Copyright
©2013 Baishideng.
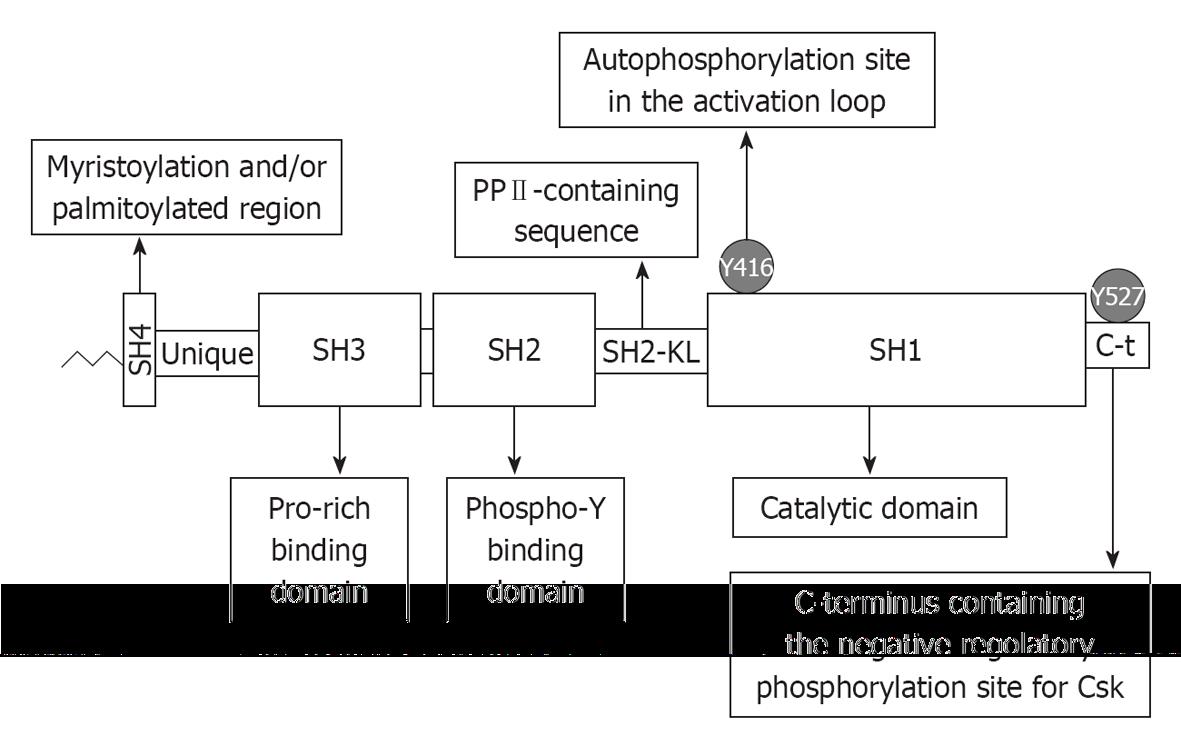
Figure 1 Diagram representing the domain organization of Src family kinases.
As reported in the text, the C-terminus (C-t in the figure) when phosphorylated at Tyr527 binds to the Src homology (SH)2 domain and the polyproline type II helical motif (PPII) motif in the SH2 kinase linker (SH2-KL in the figure) engages the SH3 domain, thus inducing an inactive conformation. Disruption of these inhibitory interactions, in the case of viruses mostly induced by proteins bearing tyrosine phosphorylated or proline-rich motifs, leads to the full activation of Src family kinases.
- Citation: Pagano MA, Tibaldi E, Palù G, Brunati AM. Viral proteins and Src family kinases: Mechanisms of pathogenicity from a “liaison dangereuse”. World J Virol 2013; 2(2): 71-78
- URL: https://www.wjgnet.com/2220-3249/full/v2/i2/71.htm
- DOI: https://dx.doi.org/10.5501/wjv.v2.i2.71