Copyright
©2013 Baishideng.
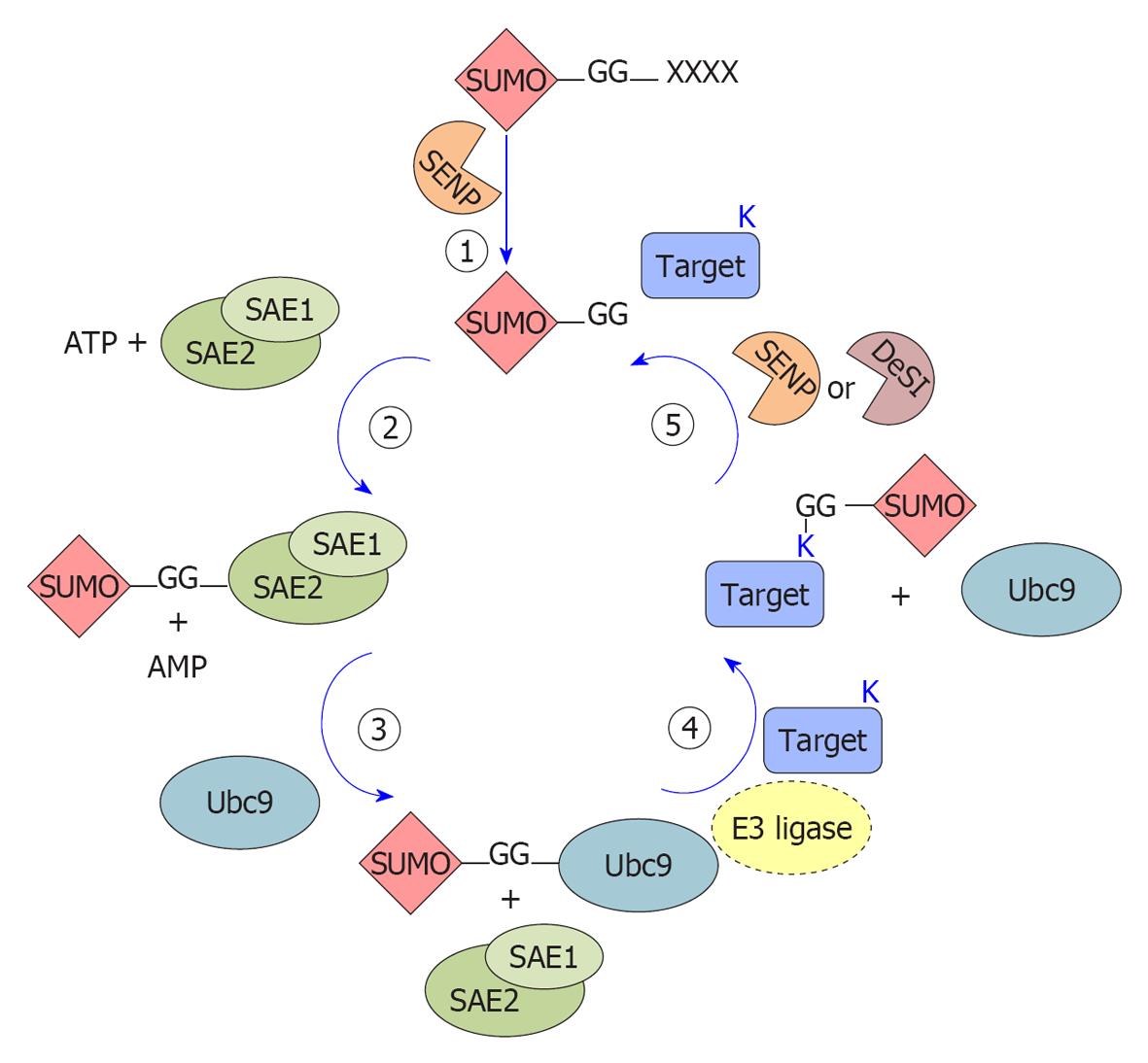
Figure 1 Schematic representation of the small ubiquitin-like modifier conjugation enzymatic cascade.
The cartoon schematically represents the enzymatic steps of small ubiquitin-like modifier (SUMO) protein conjugation on lysines of substrate proteins. 1: SUMO protein precursors are processed by SUMO proteases (SENP) that remove the C-terminal tetrapeptide (X) and free the diglycine motif (-GG); 2: The mature form of SUMO is activated by adenylation at the C-terminal diglycine motif by the E1 enzyme (the SUMO activating enzyme, SAE1-SAE2 or AOS1-UBA2) promoting a thioester bond with a conserved Cys of the E1 enzyme; 3: SUMO is then transferred to a Cys on the E2 conjugating enzyme (Ubc9) forming an E2-SUMO thioester; 4: An isopeptide bond is formed between the diglycine motif of SUMO and a lysine (K) residue in the substrate. E3 ligases are dispensable in vitro but most likely required in vivo; 5: SUMO proteins are removed from substrates by the action of SUMO proteases (SENPs) or DeSumoylating-isopeptidase (DeSI) and free SUMO proteins are available for another cycle of conjugation.
- Citation: Mattoscio D, Segré CV, Chiocca S. Viral manipulation of cellular protein conjugation pathways: The SUMO lesson. World J Virol 2013; 2(2): 79-90
- URL: https://www.wjgnet.com/2220-3249/full/v2/i2/79.htm
- DOI: https://dx.doi.org/10.5501/wjv.v2.i2.79