 0.057 | |||
| TUP | year 1 vs baseline | 6.01 | 0.003 | ||
| year 2 vs year 1 | 0.16 | 0.851 | |||
| year 3 vs year 2 | 3.11 | 0.048 |
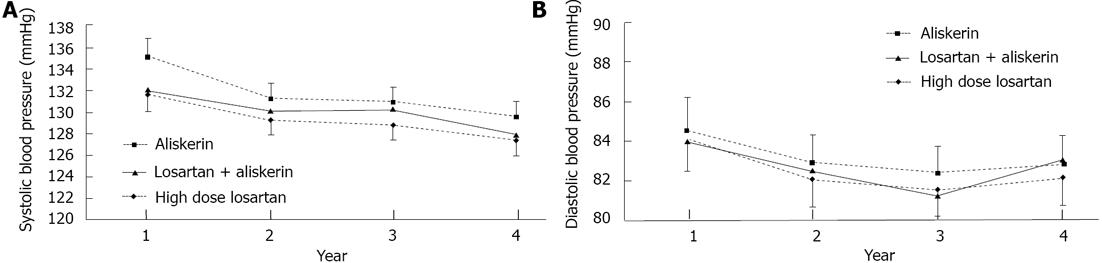
The changes in eGFR from baseline to each year were not significantly differently between the 3 therapeutic groups (Figure 1A, Table 2). For TUP, ARB high dose distinctively showed a bigger drop from baseline value, compared to the rest at years 1 and 2 (Figure 1B, Table 2). There was a trend towards improvement in eGFR from year 2 to year 3 in High dose ARB group as opposed to Combined Aliskiren and ARB group, and Aliskiren alone group which both showed a drop in eGFR (Figure 1A, P = 0.057 as seen in Table 2). This is also shown in Figure 2 which depicted the yearly loss of eGFR (loss from previous year) in the various groups. For TUP the yearly changes were significantly higher in high dose ARB group compared to Combined Aliskiren and ARB group and Aliskiren alone group (Figure 1B, Table 2). The Bp levels, Systolic and Diastolic over the 3 years for the 3 drug groups are displayed graphically in Figure 3A and 3B respectively. There were no significant differences between the 3 drug groups throughout the 3 years.
The incidence of hyperkalaemia (> 5.5 mmol/L) was 14.2% (7/49) in the combined Aliskiren and ARB group, 8.7% (4/46) in the Aliskiren alone group and 6.3% (3/48) in the high dose ARB group (P < 0.001). For hyperkalaemia ≥ 6.0 mmol/L it was 4.1% (2/49) in the Combined Aliskiren and ARB group, 0% (0/46) in the Aliskiren alone group and 2.1% (1/48) in the High dose ARB group (P < 0.07).
The above data from our study showed that among patients with non-diabetic CKD, those treated with High dose ARB tended to have higher eGFR and less proteinuria at the end of the trial compared to the other 2 treatment groups on Combined Aliskiren and ARB and those on Aliskiren alone. The decrease in eGFR in the high dose ARB group tended to be less compared to the Combined Aliskiren and ARB group and the group on Aliskiren alone respectively though this difference was not significant (P < 0.119). Multivariate analyses showed no confounding factors to account for the above differences. The Bp of all 3 drug groups showed no significant differences and did not influence any of the above data.
This retrospective study was initiated because of the recent early termination of the Aliskiren trial in type 2 diabetes using Cardio-Renal Endpoints (ALTITUDE)[11] as the results showed that there was no benefit with Aliskiren and that there were more cases of stroke, renal complications, hyperkalaemia and hypotension in patients who received Aliskiren compared with patients who received a placebo.
In view of futility of meeting the final endpoint and the substantial safety concerns through the preliminary analyses of the interim results, the Data Monitoring Committee recommended that all subjects cease treatment with Aliskiren. Additional analyses from ALTITUDE by Norvatis are ongoing[12].
