Published online Jul 25, 2022. doi: 10.5527/wjn.v11.i4.115
Peer-review started: April 4, 2022
First decision: June 15, 2022
Revised: June 24, 2022
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: July 25, 2022
Processing time: 106 Days and 13.6 Hours
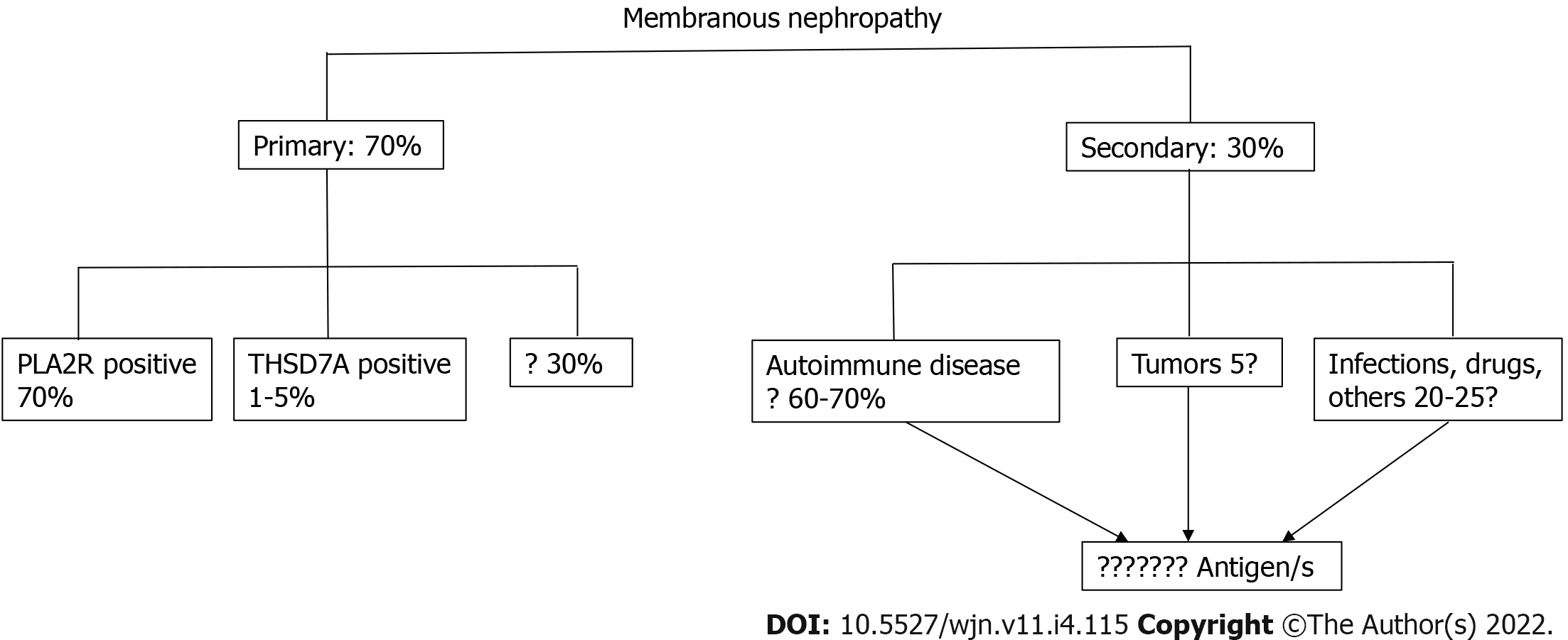
When the physiopathology of membranous nephropathy was first described, almost 30% of cases were recognized to be secondary to well-known diseases such as autoimmune diseases, tumors or infections. The remaining 70% cases were called primary membranous nephropathy as the exact mechanism or pathogenic factor involved was unknown. The discovery of the M type phospholipase A2 receptor and thrombospondin type 1 domain containing 7A as causative antigens in these “so called” primary membranous nephropathies provided new insights into the effective causes of a large proportion of these cases. Novel techniques such as laser microdissection and tandem mass spectrometry as well as immunochemistry with antibodies directed against novel proteins allowed the confirmation of new involved antigens. Finally, using confocal microscopy to localize these new antigens and immunoglobulin G and Western blot analysis of serum samples, these new antigens were detected on the glomerular membrane, and the related antibodies were detected in serum samples. The same antigens have been recognized in some cases of secondary membranous disease due to autoimmune diseases, tumors and infections. This has allowed examination of the relationship between antigens in primary membranous nephropathy and their presence in some secondary nephropathies. The aim of this study is to describe the characteristics of the new antigens discovered and their association with other diseases.
Core Tip: The pathophysiological mechanisms of membranous nephropathy have been partially known for a long time. Novel techniques have allowed identifying several antigens and the corresponding antibodies as the main cause of a large part of these diseases. Therefore, a large part of membranous nephropathy, once called primary, are due to immune complexes whose components are now recognized. The same antigens have been recognized in a part of secondary membranous disease, which are due to autoimmune diseases, tumors and infections, diseases. This fact allows a relationship between antigens found either in primary membranous nephropathy or in some forms of secondary nephropathies.
- Citation: Salvadori M, Tsalouchos A. New antigens involved in membranous nephropathy beyond phospholipase A2 receptor. World J Nephrol 2022; 11(4): 115-126
- URL: https://www.wjgnet.com/2220-6124/full/v11/i4/115.htm
- DOI: https://dx.doi.org/10.5527/wjn.v11.i4.115
Membranous nephropathy (MN) is a rare disease that affects podocytes and is characterized by the accumulation of immune deposits on the subepithelial side of the glomerular capillary wall. These immune deposits consist of immunoglobulin (Ig) G directed against antigens that have long remained unknown. MN is referred to as primary MN when there is no association with a known disease (70% of cases) or secondary MN when MN occurs in association with clinical conditions such as autoimmune diseases, tumors, infections and hepatitis B (30% of cases). Two studies in 2009 and 2014 allowed us to identify causal antigens involved in primary MN[1,2]. A related study reported the first human podocyte antigen in a rare subset of infants born with MN that developed because the mother was deficient in neutral endopeptidase (NEP) due to a truncating mutation in the MME gene coding for NEP[3,4]. The first antigen, identified in 2009, is the M type phospholipase A2 receptor 1. The antigen recognized in the 2014 study is thrombospondin type 1 domain containing 7A (THSD7A)[1,2]. Using Western blotting and mass spectrometry, THSD7A was identified in serum samples from patients with MN. Additionally, immunohistochemical analysis of biopsy samples from the same patients revealed that THSD7A localized to podocytes, and immunoglobulin G (IgG) eluted from these samples was specific for THSD7A.
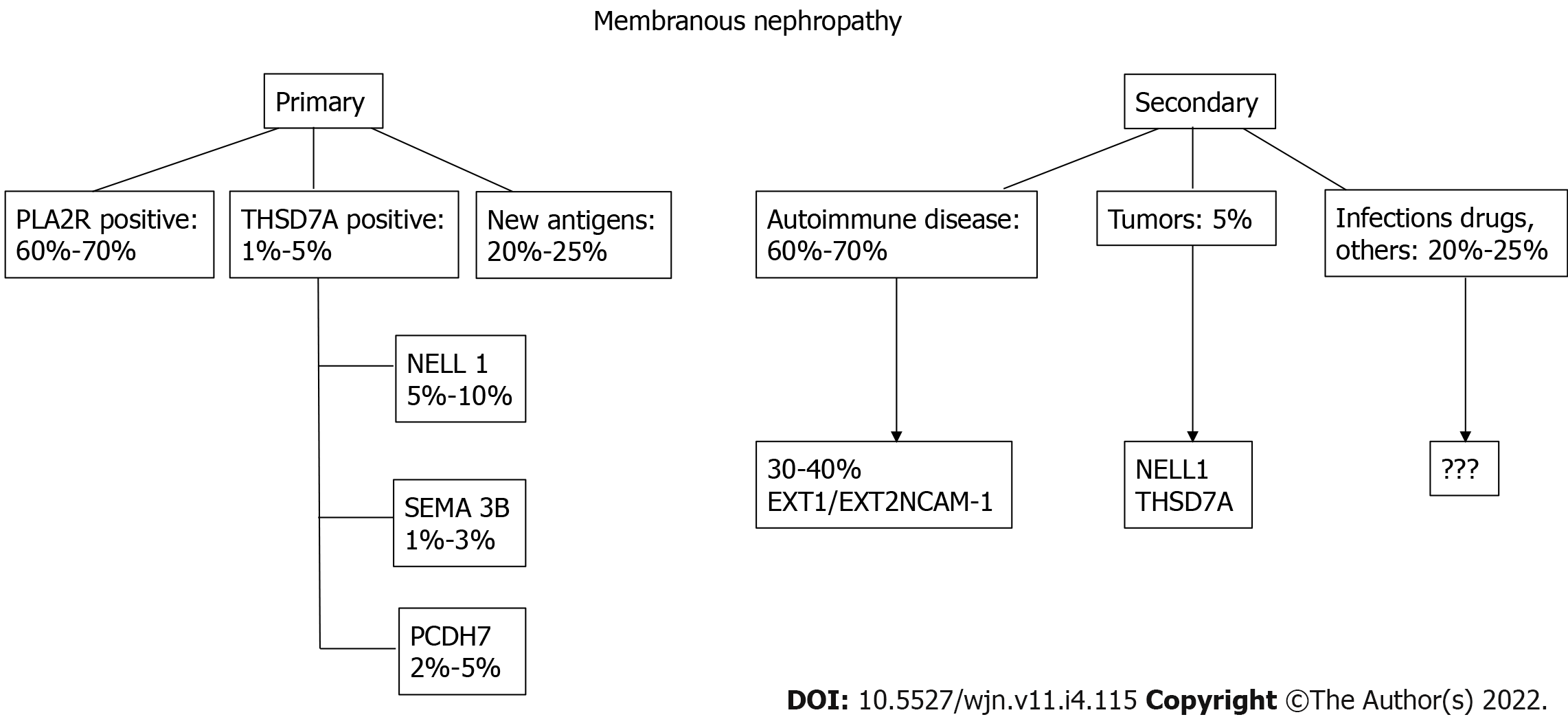
Phospholipase A2 receptor (PLA2R) and THSD7A are involved in 70% and 5% of primary MN cases respectively (Figure 1).
These antigens were thought to be specific to primary MN, but were also later found in patients with MN related to hepatitis B infection and sarcoidosis[5-7].
Recently, an approach using laser microdissection and tandem mass spectrometry (MS/MS) enabled the detection of novel proteins in glomerular diseases. MS/MS can identify approximately 1500-2000 proteins in glomerular extracts and allows semiquantitative measurements.
Briefly, these techniques were used to identify proteins with high spectral counts in PLA2R-negative MN patients and control patients with different nephropathies. This new protein was identified, using immunochemistry with antibodies directed against the new protein, which revealed membranous staining along the glomerular basement membrane (GBM), confirming a new antigen involved in MN.
Finally, using confocal microscopy to localize the new antigen and IgG and Western blot analysis of serum samples, we detected the new antigen on the glomerular membrane and the related antibody in serum samples[8].
The aim of this study is to describe the characteristics of the new antigens discovered principally thanks to these novel techniques and to clarify their association with other diseases.
An examination of both serum samples and glomerular eluates from patients with the so- called idiopathic MN negative for PLA2R with these new techniques revealed the first novel antigens, namely exostosin 1 and exostosin 2 (EXT1 and EXT2, respectively).
EXT1/EXT2-positive MN cases were more common in females (80.9 with a mean age of 35.7 years)[8]. In the first report, the mean serum creatinine and proteinuria levels at presentation were 1 mg% and 5.9 g/24 h, respectively. A total of 70.8% of patients had abnormal laboratory values for antinuclear antibodies, double-stranded DNA antibodies, anti-Smith antibodies or anti-Sjogren syndrome-related antigen A or B[9]. Thirty-four percent of patients had a clinical diagnosis of systemic lupus erythematosus.
Based on MS analysis, all four classes of IgG were present in patients with EXT1/EXT2-positive MN with IgG1 the most abundant, followed by IgG2, IgG3 and IgG4. In addition to IgG and C3, 84% of patients exhibited staining for IgA or IgM. Seventy-three percent of patients showed staining for C1q on immunofluorescence, and all patients showed subepithelial deposits. Mesangial deposits were also present in 96% of patients. Subendothelial deposits were less frequently found.
Tubulointerstitial inclusions were present in 80% of patients.
The GBM is composed of mostly type IV collagen, laminin, nidogen and heparan sulfate proteoglycans. Agrin and perlecan are the main heparan sulfate proteoglycans in GBM. Heparan sulfate proteoglycans are present in the basement membrane and matrix and on cell surfaces.
EXTs are glycosyltransferases responsible for the synthesis of heparansulfate, through the addition of glycosaminoglycan residues to the core protein. The result is the generation of complex polysaccharides, which explains why these two proteins are found together[10,11].
EXT1 and EXT2 show structural similarities, and EXT1 and EXT2 can exist as a heterodimers and act as a copolymerases in heparan sulfate chain elongation. The EXT1/EXT2 heterodimer also has increased stability and activity compared to those of the individual proteins, which are transmembrane proteins.
EXTs are secreted into the extracellular medium in a truncated form[12]. Five genes encode EXT proteins: EXT1, EXT2, EXTL1, EXTL2 and EXTL3[13]. Mutations in EXT1 and EXT2 generate hereditary multiple exostoses, one of the most common inherited skeletal disorders[14].
To date, it is still difficult to detect circulating anti-EXT1/EXT2 antibodies. This difficulty may be because serum antibodies target truncated EXT proteins or are present at a very low titer.
In a recent study, EXT1/EXT2 were present in 33% of a cohort of patients with membranous lupus nephritis[15]. Compared with EXT1/EXT2-negative membranous lupus nephritis, EXT1/EXT2-positive disease appears to represent a subgroup with favorable kidney biopsy findings with respect to chronicity indices. Indeed, cases of membranous lupus nephritis that are EXT1/EXT2 negative are more likely to progress to end-stage kidney disease (ESKD) than those that are EXT1/EXT2 positive.
In conclusion, using proteomics and immunochemistry, the authors found EXT1/EXT2 in the GBM of PLA2R-negative MN patients. Clinical and biopsy findings showed features of autoimmune disease, including lupus nephritis in 8% of patients[16].
Neural cell adhesion molecule 1 (NCAM1) is a member of the IgG superfamily of proteins that was identified using the techniques described above[17]. NCAM1 colocalizes with IgG within glomerular immune deposits, and antibodies against NCAM1 could be detected in patient sera. NCAM1 was predominantly expressed in membranous lupus nephritis patients but was also found in 2% of primary MN patients. Many lupus nephritis patients with NCAM1 were also positive for EXT2. NCAM patients were also positive for IgA, IgM and C1q. Neuropsychiatric disease occurred in 40% of NCAM-positive patients, probably due to NCAM1 expression in the central nervous system[18].
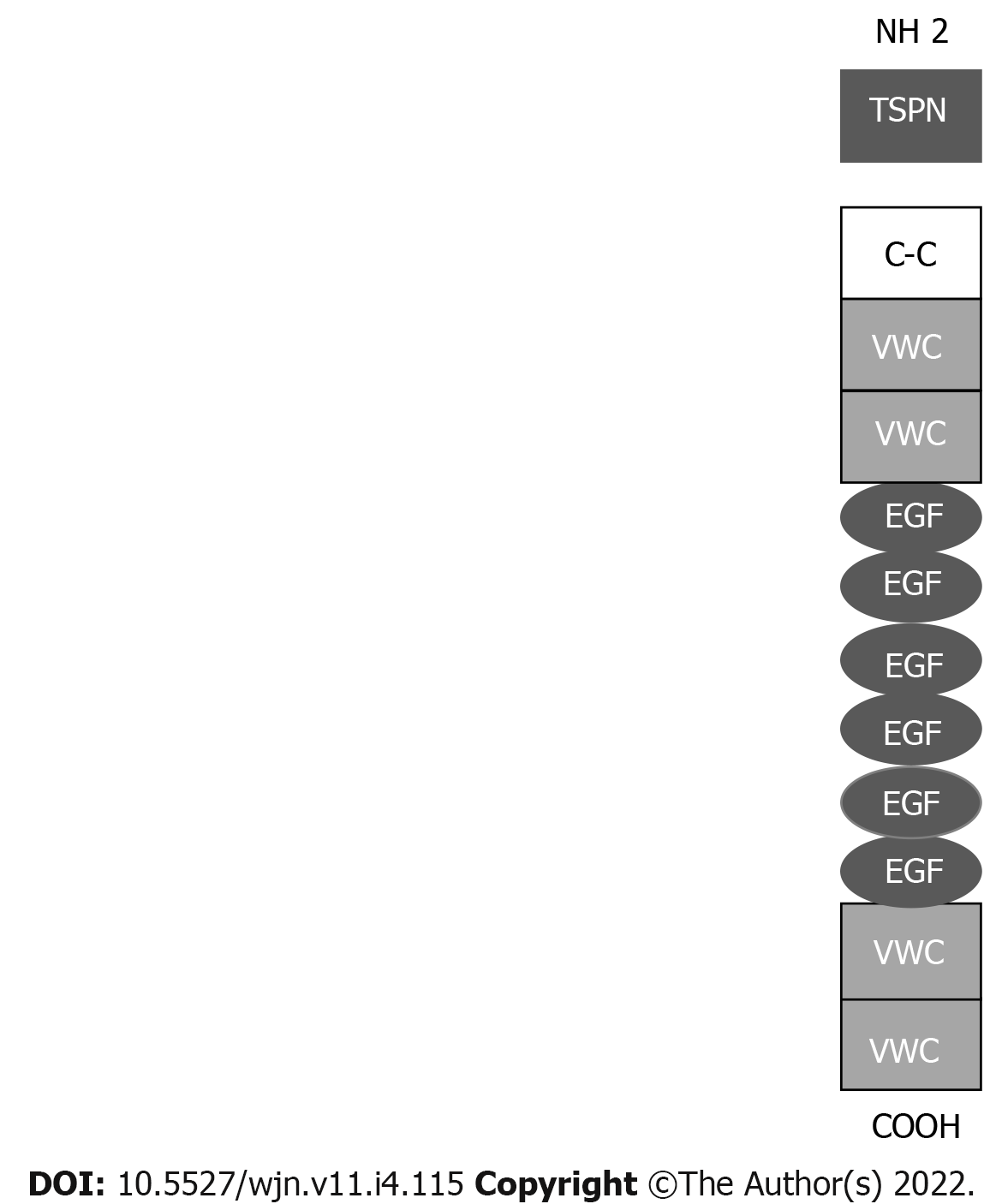
Neural epidermal growth factor-like 1 protein (NELL-1) is a secreted, 90-kDa protein expressed in osteoblasts that promotes bone regeneration[19]. The NELL-1 gene is named after its similarity to a gene called Nel that is strongly expressed in neural tissue and encodes a protein with epidermal growth factor (EGF)-like repeats (Figure 2)[20].
In the kidney, NELL-1 expression is increased in tubules and detectable in the glomeruli, as 20% of glomerular cells express NELL-1 at the RNA level[21,22].
Sethi et al[23] suggested that NELL-1 is associated with MN.
The authors selected PLA2R-negative MN patients and identified this novel NELL-1 protein by laser microdissection and MS. Granular anti -NELL-1 GBM staining was documented using immunohistochemistry, and NELL-1 and IgG colocalization was observed by confocal microscopy. Finally, serum antibodies against NELL-1 were detected by Western blotting. Sethi et al[23] concluded that NELL-1 positive MN is a distinct type of MN. The authors suggested that NELL-1 is shed from podocytes rather than entrapped from circulating antigens or immune complexes[24].
Most importantly, Sethi’s finding was confirmed by validation in a French cohort and a Belgian cohort.
Kidney biopsy specimens from patients with NELL-1-associated MN showed features of MN with a thickened GBM as well as IgG and C3 expression. IgG subtyping revealed predominantly IgG1. In a subset of the biopsy specimens, a segmental GBM distribution of immune deposits was observed by immunofluorescence and electron microscopy[25].
In particular, Kudose et al[25] examined 2003 MN patients without lupus. Fifty of them showed segmental MGN (sMGN) defined by subepithelial deposits involving 25%-75% of the GBM. Among these cases with sMGNs, NELL-1 staining was present in 25%. PLA2R, THSD7A and EXT 1 were negative in all cases evaluated.
Among 21 patients with sMGN at follow-up, 7/21 patients had received immunosuppression, 86% had stable improved renal function, and 60% had complete (45%) or partial (15%) remission of proteinuria. Accordingly, MGN is a rare PLA2R-negative variant of MN with NELL-1 positivity in 29% of patients and favorable prognosis, even in the absence of immunosuppressive treatment.
According to this study, NELL-1 appears to be the first antigen in segmental MN.
In a recent study, Caza et al[26] found that NELL-1 is a target antigen associated with MN malignancy. They found active or metastatic malignancy in 33% of patients with NELL-1-associated MN. Additionally, they found NELL-1 positivity within glomeruli and a tumor from the same patient affected by invasive ductal carcinoma of MN in the breast.
The authors concluded that NELL-1, a recently identified antigen in MN, is enriched in patients with malignancy-associated MN, and anti-NELL-1 antibodies can be detected within the serum of these patients.
Finally, a recent study by Spain et al[27] found that in patients administered lipoic acid for different conditions, high-grade proteinuria could appear. These patients may have NELL-1-associated MN, and the discontinuation of lipoic acid could result in proteinuria remission.
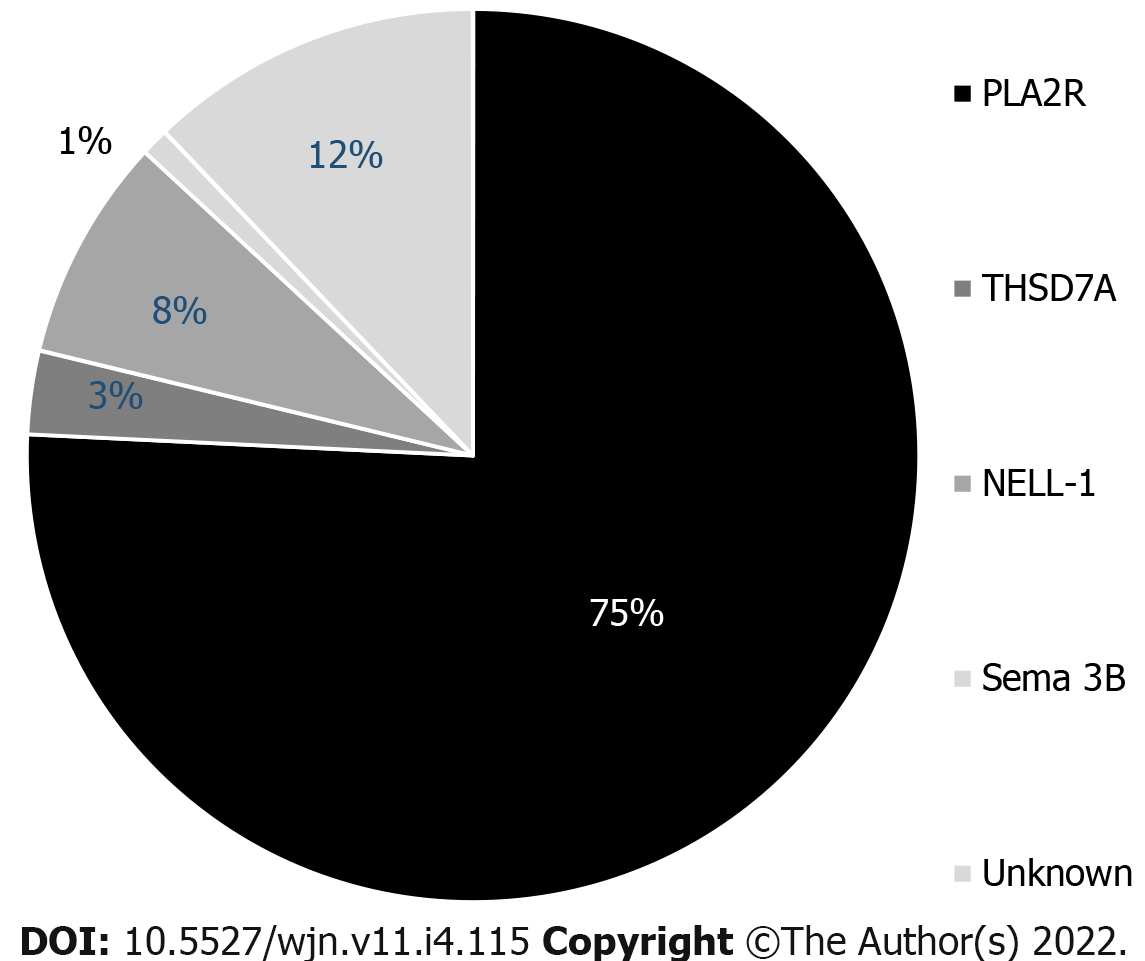
Semaphorin 3B (SEMA 3B) is a recently discovered target antigen that has been principally detected in pediatric patients, particularly very young children[8]. The mean age of these pediatric patients was 6.9 years, and approximately half of SEMA 3B-associated MN was detected in children < 2 years. Among all patients, SEMA 3B-associated MN is rare and accounts for 1%-3% of all MNs. In the pediatric group, it accounts for approximately 15% of MN cases.
After the initial identification of three pediatric patients, an additional eight cases of SEMA 3B-associated MN were identified in validation cohorts from France and Italy[8]. To date, 11 patients with SEMA 3B-associated MN, including three adults have been identified[28].
Using laser dissection and MS/MS, SEMA3B was detected in PLA2R-negative MN biopsies[29].
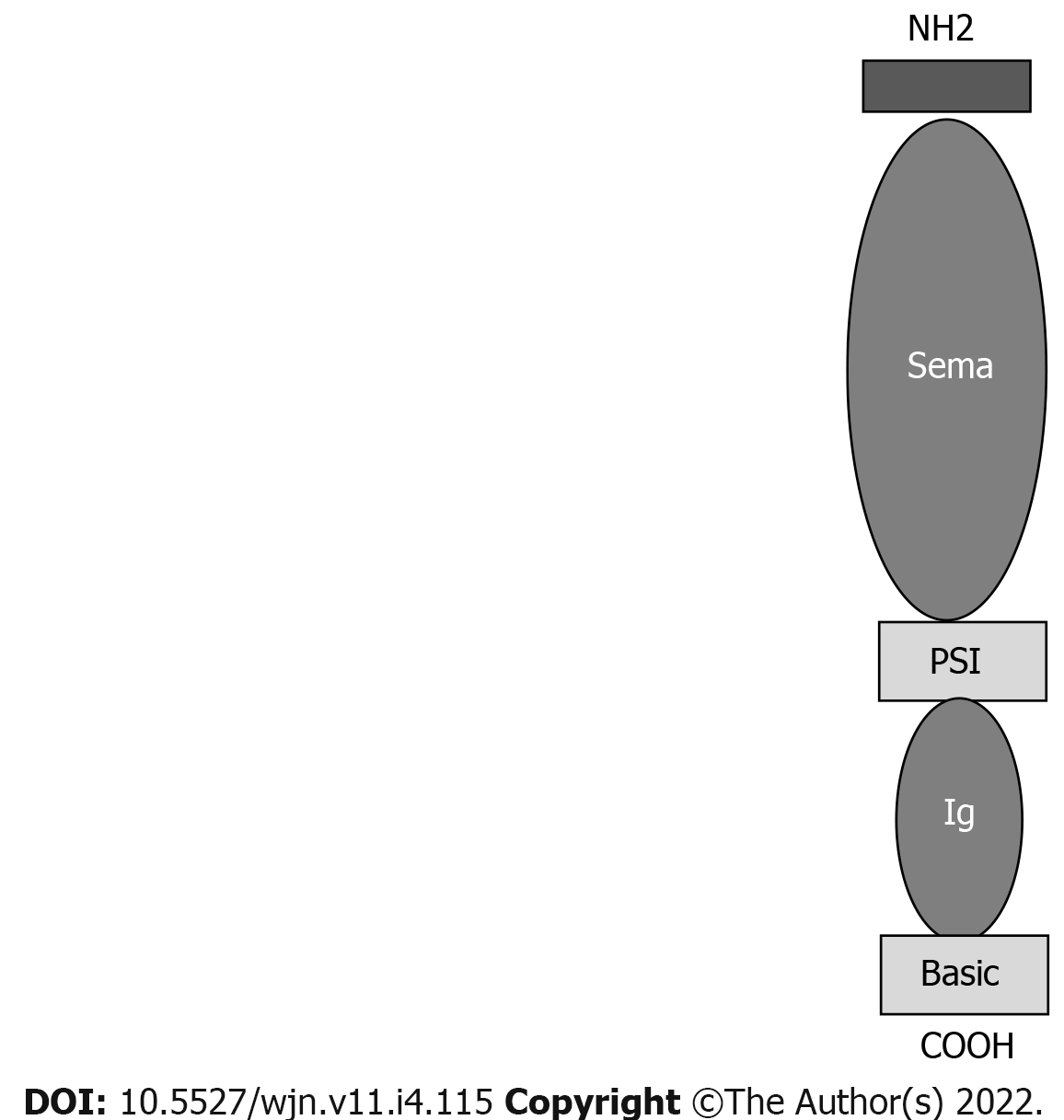
Semaphorins are a group of secreted and transmembrane/membrane-bound proteins containing a conserved extracellular semaphorin (sema) domain of approximately 500 amino acids that is characterized by highly conserved cysteine residues[30-32].
More than 20 semaphorins have been identified and divided into 8 subclasses. SEMA 3B is a secreted protein with a sema domain, a plexum-semaphorin-integrin domain, an Ig domain and a basic domain (Figure 3).
The semaphorin 3 family and its receptors have been detected in endothelial cells, podocytes and tubular epithelial cells[33]. In SEMA 3B-associated MN, bright granular capillary wall staining for SEMA 3B along the GBM have been documented, and SEMA 3B may be found using immunofluorescence microscopy. Confocal immune fluorescence microscopy analysis has shown the colocalization of SEMA 3B and IgG in glomerular immune deposits.
Using Western blot analysis, anti-SEMA 3B antibodies have been detected in patients with SEMA 3B-associated MN. The SEMA 3B autoantibody can recognize a cryptic epitope that is unmasked by disruption of disulfide bonds.
Clinical findings in SEMA 3B-associated MN are shown in Table 1, and proteinuria remission may be obtained either spontaneously or with immunosuppressive treatment.
| Case | Age (yr)/sex | UP (g/24) | Serum CR (mg/dL) | Remission | Serum CR/UP/24 h |
| 1 | 41/F | 7.9 | 0.74 | Spontaneous | 0.6/no proteinuria (16 mo) |
| 2 | 26/F | 6.2 | 0.4 | Spontaneous | 0.43/400 mg (18 mo) |
| 3 | 2/M | 5 | 0.5 | Immunosuppressive | 0.35/150 mg (24 mo) |
| 4 | 40/F | 17.3 | 0.9 | Immunosuppressive | 0.6/no proteinuria (10 yr) |
| 5 | 19 mo/M | 0.4 | 0.7 | Immunosuppressive | 0.9/400 mg (18 mo) |
| 6 | 2/F | UP/CR ratio 6.81 | 0.21 | Immunosuppressive | UP/CR ratio 0.23 (13 mo) |
| 7 | 17/M | UP/CR ratio 0.78 | 0.6 | Immunosuppressive | UP/CR ratio 0.1 (19 mo) |
| 8 | 9 mo/M | UP/CR ratio 0.94 | 0.45 | Immunosuppressive | UP/CR ratio 0.09 /14 yr) |
| 9 | 2/M | UP/CR ratio 1.95 | 0.13 | Immunosuppressive | UP/CR ratio 0.12 (5 yr) |
| 10 | 14/M | 3 | 0.64 | Lost to follow up | n/a |
| 11 | 16/M | 12 | 0.83 | Immunosuppressive | Dialysis |
Recurrence of anti-SEMA 3B-mediated MN after kidney transplantation was recently reported[34]. Kidney biopsy confirmed histological MN recurrence with the colocalization of SEMA 3B antigen and IgG. Treatment with rituximab was effective, and the disappearance of anti-SEMA 3B antibodies was noted 40 days after rituximab treatment.
Given the discovery of the antigen SEMA 3B, the distribution of podocyte antigens in patients with “primary” MN is presented in Figure 4.
Chauhan et al[35] performed laser microdissection and MS/MS in kidney biopsies from patients with PLA2R-negative MN and detected a unique protein, namely, protocadherin 7 (PCDH7) in glomeruli from 10 patients who were also negative for THSDF7A, EXT1/EXT2, NELL 1 and SEMA 3B. Additionally, in a validation cohort from the UCLouvain Kidney Disease Network in Belgium, four additional patients were identified[36]. In all patients, immunohistochemistry showed bright granular staining along the GBM. Confocal microscopy showed colocalization of PCDH7 and IgG along the GBM, and Western blot analysis using sera revealed antibodies against PCDH7.
Cadherins are a large group of transmembrane proteins that mediate cell-cell recognition and adhesion[37]. Cadherins are classified into subfamilies on the basis of the number and arrangement of extracellular cadherin (EC) domains. Therefore, cadherins are subdivided into classic cadherins, closely related cadherins, desmosomal cadherins and protocadherins[38]. PCDH7 is a 16-kDa glycosylated protocadherin with seven EC repeats. Its function is unknown, but it is likely important in cell signaling[39]. PCDH7 is mostly present in older patients. Complement activation is minimal in these patients, and spontaneous remission frequently occurs without immunosuppressive treatment. In addition, the MS/MS complement profile of PCDH7-associated MN is lower than that of other antigen-associated MNs[40]. The clinical and pathologic findings in PCDH7-associated MN are described in Table 2.
| Patient | Age (yr) | Urinary protein (g/24h) | Serum creatinine (mg/dL) | Immunofluorescence | IgG |
| 1 | 73 | 3.2 | 1.1 | IgG 3+; C1q 1+ | IgG1 2+; IgG3 1+; IgG4 1+ |
| 2 | 66 | 9.6 | 1.3 | IgG 2+ | IgG1 1+; IgG4 2+ |
| 3 | 68 | NA | 1.1 | IgG 2+ | IgG4 2+ |
| 4 | 59 | 3 | 1.1 | IgG 2+; C3 1+ | 1gG 1+; IgG2 2+; IgG3 2+; IgG4 3+ |
| 5 | 61 | 7 | 1.9 | IgG 3+; C3 1+ | IgG1 2+; IgG3 2+ |
| 6 | 38 | 3 | 1 | IgG 1+ | NA |
| 7 | 37 | 1.4 | 1.76 | IgG3 + | IgG4 1+ |
| 8 | 67 | 4.3 | 1.2 | IgG 3+ | NA |
| 9 | 75 | 7 | 1 | IgG 3+; C3 1+ | NA |
| 10 | 70 | 1 | 1.6 | IgG 3+; C3 1+ | NA |
| 11 | 64 | 8.4 | 1.2 | IgG 3+; C3 3+ | IgG3 2+; IgG2 2+ |
| 12 | 61 | 3.9 | 1 | IgG 3+ | IgG4 2+; IgG2 1+ |
| 13 | 66 | 23.3 | 3.8 | IgG 3+; IgA 2+ | IgG4 2+; IgG2 2+ |
| 14 | 72 | 21 | 1.3 | IgG 1+ | NA |
In conclusion, PCDH7 has been identified as a novel antigen along with circulating anti- PCDH7 autoantibodies, in a subset of adult patients with PLA2R-negative MN.
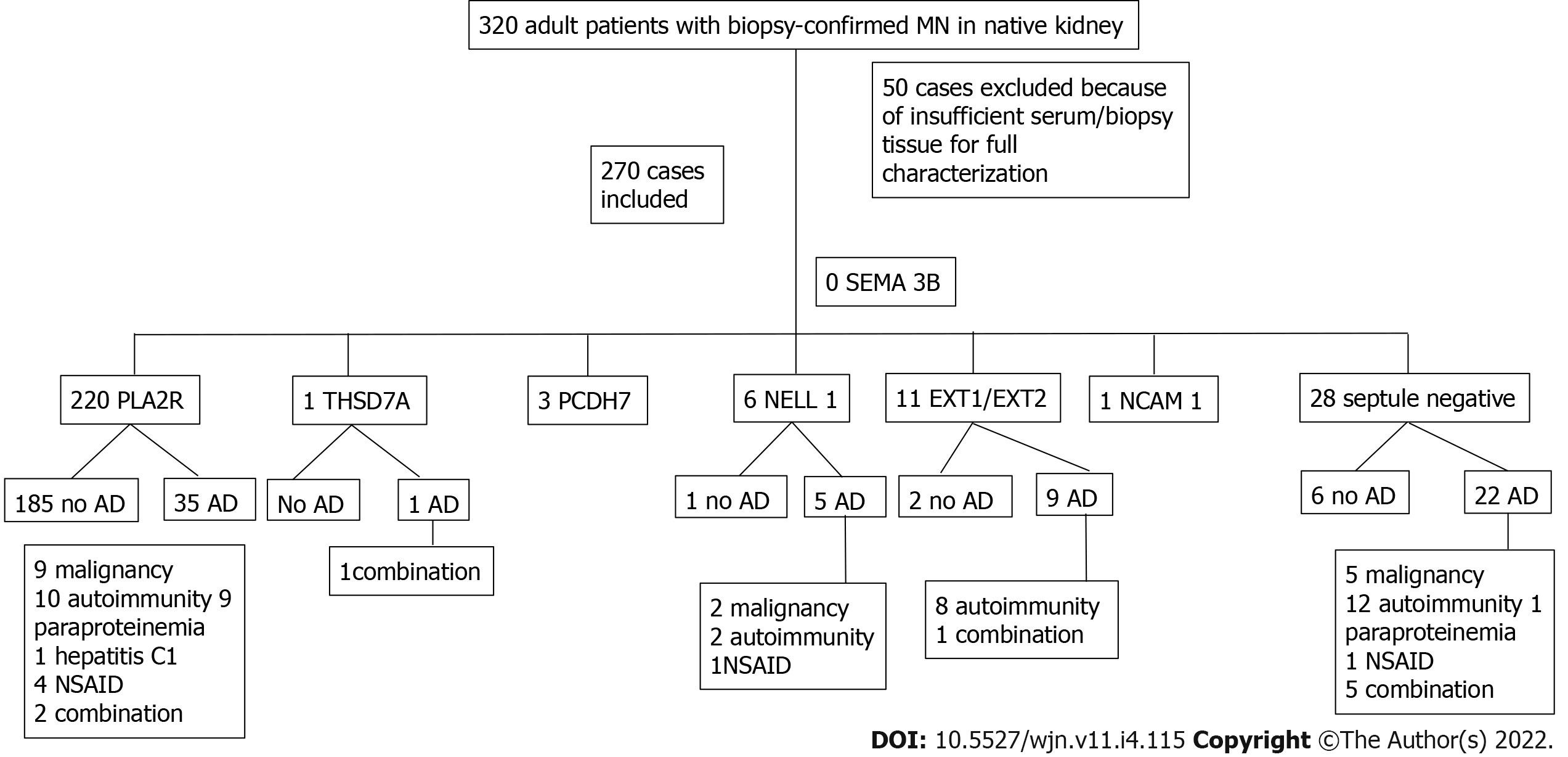
Interestingly, Bobart et al[41] analyzed a total of 320 adult patients with MN in native kidneys between 2015 and 2020. Overall, they found three patients with PCDH7-associated MN. This study is interesting as the authors presented a patient distribution based on the antigen found and the presence of associated diseases (Figure 5). Similarly, the authors presented a table reporting the demographic and clinical characteristics based on the antigen involved (Table 3).
| Total 270 | PLA2R 220 | EXT1/EXT2 11 | NELL 1 6 | PCDH7 3 | THSD7A 1 | NCAM-1 1 | Septule negative 28 |
| Age | 54.0 (43.2-61.0) | 40.0 (25.0-48.0) | 57.0 (36.7-66.5) | 73 (69.0-74.0) | 67.0 | 46 | 52 (44.5-66.5) |
| Male sex % (n) | 75.0 (165/220) | 27.2 (3/11) | 66.6 (4/6) | 33.3 (1/3) | 100 (1/1) | 0 (0/1) | 60.7 (17/28) |
| Serum creatinine (mg/dL) | 1.1 (0.9-1.4) | 1.0 (0.7-1.1) | 1.9 (1.0-4.9) | 1.1 (1.03-1.48) | 0.9 | 0.6 | 1.2 (0.9-1.7) |
| eGFR (mL/min/1.73 m2) | 68 (49.9-91.0) | 85.0 (65.2- 113.4) | 38.3 (14.5-75.7) | 57 (39.8-66.6) | 89.5 | 114.0 | 67 (40.0-95.0) |
| Proteinuria (g/24 h) | 8.0 (5.2-12.0) | 5.6 2.6-9.3) | 11.0 (6.8-16.1) | 3.2 ( 1.55-6.05) | 14.4 | 5.7 | 4.5 (3.2-9.9) |
| Malignancy % | 5.0 (11/220) | 9.0 (1/11) | 33.3 (2/6) | - | 100 (1/1) | - | 25.0 (7/28) |
| Autoimmunity % | 5.4 (12/220) | 81.8 (9/11) | 33.3 (2/6) | - | - | - | 46.4 (13/28) |
| Paraproteinmemia % | 4.0 (9/220) | - | - | - | 100 (1/1) | - | 35 (1/28) |
| Infection % | 0.4 (1/220) | - | - | - | - | - | - |
| NSAID % | 1.8 (4/220) | - | 16.6 (1/6) | - | - | - | 14.2 (6/28) |
| No associated disease % | 84.0 (185/220) | 18.1 (2/11) | 16.6 (1/6) | 100 (3/3) | - | 100 (1/1) | 21.4 (6/28) |
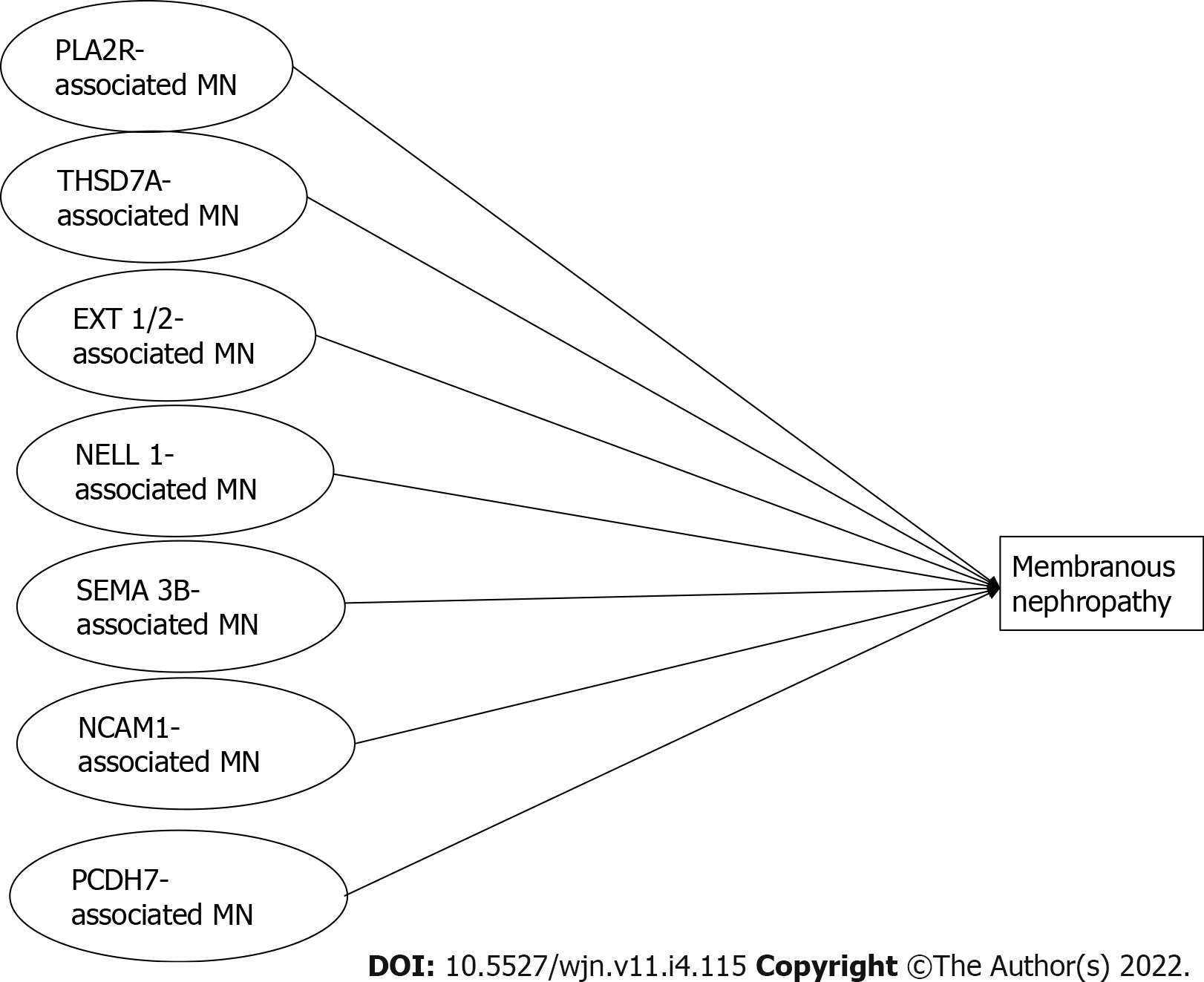
The new classification of MNs is shown in Figure 6.
Sethi[42] suggested that MN is not a single disease but rather a pattern of injury resulting from different diseases. Each antigen associated with the MN pattern should be considered as representative of a specific disease, and each disease results in an MN pattern of injury (Figure 7).
As described in Figure 6, other antigens and immune complexes are probably less frequently involved. Recently, Contactin 1 was shown to be a novel target antigen in MN associated with chronic inflammatory demyelinating polyneuropathy (CIDP)[43].
In 2018, Hashimoto et al[44] described a patient with chronic inflammatory demyelinating polyneuropathy with concurrent MN. CIDP may be due to autoantibodies against paranodal proteins, such as neurofascin 155 (NF155) and contactin-1 (CNTN1).
Autoantibody assays revealed the presence of IgG4- and IgG1-reactive anti- CNTN1 in MN. The authors hypothesized that CIDP with MN, can be detected by anti-CNTN1 antibodies in some cases.
More recently, Xu et al[45] described a 57-year-old man admitted to the hospital for limb numbness, weakness and sensory disorder. This man had MN and was diagnosed with anti-CNTN1 antibody-associated autoimmune nodopathy. Reviewing the literature, the authors found 22 cases of CIDP with MN, five of which were associated with the anti-CNTN1 antibody[46-49]. However, given the limited available research, no conclusions regarding a common antigen can be drawn.
In their recent study, Le Quintrec et al[43] looked for a novel target antigen by analyzing kidney biopsies from 5 patients positive for anti-contactin 1 antibodies who presented with MN combined with chronic inflammatory demyelinating polyneuropathy.
Western blot analysis revealed contactin 1 expression in kidney glomeruli. Confocal microscopic analysis showed the presence and colocalization of contactin 1 and IgG4 on the GBM. Eluted IgG could bind paranodal tissue and colocalized with commercial anti-contactin 1 antibody. Based on these findings, contactin 1 is a novel common antigenic target in MN associated with chronic inflammatory demyelinating polyneuropathy.
CTNT 1 is a glycosylphosphatidylinositol-anchored cell membrane protein expressed on the extracellular side.
Anti-CNTN1 predominantly comprises the IgG4 subclass. IgG4 deposits were found to colocalize with CNTN 1 or PLA2R1 in kidney biopsies. IgG4-PLA2R1-MN is considered a kidney autoimmune disease. After the formation of immune complexes, complement may be activated[50].
In conclusion, CNTN1 is the first discovered target involved in combined MN and anti-CNTN1-related CIDP.
Le Quintrec et al[43] detected three proteins (CNTN1, CASPR1, and NF155) in human glomerular extracts by immunoblotting and mass spectrometry. The authors were unable to show CNTN1 staining in podocytes in the normal human kidney. This finding could be ascribed to the fact that epitopes recognized by anti-CNTN1 antibodies are accessible only under pathological conditions[51].
MN has long been classified as primary MN (70%) with no disease association, and secondary MN (30%) with an underlying disease such as autoimmune disorders, tumors or infections. The principal known antigens involved as targets in primary MN were phospholipase A2 receptor and THSD7A. The availability of new techniques has allowed the discovery of new antigens and antibodies that are less frequently involved. Preliminary studies of patients at follow-up have shown different pathological findings and different outcomes associated with each of these new antigens. Now, it is possible that each new-type of antigen associated MN represents a distinct disease that causes the deposition of immune deposits along the GBM. The thickening of the GBM is the common result of these different diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alsaidan A, Saudi Arabia; Fujigaki Y, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1500] [Cited by in F6Publishing: 1535] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 2. | Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277-2287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 614] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 3. | Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053-2060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 348] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Debiec H, Nauta J, Coulet F, van der Burg M, Guigonis V, Schurmans T, de Heer E, Soubrier F, Janssen F, Ronco P. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet. 2004;364:1252-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Xie Q, Li Y, Xue J, Xiong Z, Wang L, Sun Z, Ren Y, Zhu X, Hao CM. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol. 2015;41:345-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Berchtold L, Zanetta G, Dahan K, Mihout F, Peltier J, Guerrot D, Brochériou I, Ronco P, Debiec H. Efficacy and Safety of Rituximab in Hepatitis B Virus-Associated PLA2R-Positive Membranous Nephropathy. Kidney Int Rep. 2018;3:486-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Stehlé T, Audard V, Ronco P, Debiec H. Phospholipase A2 receptor and sarcoidosis-associated membranous nephropathy. Nephrol Dial Transplant. 2015;30:1047-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Sethi S. New 'Antigens' in Membranous Nephropathy. J Am Soc Nephrol. 2021;32:268-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 130] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 9. | Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, Hummel AM, Specks U, Fervenza FC, Ronco P. Exostosin 1/Exostosin 2-Associated Membranous Nephropathy. J Am Soc Nephrol. 2019;30:1123-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 10. | Busse-Wicher M, Wicher KB, Kusche-Gullberg M. The exostosin family: proteins with many functions. Matrix Biol. 2014;35:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Ahn J, Lüdecke HJ, Lindow S, Horton WA, Lee B, Wagner MJ, Horsthemke B, Wells DE. Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nat Genet. 1995;11:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 306] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989;264:17615-17618. [PubMed] [Cited in This Article: ] |
| 13. | Busse M, Feta A, Presto J, Wilén M, Grønning M, Kjellén L, Kusche-Gullberg M. Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem. 2007;282:32802-32810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Cook A, Raskind W, Blanton SH, Pauli RM, Gregg RG, Francomano CA, Puffenberger E, Conrad EU, Schmale G, Schellenberg G. Genetic heterogeneity in families with hereditary multiple exostoses. Am J Hum Genet. 1993;53:71-79. [PubMed] [Cited in This Article: ] |
| 15. | Ravindran A, Casal Moura M, Fervenza FC, Nasr SH, Alexander MP, Fidler ME, Herrera Hernandez LP, Zhang P, Grande JP, Cornell LD, Gross LA, Negron V, Jenson GE, Madden BJ, Charlesworth MC, Sethi S. In Patients with Membranous Lupus Nephritis, Exostosin-Positivity and Exostosin-Negativity Represent Two Different Phenotypes. J Am Soc Nephrol. 2021;32:695-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | Anders HJ. Nephropathic autoantigens in the spectrum of lupus nephritis. Nat Rev Nephrol. 2019;15:595-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Caza TN, Hassen SI, Kuperman M, Sharma SG, Dvanajscak Z, Arthur J, Edmondson R, Storey A, Herzog C, Kenan DJ, Larsen CP. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 2021;100:171-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 18. | Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7696] [Cited by in F6Publishing: 9355] [Article Influence: 1039.4] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Zara J, Siu RK, Ting K, Soo C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res. 2010;89:865-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, Hori K. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn. 1995;203:212-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, Nakamura Y, Hirai Y, Maekawa H, Takahashi Ei. Cloning and characterization of two novel human cDNAs (NELL-1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | The Human Protein Atlas: NELL-1. [cited 20 April 2022]. Available from: www.proteinatlas.org/ENSG00000165973-NELL-1/tissue/kidney. [Cited in This Article: ] |
| 23. | Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, Ravindran A, Buob D, Jadoul M, Fervenza FC, Ronco P. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 24. | Ahmad SB, Appel GB. Antigens, antibodies, and membranous nephropathy: a decade of progress. Kidney Int. 2020;97:29-31. [PubMed] [Cited in This Article: ] |
| 25. | Kudose S, Santoriello D, Debiec H, Canetta PA, Bomback AS, Stokes MB, Batal I, Ronco P, D'Agati VD, Markowitz GS. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. 2021;99:247-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Caza TN, Hassen SI, Dvanajscak Z, Kuperman M, Edmondson R, Herzog C, Storey A, Arthur J, Cossey LN, Sharma SG, Kenan DJ, Larsen CP. NELL-1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99:967-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 27. | Spain RI, Andeen NK, Gibson PC, Samuels MH, Morris CD, Solomon AJ, Solomon R, Waslo C, Avasare RS. Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL-1)-associated membranous nephropathy. Kidney Int. 2021;100:1208-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Ronco P, Debiec H. Membranous nephropathy: current understanding of various causes in light of new target antigens. Curr Opin Nephrol Hypertens. 2021;30:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A, Gross L, Ulinski T, Buob D, Tran CL, Emma F, Diomedi-Camassei F, Fervenza FC, Ronco P. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020;98:1253-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 30. | Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int. 2006;69:1564-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Alto LT, Terman JR. Semaphorins and their Signaling Mechanisms. Methods Mol Biol. 2017;1493:1-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 32. | Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 33. | Tapia R, Guan F, Gershin I, Teichman J, Villegas G, Tufro A. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2008;73:733-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Fila M, Debiec H, Perrochia H, Djouadi N, Verpont MC, Buob D, Ronco P. Recurrence of Anti-Semaphorin 3B-Mediated Membranous Nephropathy after Kidney Transplantation. J Am Soc Nephrol. 2022;33:503-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Chauhan V, Anis A, Chauhan A. Effects of Starvation on the Levels of Triglycerides, Diacylglycerol, and Activity of Lipase in Male and Female Drosophila Melanogaster. J Lipids. 2021;2021:5583114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 36. | Hanset N, Aydin S, Demoulin N, Cosyns JP, Castanares-Zapatero D, Crott R, Cambier JF, Pochet JM, Gillerot G, Reginster F, Houssiau F, Debiec H, Ronco P, Jadoul M, Morelle J; UCLouvain Kidney Disease Network. Podocyte Antigen Staining to Identify Distinct Phenotypes and Outcomes in Membranous Nephropathy: A Retrospective Multicenter Cohort Study. Am J Kidney Dis. 2020;76:624-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 2012;22:299-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 38. | Khalili-Shirazi A, Kaisar M, Mallinson G, Jones S, Bhelt D, Fraser C, Clarke AR, Hawke SH, Jackson GS, Collinge J. Beta-PrP form of human prion protein stimulates production of monoclonal antibodies to epitope 91-110 that recognise native PrPSc. Biochim Biophys Acta. 2007;1774:1438-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199-3214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 738] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 40. | Ravindran A, Madden B, Charlesworth MC, Sharma R, Sethi A, Debiec H, Cattran D, Fervenza FC, Smith RJ, Ronco P, Sethi S. Proteomic Analysis of Complement Proteins in Membranous Nephropathy. Kidney Int Rep. 2020;5:618-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Bobart SA, Tehranian S, Sethi S, Alexander MP, Nasr SH, Moura Marta C, Vrana JA, Said S, Giesen CD, Lieske JC, Fervenza FC, De Vriese AS. A Target Antigen-Based Approach to the Classification of Membranous Nephropathy. Mayo Clin Proc. 2021;96:577-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 42. | Sethi S. Membranous nephropathy: a single disease or a pattern of injury resulting from different diseases. Clin Kidney J. 2021;14:2166-2169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Le Quintrec M, Teisseyre M, Bec N, Delmont E, Szwarc I, Perrochia H, Machet MC, Chauvin A, Mavroudakis N, Taieb G, Lanfranco L, Rigothier C, José B, Concetta C, Geneste C, Pernin V, Larroque C, Devaux J, Beyze A. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int. 2021;100:1240-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Hashimoto Y, Ogata H, Yamasaki R, Sasaguri T, Ko S, Yamashita K, Xu Z, Matsushita T, Tateishi T, Akiyama S, Maruyama S, Yamamoto A, Kira JI. Chronic Inflammatory Demyelinating Polyneuropathy With Concurrent Membranous Nephropathy: An Anti-paranode and Podocyte Protein Antibody Study and Literature Survey. Front Neurol. 2018;9:997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Xu Q, Liu S, Zhang P, Wang Z, Chang X, Liu Y, Yan J, He R, Luo X, Zou LY, Chu X, Guo Y, Huang S, Fu X, Huang Y. Characteristics of Anti-Contactin1 Antibody-Associated Autoimmune Nodopathies With Concomitant Membranous Nephropathy. Front Immunol. 2021;12:759187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Doppler K, Appeltshauser L, Wilhelmi K, Villmann C, Dib-Hajj SD, Waxman SG, Mäurer M, Weishaupt A, Sommer C. Destruction of paranodal architecture in inflammatory neuropathy with anti-contactin-1 autoantibodies. J Neurol Neurosurg Psychiatry. 2015;86:720-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 47. | Witte AS, Burke JF. Membranous glomerulonephritis associated with chronic progressive demyelinating neuropathy. Neurology. 1987;37:342-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Kohli A, Tandon P, Kher V. Chronic inflammatory demyelinating polyradiculoneuropathy with membranous glomerulonephritis: report of one case. Clin Neurol Neurosurg. 1992;94:31-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Plemel DJA, Nazarali SA, Damji KF, Chen SH. Tube shunt surgery in pig eyes: a wet lab teaching model. Can J Ophthalmol. 2019;54:585-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Brglez V, Boyer-Suavet S, Seitz-Polski B. Complement Pathways in Membranous Nephropathy: Complex and Multifactorial. Kidney Int Rep. 2020;5:572-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Debiec H, Ronco P. When contactin antibodies hit the podocyte: a new neurorenal syndrome. Kidney Int. 2021;100:1163-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |