Copyright
©The Author(s) 2016.
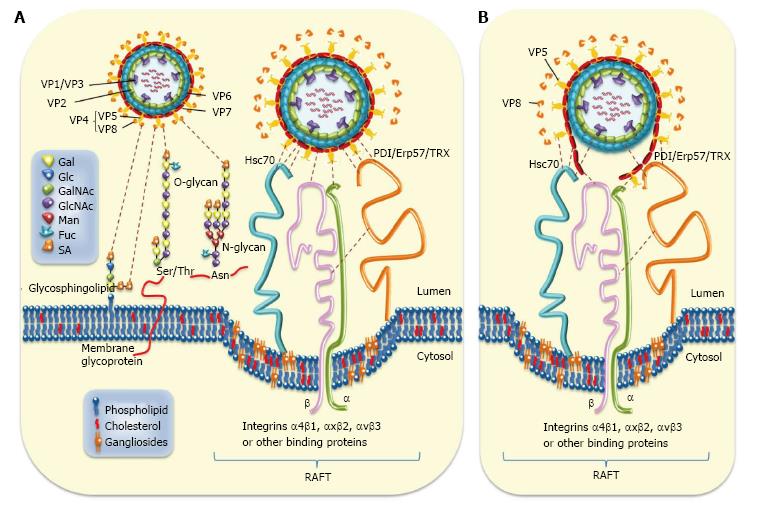
Figure 1 Rotavirus-cell surface interactions during entry.
A: The rotavirus particle-associated proteins (VP1/2/3/4/5/6/7) that enclose the viral genome are represented. Cell surface molecules including sialic acid (SA), Hsc70, PDI, Erp57, thioredoxin (TRX), and integrins α4β1, αxβ2, and αvβ3 are also represented. Infection is initiated by the VP8*-mediated binding (attachment) of virion to terminal or non-terminal (neuraminidase -resistant) SAs located on cell surface glycolipids including gangliosides or to SAs located on cell surface glycoproteins. The N- and O-substituted derivatives of neuraminic acid (SAs) are indicated. Neuraminidase-resistant rotavirus strains can bind directly to integrin α2β1 through VP5* DGE sequence. SA-dependent strains bind first through VP8* to SA before interacting with integrin α2β1 through VP5*. A putative caveolae containing raft-associated cell surface receptors is depicted. The sequence of virion-cell interactions taking place after binding to α2β1 has not yet been established. However, several interactions involving rotavirus structural protein (VP5*, VP7 and VP6) and raft-associated cell surface receptors (Hsc70, PDI and integrins α4β1, αxβ2 and αvβ3) have been documented. Interactions between rotavirus structural proteins and cell surface molecules are illustrated; B: Disruption of rotavirus proteins (VP5*, VP7 and VP6) caused by cell surface-associated chaperone (Hsc70, PDI) and oxido-reductase activities (PDI, integrin αvβ3) is depicted. Hsc70: Heat shock cognate protein 70; PDI: Protein disulfide isomerase.
- Citation: Guerrero CA, Acosta O. Inflammatory and oxidative stress in rotavirus infection. World J Virol 2016; 5(2): 38-62
- URL: https://www.wjgnet.com/2220-3249/full/v5/i2/38.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i2.38