To date, the Health Science Authority (HSA) of Singapore[13] has received 14 suspected adverse reaction reports associated with the use of Aliskiren, of which 4 involved CV events (1 case of hypotension, myocardial infarction and stroke and 3 cases of hypotension). HSA has recommended that Aliskiren or Aliskiren combination with ACE Inhibitors (ACEI) or ARBs should not be used in Diabetics or patients with severe renal failure (eGFR < 30 mL/min)[13].
Parving et al[14] in 2008, published the results of a double blind randomised controlled trial of Aliskiren combined with Losartan in 599 patients with type 2 Diabetes with nephropathy (AVOID Study) over a 6 mo period. Patients on the Aliskiren arm (n = 301) were prescribed a dose of Aliskiren of 150 mg/d for 3 mo and then increased to 300 mg/d for the next 3 mo in combination with Losartan 100 mg/d. There were 298 patients in the placebo arm. The primary outcome was a reduction in the ratio of albumin to creatinine, measured in an early morning urine sample at 6 mo. The results of the study showed that the decline in eGFR was the same in the treatment and placebo group but the decline in the treatment group tended to be less than in the placebo group at 6 mo. The reduction of albuminuria by 50% occurred twice as often in the treatment group compared to the placebo group. The authors concluded that Aliskiren appeared to have a renoprotective effect independent of its Bp lowering effect in patients with type 2 diabetes who were receiving maximal renoprotective treatment and optimal antihypertensive therapy. Hyperkalamia, based on a single measurement of serum potassium > 5.5 mmol/L was more frequent in the Aliskiren treated group (41/301 or 13.7%) compared with the placebo group (32/298 or 10.8%) (P = 0.07). Severe hyperkalaemia (serum potassium > 6.0 mmol/L) occurred in 14 patients in the Aliskiren treated group (4.7%) compared to 5 placebo treated patients (1.7%) (P = 0.113). Symptoms of hypotension were not a frequent adverse event, with no difference in the 2 groups. Parving et al[14] in a post hoc analysis of Parving’s AVOID trial[14] concluded that Aliskiren added to Losartan reduced albuminuria and renal dysfunction and was well tolerated, except for hyperkalaemia in stage 3 CKD patients, independent of baseline CKD stage in patients with type 2 diabetes, hypertension and nephropathy[15].
In an open labelled pilot study by Tang et al[16] in 25 consecutive patients where Aliskiren (300 mg/d) was prescribed despite being on maximum ARB therapy with Losartan (100 mg/d) for 3 mo in patients with IgA nephropathy (stage 3 CKD with proteinuria > 1 mg/d) over a 12 mo period, there was a 22% reduction in proteinuria at 6 mo and 26% reduction at 12 mo. This was associated with significant reductions in plasma renin activity, serum interlukin-6 and transforming growth factor β levels compared to baseline levels. Two patients developed mild allergic reactions and 6 (24%) patients had transient hyperkalaemia (serum K+ > 5.5 mmol/L). The authors concluded that Aliskiren conferred an antiproteinuric effect in patients with IgA nephropathy with significant residual proteinuria, despite receiving the recommended renoprotective treatment.
In a systemic review and meta-analyses of aliskiren and angiotensin receptor blockers in the management of essential hypertension, 7 randomised controlled trials, duration of follow up for at least 4 wk by Zheng et al[17] , no differences were found between the two groups.
The trials of Uresin et al[18] and that of Oparil et al[19] , gave no indication regarding the renoprotective efficacy of Aliskiren, the initial trials of Aliskiren involved patients with hypertension, being first developed as an antihypertensive agent. They were short trials in hypertensive patients[20,21] lasting 8 wk and did not address the question of renoprotection.
The ALTITUDE study was able to unmask serious adverse events like ischemic heart disease and strokes because it had included Cardio-Renal Endpoints among its primary end points.
It would be advisable to require future trials on drugs which could impact on the kidneys, heart and brain to have similar Cardio-Renal Endpoints or Cardio-Neuro- Renal End points to further ensure therapeutic safety of the trial drug.
In conclusion, our present study in 143 patients with CKD over 3 years showed that the use of Combination therapy of Aliskiren with ARB Losartan or Aliskiren alone were efficacious as an antiproteinuric drug when compared to High dose Losartan. But like Parving[14], our study showed that the incidence of hyperkalaemia (> 5.5 mmol/L) was 14.2% in the Combined Aliskiren and ARB group, 8.7% in the Aliskiren alone group and 6.3% in the High dose ARB group (P < 0.001).
The problem of hyperkalaemia was 36.9% in the Aliskiren group versus 27.1% in the placebo group in the ALTITUDE study. Parving’s AVOID study[14] showed that patients on combined Aliskiren and ARB had 13.7% with Hyperkalaemia > 5.5 mmol/L compared to 10.8% for Placebo group and 4.7% and 1.7% respectively for serum K+≥ 6mmol/L (P < 0.113). Like Parving[14], all our patients had stage 3 CKD but we were only using 150 mg/d of Alisikiren compared to 300mg/d in Parving’s study[14].
Our modest study compared to the magnitude of the ALTITUDE study still managed to detect the problem of hyperkalaemia in the group treated with Combination therapy with Aliskiren and ARB like those of Parving[14] and Tang’s[16]. Based on our study it would appear that the findings of the ALTITUDE study would also apply to non-diabetic CKD patients.
All the authors declared no competing interests. The authors would like to acknowledge M/s Irene Ow, M/s Tan Hwee Boon and M/s Chin Yok Mooi for Administrative and other support.
For the future, Aldosterone blockade and the mineralocorticoid receptor antagonist would probably be the new emerging therapy in the management of Chronic Kidney Disease (CKD). Hitherto, the major therapeutic intervention to delay progression of CKD and the risk of endstagerenaldisease has been the use of Angiotensin-Converting Enzyme Inhibitors/Angiotensin receptor Blockers and more recently the use of direct renin inhibitors. But in The authors’ opinion, the release of the Trial in Type 2 Diabetes using Cardio-Renal Endpoints report may have effectively tolled the bell for the use of Combination therapy involving Aliskiren as renin inhibitors for now.
The authors have used Aliskiren in a dose of 150 mg/d for the treatment of CKD patients with proteinuria. The maximum therapeutic dose is 300 mg/d. Perhaps, if the authors had used Aliskiren in a dose of 300 mg/d, the effects would have been better and comparable to high dose Angiotensin receptor blockers (ARB) therapy. However, the authors would have to contend with the major side effect of hyperkalaemia which is a disadvantage of combination therapy of Aliskiren and ARB.
Treatment of Patients with CKD with proteinuria. The authors believe that Aliskiren, by itself is still an effective and innovative therapy for treatment of hypertension. It was first introduced as an anti- hypertensive drug and still is widely used as such.
Aliskiren, a direct renin inhibitor, hypertensive drug. Also renoprotective as it reduces proteinuria, but major side effect of hyperkalaemia.
This retrospective study has its limitations. It is not a randomised controlled trial and though the statistics show that it is adequately powered, the number of patients entered in the study are small. The dose of Aliskiren employed in most studies is 300 mg/d. Here the investigators have chosen a dose of 150 mg/d. Perhaps with a dose of 300 mg/d, Aliskiren may prove to be more efficacious.
P- Reviewer: Hu B S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Strippoli GF, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15:411-419. [PubMed] [Cited in This Article: ] |
| 2. | Chen Y, Schieppati A, Cai G, Chen X, Zamora J, Giuliano GA, Braun N, Perna A. Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials. Clin J Am Soc Nephrol. 2013;8:787-796. [PubMed] [Cited in This Article: ] |
| 3. | Kamilic J, Hamming I, Lely AT, Korstanje R, Schulze U, Poppinga WJ, Turner AJ, Clarke NE, van Goor H, Navis GJ. Rat Ace allele variation determines susceptibility to AngII-induced renal damage. J Renin Angiotensin Aldosterone Syst. 2011;12:420-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Wiggins KJ, Kelly DJ. Aliskiren: a novel renoprotective agent or simply an alternative to ACE inhibitors? Kidney Int. 2009;76:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Rahuel J, Rasetti V, Maibaum J, Rüeger H, Göschke R, Cohen NC, Stutz S, Cumin F, Fuhrer W, Wood JM. Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human rennin. Chem Biol. 2000;7:493-504. [PubMed] [Cited in This Article: ] |
| 7. | Woo KT, Chan CM, Tan HK, Choong HL, Foo M, Vathsala A, Lee EJ, Tan CC, Lee GS, Tan SH. Beneficial effects of high-dose losartan in IgA nephritis. Clin Nephrol. 2009;71:617-624. [PubMed] [Cited in This Article: ] |
| 8. | Luke RG. Hypertensive nephrosclerosis. Kidney Int. 2006;70:1383; author reply 1383-1384. [PubMed] [Cited in This Article: ] |
| 9. | Busch M, Franke S, Wolf G, Rohde RD, Stein G. Serum levels of the advanced glycation end products Nepsilon-carboxymethyllysine and pentosidine are not influenced by treatment with the angiotensin receptor II type 1 blocker irbesartan in patients with type 2 diabetic nephropathy and hypertension. Nephron Clin Pract. 2008;108:c291-c297. [PubMed] [Cited in This Article: ] |
| 10. | Botev R, Mallié JP, Couchoud C, Schück O, Fauvel JP, Wetzels JF, Lee N, De Santo NG, Cirillo M. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4:899-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Early termination of aliskiren study due to adverse events, 2012 Apr. Available from: http://www.hsa.gov.sg/publish/hsaportal/en/health_products_regulation/safety_information/product_safety_alerts/Safety_Alerts_2012/early_termination.html. [Cited in This Article: ] |
| 12. | Data Monitoring Committee’s recommendation letter for ALTITUDE, 2011 Dec. Available from: http: //hc-gc.caldhp-mps/medeeff/advisories-avis/prof/_2012/rasilez_hpc-cps-eng.php. [Cited in This Article: ] |
| 13. | Health Sciences Authority (HAS), Adverse Drug Reaction News, 2012 Apr. Available from: http: //www.hsa.gov.sg. [Cited in This Article: ] |
| 14. | Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433-2446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 716] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 15. | Motin VG, Iasnetsov VV. [Effect of synthetic analogs of enkephalins, morphine and their antagonists on the course of experimental traumatic shock]. Farmakol Toksikol. 1986;49:103-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Tang SC, Lin M, Tam S, Au WS, Ma MK, Yap DY, Ho YW, Lai KN. Aliskiren combined with losartan in immunoglobulin A nephropathy: an open-labeled pilot study. Nephrol Dial Transplant. 2012;27:613-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Zheng Z, Shi H, Jia J, Li D, Lin S. A systematic review and meta-analysis of candesartan and losartan in the management of essential hypertension. J Renin Angiotensin Aldosterone Syst. 2011;12:365-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Uresin Y, Taylor AA, Kilo C, Tschöpe D, Santonastaso M, Ibram G, Fang H, Satlin A. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. J Renin Angiotensin Aldosterone Syst. 2007;8:190-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 20. | Gao D, Ning N, Niu X, Wei J, Sun P, Hao G. Aliskiren vs. angiotensin receptor blockers in hypertension: meta-analysis of randomized controlled trials. Am J Hypertens. 2011;24:613-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Zhu JR, Sun NL, Yang K, Hu J, Xu G, Hong H, Wang R, Tu YM, Ritter S, Keefe D. Efficacy and safety of aliskiren, a direct renin inhibitor, compared with ramipril in Asian patients with mild to moderate hypertension. Hypertens Res. 2012;35:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |